Redox Homeostasis and Non-Invasive Assessment of Significant Liver Fibrosis by Shear Wave Elastography
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalifa, A.; Rockey, D.C. The utility of liver biopsy in 2020. Curr. Opin. Gastroenterol. 2020, 36, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.K.; Torbenson, M.S. Liver fibrosis quantification. Abdom. Radiol. 2022, 47, 1032–1052. [Google Scholar] [CrossRef]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef] [PubMed]
- Elpek, G.O. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J. Gastroenterol. 2014, 20, 7260–7276. [Google Scholar] [CrossRef]
- Gressner, A.M.; Weiskirchen, R.; Breitkopf, K.; Dooley, S. Roles of TGF-beta in hepatic fibrosis. Front. Biosci. 2002, 7, d793–d807. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta 2007, 381, 107–113. [Google Scholar] [CrossRef]
- Guechot, J.; Laudat, A.; Loria, A.; Serfaty, L.; Poupon, R.; Giboudeau, J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin. Chem. 1996, 42, 558–563. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Karsdal, M.A.; Leeming, D.J.; Genovese, F. Molecular serum markers of liver fibrosis. Biomark. Insights 2012, 7, 105–117. [Google Scholar] [CrossRef]
- Berzigotti, A.; Ashkenazi, E.; Reverter, E.; Abraldes, J.G.; Bosch, J. Non-invasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Dis. Mark. 2011, 31, 129–138. [Google Scholar] [CrossRef]
- Papastergiou, V.; Tsochatzis, E.; Burroughs, A.K. Non-invasive assessment of liver fibrosis. Ann. Gastroenterol. 2012, 25, 218–231. [Google Scholar]
- Lurie, Y.; Webb, M.; Cytter-Kuint, R.; Shteingart, S.; Lederkremer, G.Z. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J. Gastroenterol. 2015, 21, 11567–11583. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Li, K.; Beard, M.R.; Showalter, L.A.; Scholle, F.; Lemon, S.M.; Weinman, S.A. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002, 122, 366–375. [Google Scholar] [CrossRef]
- Abrahamovych, M.; Abrahamovych, O.; Fayura, O.; Tolopko, S. Relation between redox homeostasis blood parameters in cirrhotic patients and endothelial dysfunction development. Min. Gastroenterol. Dietol. 2020, 66, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Kasper, D.; Fitting, D.; Knop, V.; Vermehren, A.; Sprinzl, K.; Hansmann, M.L.; Herrmann, E.; Bojunga, J.; Albert, J.; et al. Assessment of liver fibrosis with 2-D shear wave elastography in comparison to transient elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultras Med. Biol. 2015, 41, 2350–2359. [Google Scholar] [CrossRef]
- Virarkar, M.; Morani, A.C.; Taggart, M.W.; Bhosale, P. Liver Fibrosis Assessment. Semin. Ultrasound CT MRI 2021, 42, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Branchi, F.; Conti, C.B.; Baccarin, A.; Lampertico, P.; Conte, D.; Fraquelli, M. Non-invasive assessment of liver fibrosis in chronic hepatitis B. World J. Gastroenterol. 2014, 20, 14568–14580. [Google Scholar] [CrossRef]
- Boursier, J.; de Ledinghen, V.; Leroy, V.; Anty, R.; Francque, S.; Salmon, D.; Lannes, A.; Bertrais, S.; Oberti, F.; Fouchard-Hubert, I.; et al. A stepwise algorithm using an at-a-glance first-line test for the non-invasive diagnosis of advanced liver fibrosis and cirrhosis. J. Hepatol. 2017, 66, 1158–1165. [Google Scholar] [CrossRef]
- Bota, S.; Sporea, I.; Sirli, R.; Focsa, M.; Popescu, A.; Danila, M.; Strain, M. Can ARFI elastography predict the presence of significant esophageal varices in newly diagnosed cirrhotic patients? Ann. Hepatol. 2012, 11, 519–525. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2021, 71, 1006–1019. [Google Scholar] [CrossRef]
- Sebastiani, G.; Halfon, P.; Castera, L.; Mangia, A.; Di Marco, V.; Pirisi, M.; Voiculescu, M.; Bourliere, M.; Alberti, A. Comparison of three algorithms of non-invasive markers of fibrosis in chronic hepatitis C. Aliment. Pharmacol. Ther. 2012, 35, 92–104. [Google Scholar] [CrossRef]
- Piscaglia, F.; Salvatore, V.; Mulazzani, L.; Cantisani, V.; Schiavone, C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016, 37, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Parekh, P.; Levitov, A.B.; Filice, C. Shear Wave Elastography for Evaluation of Liver Fibrosis. J. Ultrasound Med. 2014, 33, 197–203. [Google Scholar] [CrossRef]
- Kleiner, D.; Szilvas, A.; Szentmihalyi, K.; Sule, K.; Blazovics, A. Changes of erythrocyte element status of colectomysed cancerous patients: Retrospective study. J. Trace Elem. Med. Biol. 2016, 33, 8–13. [Google Scholar] [CrossRef]
- Ellman, G.L.; Lysko, H. Disulfide and sulfhydryl compounds in TCA extracts of human blood and plasma. J. Lab. Clin. Med. 1967, 70, 518–527. [Google Scholar]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Blázovics, A.; Sárdi, É. Methodological repertoire development to study the effect of dietary supplementation in cancer therapy. Microchem. J. 2018, 136, 121–127. [Google Scholar] [CrossRef]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef]
- Luo, Q.T.; Zhu, Q. Diagnostic Performance of Transient Elastography Versus Two-Dimensional Shear Wave Elastography for Liver Fibrosis in Chronic Viral Hepatitis: Direct Comparison and a Meta-Analysis. BioMed Res. Int. 2022, 2022, 1960244. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Stasi, C.; Milani, S. Evolving strategies for liver fibrosis staging: Non-invasive assessment. World J. Gastroenterol. 2017, 23, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Crosas-Molist, E.; Fabregat, I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 2015, 6, 106–111. [Google Scholar] [CrossRef]
- Ozturk, A.; Olson, M.C.; Samir, A.E.; Venkatesh, S.K. Liver fibrosis assessment: MR and US elastography. Abdom. Radiol. 2022, 47, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, B.; Wu, H.; Lin, F.; Deng, W. Accuracy of real-time shear wave elastography in staging hepatic fibrosis: A meta-analysis. BMC Med. Imaging 2020, 20, 16. [Google Scholar] [CrossRef]
- Barr, R.G.; Wilson, S.R. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020, 296, 263–274. [Google Scholar] [CrossRef]
- Zayadeen, A.R.; Hijazeen, S.; Smadi, M.; Fayyad, L.; Halasa, M.; AlQusous, S.; AlRabadi, O.; Hijazeen, R.; Ajlouni, Y.; Tulenko, K.; et al. Comparing shear wave elastography with liver biopsy in the assessment of liver fibrosis at King Hussein Medical Center. Egypt. Liver J. 2022, 12, 24. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.-S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Stern, C.; Ngo, A.; Villela-Nogueira, C.; Thabut, D.; Ratziu, V. A Simple and Reliable 2D-Shear Wave Elastography and UltraSound Coefficient Attenuation Parameter Technique in Chronic Liver Diseases. Dig. Dis. Sci. 2024, 69, 2648–2654. [Google Scholar] [CrossRef]
- Haghgoo, S.M.; Sharafi, H.; Alavian, S.M. Serum cytokines, adipokines and ferritin for non-invasive assessment of liver fibrosis in chronic liver disease: A systematic review. Clin. Chem. Lab. Med. 2018, 57, 577–610. [Google Scholar] [CrossRef]
- Abu-Tair, L.; Doron, S.; Mahamid, M.; Amer, J.; Safadi, R. Leptin modulates lymphocytes’ adherence to hepatic stellate cells is associated with oxidative status alterations. Mitochondrion 2013, 13, 473–480. [Google Scholar] [CrossRef]
- Makri, E.S.; Evripidou, K.; Polyzos, S.A. Circulating leptin in patients with nonalcoholic fatty liver disease-related liver fibrosis: A systematic review and a meta-analysis. J. Gastroenterol. Hepatol. 2024, 39, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, W.; Honsawek, S.; Poovorawan, Y. Adiponectin as a novel biomarker for liver fibrosis. World J. Hepatol. 2018, 10, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Angin, Y.; Arslan, N.; Kuralay, F. Leptin-to-adiponectin ratio in obese adolescents with nonalcoholic fatty liver disease. Turk. J. Pediatr. 2014, 56, 259–266. [Google Scholar] [PubMed]
- Kamada, Y.; Tamura, S.; Kiso, S.; Matsumoto, H.; Saji, Y.; Yoshida, Y.; Fukui, K.; Maeda, N.; Nishizawa, H.; Nagaretani, H.; et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 2003, 125, 1796–1807. [Google Scholar] [CrossRef]
- Saxena, N.K.; Anania, F.A. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. 2015, 26, 153–161. [Google Scholar] [CrossRef]
- Dong, Z.; Su, L.; Esmaili, S.; Iseli, T.J.; Ramezani-Moghadam, M.; Hu, L.; Xu, A.; George, J.; Wang, J. Adiponectin attenuates liver fibrosis by inducing nitric oxide production of hepatic stellate cells. J. Mol. Med. 2015, 93, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Tontikidou, C.; Makri, E.S.; Evripidou, K.; Goulis, D.G.; Goulas, A.; Polyzos, S.A. Circulating adiponectin in patients with nonalcoholic fatty liver disease-related liver fibrosis: A systematic review and a meta-analysis. J. Gastroenterol. Hepatol. 2022, 37, 1853–1864. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Dhawan, A. Noninvasive biomarkers in non-alcoholic fatty liver disease: Current status and a glimpse of the future. World J. Gastroenterol. 2014, 20, 10851–10863. [Google Scholar] [CrossRef]
- Neuman, M.G.; Maor, Y.; Nanau, R.M.; Melzer, E.; Mell, H.; Opris, M.; Cohen, L.; Malnick, S. Alcoholic Liver Disease: Role of Cytokines. Biomolecules 2015, 5, 2023–2034. [Google Scholar] [CrossRef]
- Jung, Y.K.; Yim, H.J. Reversal of liver cirrhosis: Current evidence and expectations. Korean J. Intern. Med. 2017, 32, 213–228. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar] [CrossRef]
- Milito, A.; Brancaccio, M.; D’Argenio, G.; Castellano, I. Natural Sulfur-Containing Compounds: An Alternative Therapeutic Strategy against Liver Fibrosis. Cells 2019, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Konzack, A.; Pihlajaniemi, T.; Heljasvaara, R.; Kietzmann, T. Redox-fibrosis: Impact of TGFβ1 on ROS generators, mediators and functional consequences. Redox Biol. 2015, 6, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Karanjia, R.N.; Crossey, M.M.; Cox, I.J.; Fye, H.K.; Njie, R.; Goldin, R.D.; Taylor-Robinson, S.D. Hepatic steatosis and fibrosis: Non-invasive assessment. World J. Gastroenterol. 2016, 22, 9880–9897. [Google Scholar] [CrossRef]
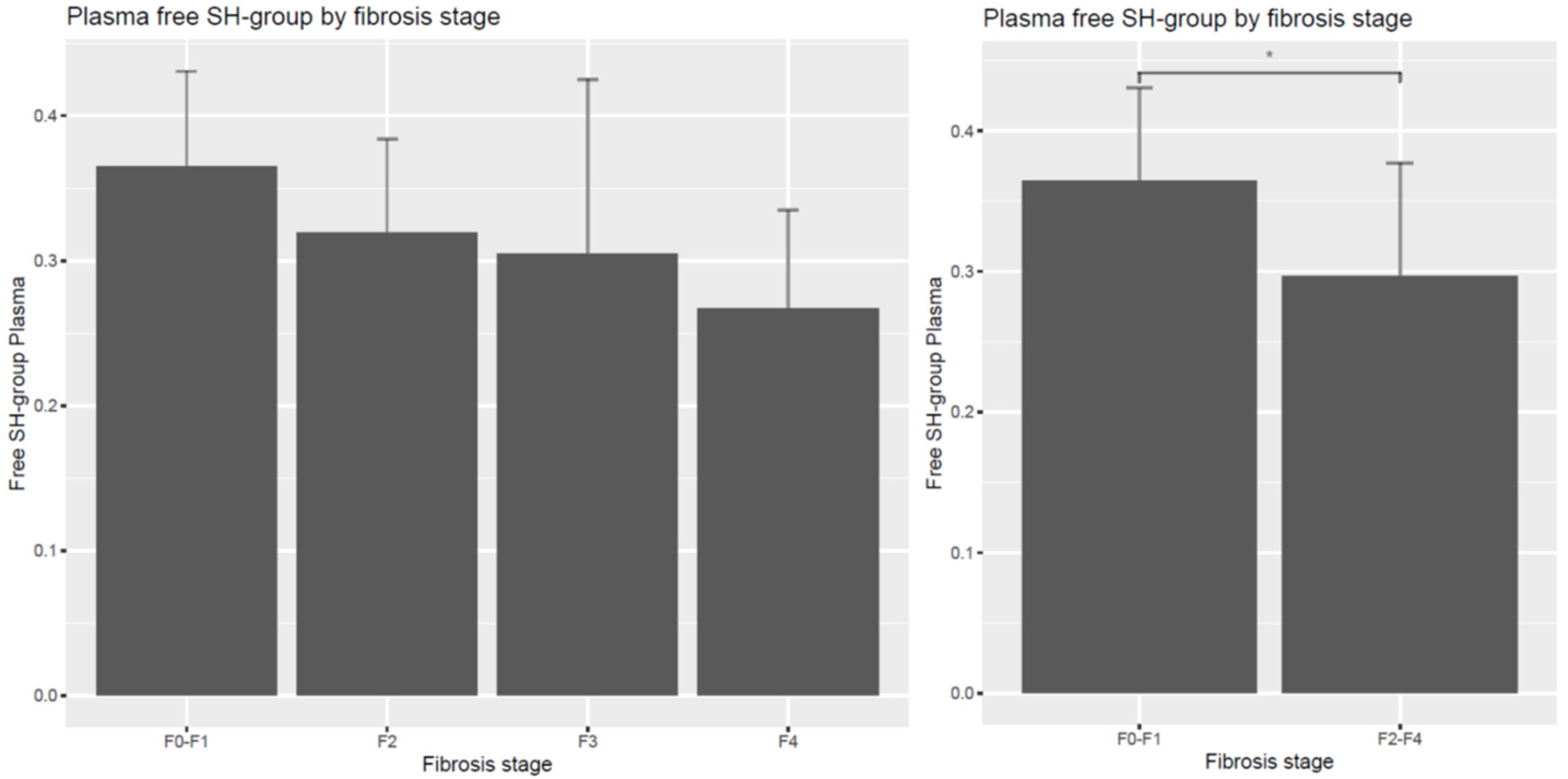
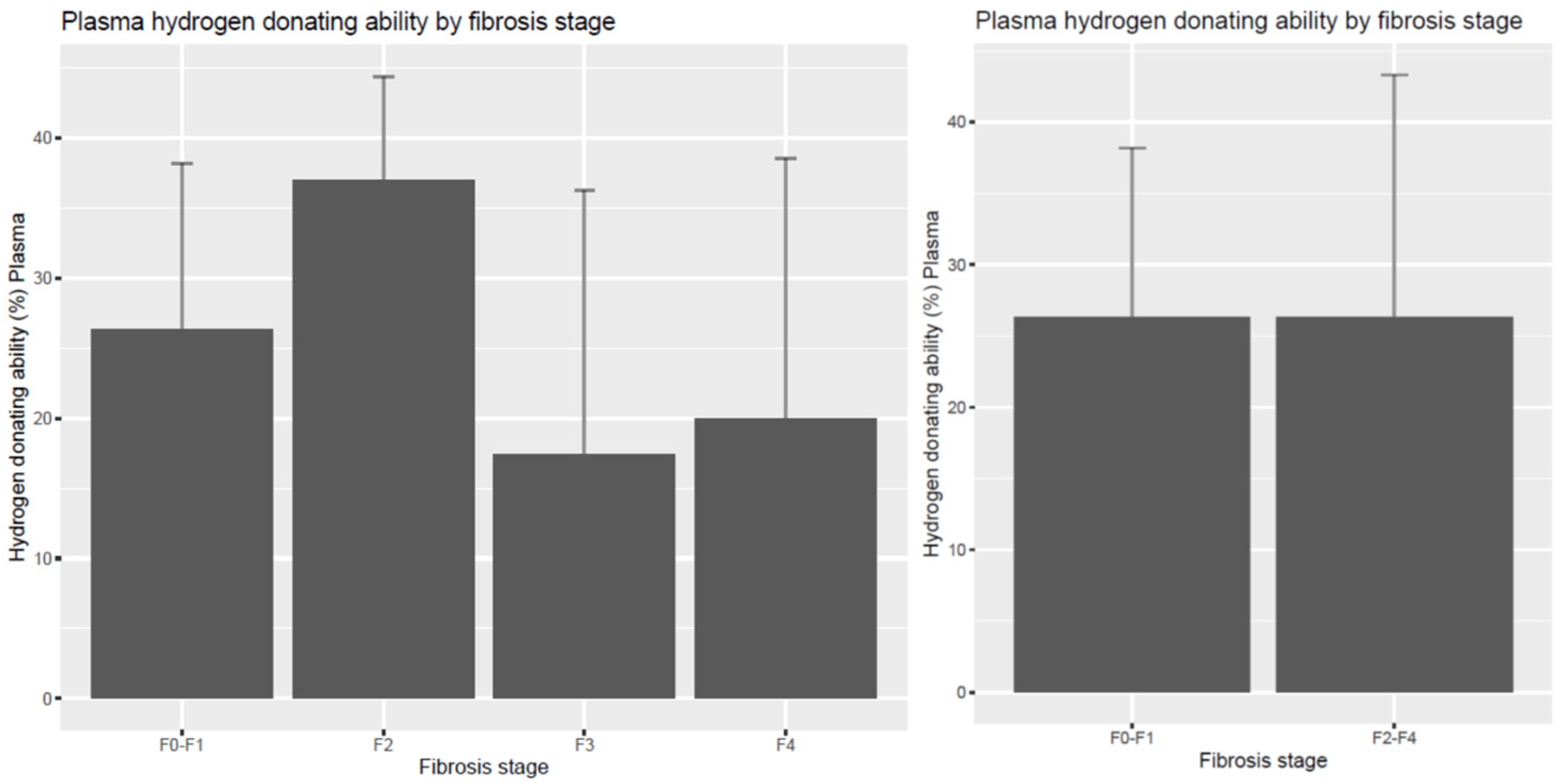
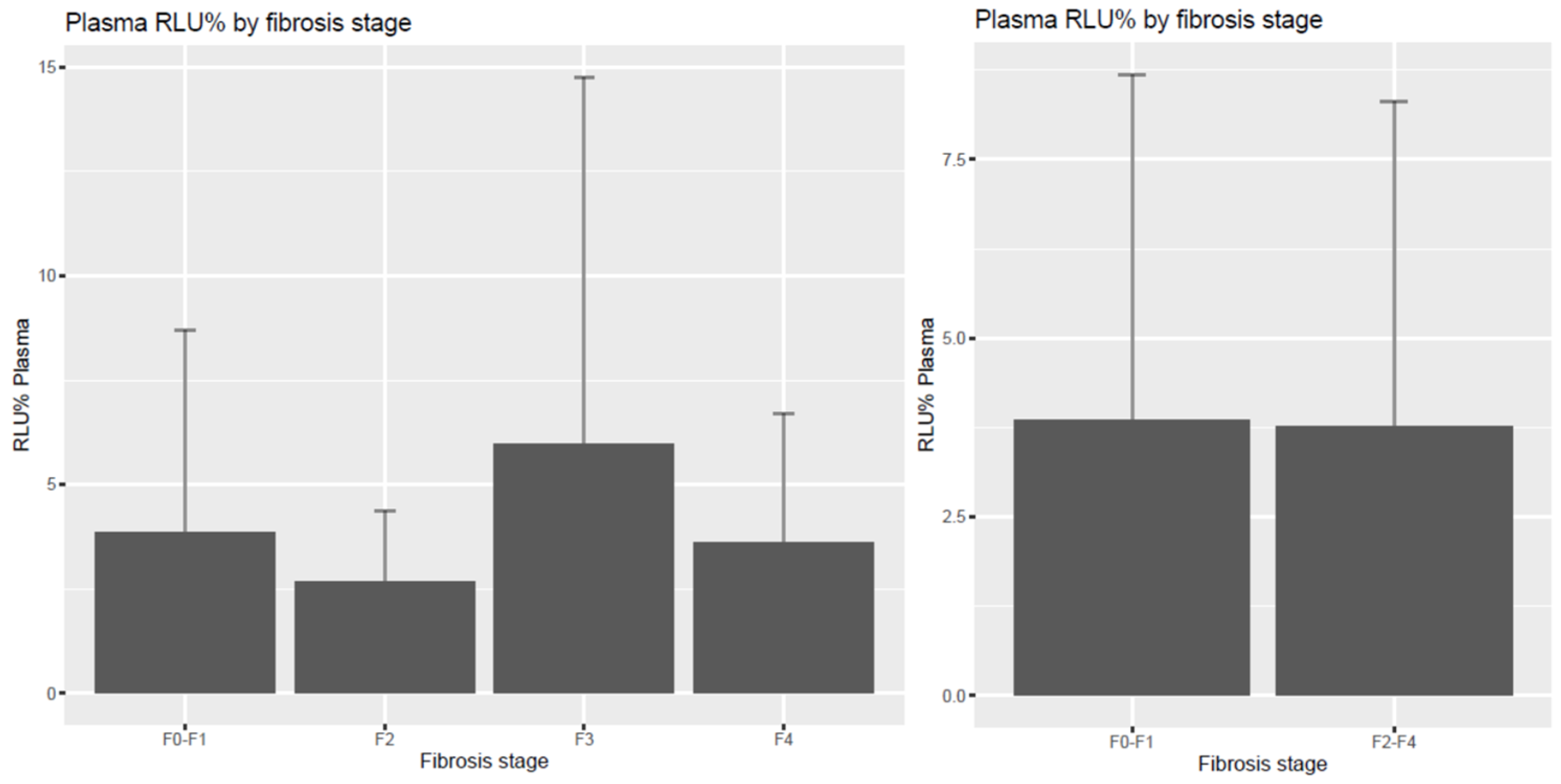

| Dimension | F0–F1 | F2 | F3 | F4 | F2–4 |
|---|---|---|---|---|---|
| Leptin (ng/mL) | 4.88 ± 7.23 | 5.25 ± 5.60 | 4.51 ± 5.59 | 6.20 ± 7.23 | 5.45 ± 5.94 |
| Adiponectin (pg/mL) | 12,374.68 ± 13,658.2 | 9560.93 ± 6356.05 | 24,665 ± 21,830.25 | 16,810 ± 12,070.07 | 15,629.7 ± 13,801.38 |
| Interleukin-6 (pg/mL) | 1.36 ± 1.19 | 1.69 ± 1.65 | 2.21 ± 2.34 | 2.45 ± 2.93 | 2.12 ± 2.28 |
| TNF-α (pg/mL) | 78.91 ± 72.89 | 155.47 ± 151.01 | 41.07 ± 28.06 | 63.65 ± 57.59 | 94.34 ± 109.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egresi, A.; Blázovics, A.; Lengyel, G.; Tóth, A.G.; Csongrády, B.; Jakab, Z.; Hagymási, K. Redox Homeostasis and Non-Invasive Assessment of Significant Liver Fibrosis by Shear Wave Elastography. Diagnostics 2024, 14, 1945. https://doi.org/10.3390/diagnostics14171945
Egresi A, Blázovics A, Lengyel G, Tóth AG, Csongrády B, Jakab Z, Hagymási K. Redox Homeostasis and Non-Invasive Assessment of Significant Liver Fibrosis by Shear Wave Elastography. Diagnostics. 2024; 14(17):1945. https://doi.org/10.3390/diagnostics14171945
Chicago/Turabian StyleEgresi, Anna, Anna Blázovics, Gabriella Lengyel, Adrienn Gréta Tóth, Barbara Csongrády, Zsuzsanna Jakab, and Krisztina Hagymási. 2024. "Redox Homeostasis and Non-Invasive Assessment of Significant Liver Fibrosis by Shear Wave Elastography" Diagnostics 14, no. 17: 1945. https://doi.org/10.3390/diagnostics14171945






