Advances in Ultrasound-Guided Surgery and Artificial Intelligence Applications in Musculoskeletal Diseases
Abstract
:1. Introduction
2. Ultrasound-Guided Surgery for Musculoskeletal Diseases
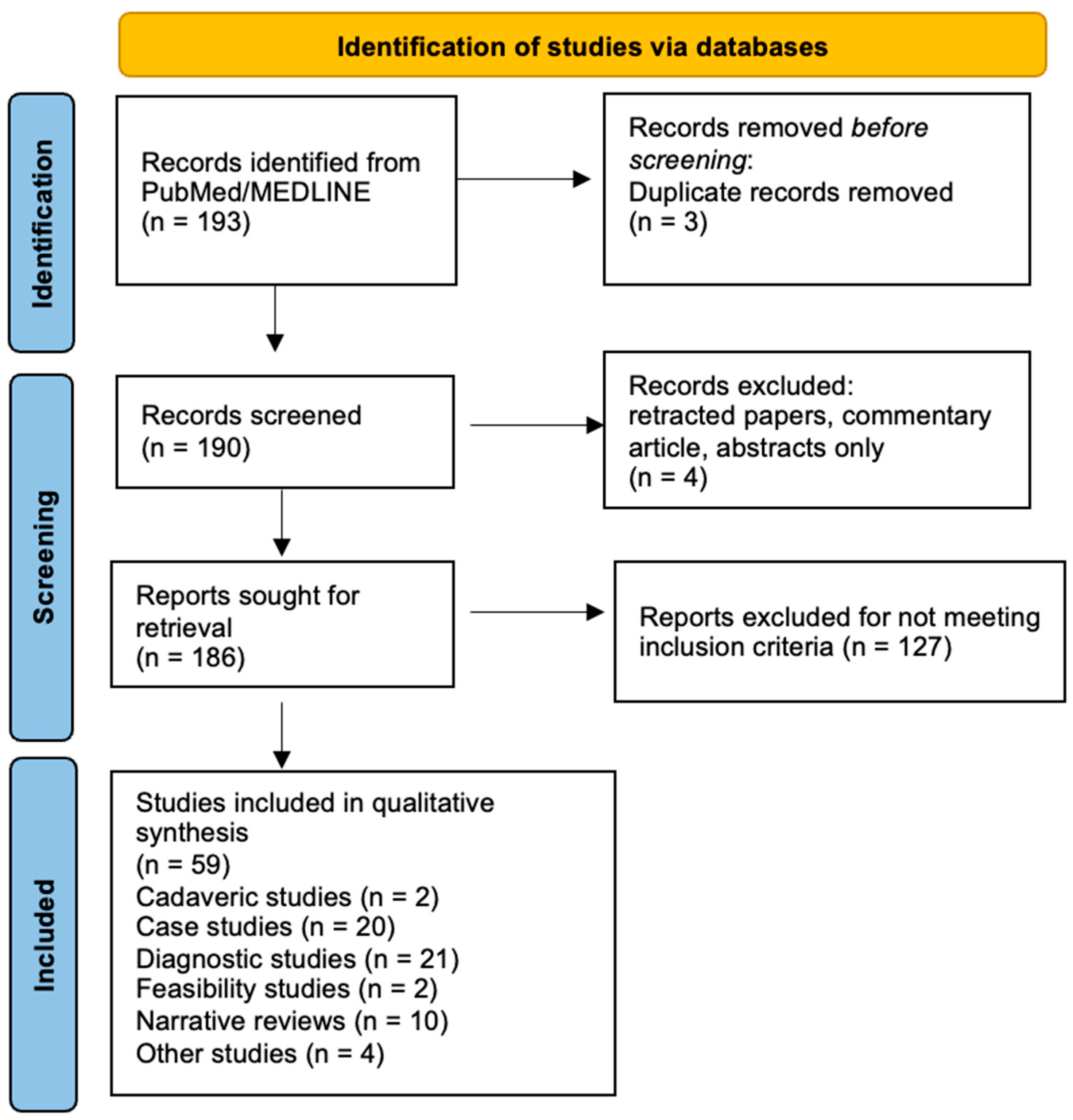
2.1. Literature Search
2.2. Classification of Studies
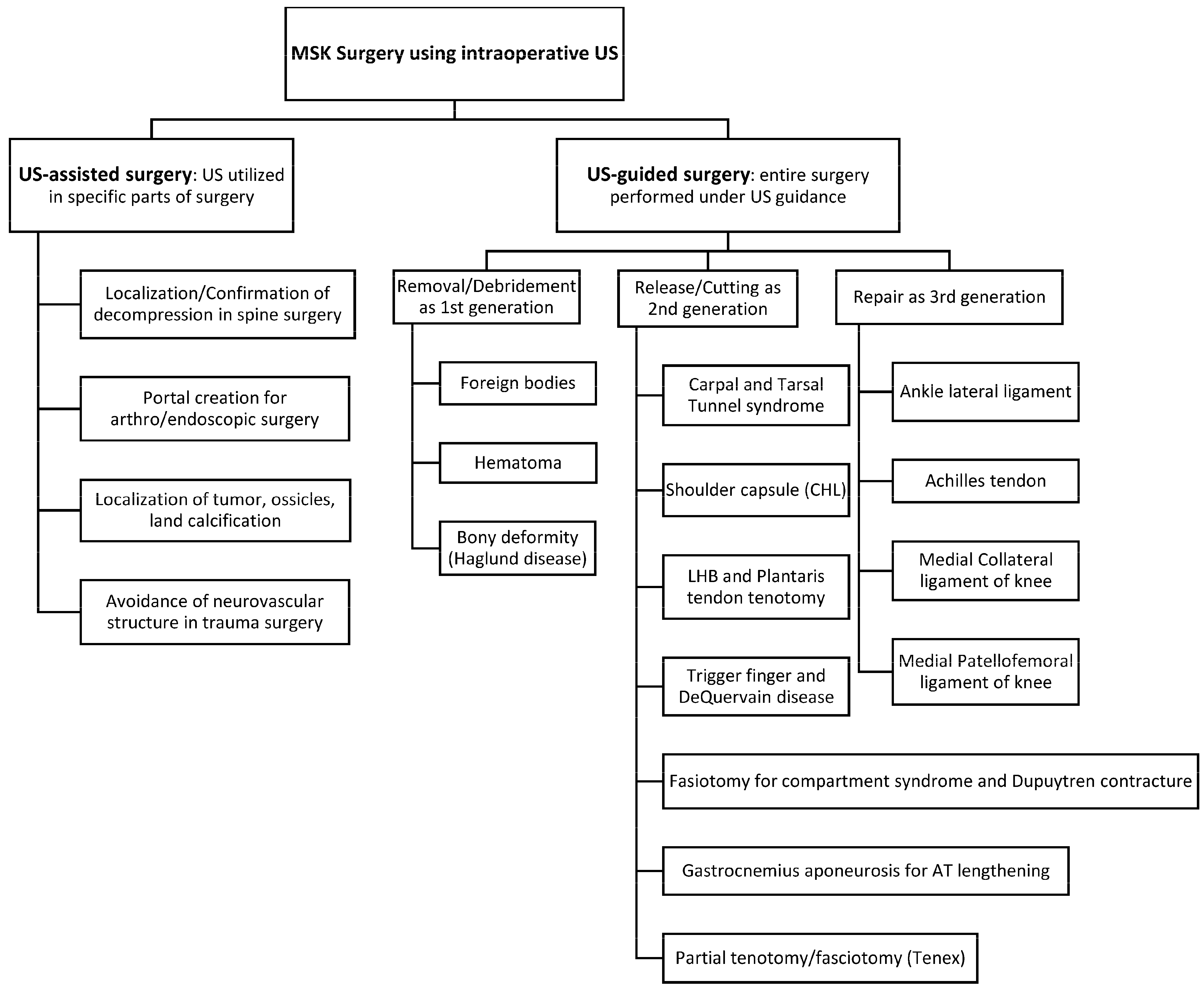
2.3. Definition of Ultrasound-Guided Surgery
2.4. Ultrasound-Assisted Surgery
2.5. Classification of Ultrasound-Guided Surgery
2.6. Cadaveric Studies, Case Reports/Technical Notes, and Case Series
2.7. Clinical Studies above Evidence Level 3
2.8. Randomized Control Trials and Meta-Analysis
2.9. Strength of Ultrasound-Guided Surgery for Musculoskeletal Pathologies
3. Artificial Intelligence and Musculoskeletal Ultrasound
3.1. Literature Search
3.2. Classification of Studies
3.3. Inclusion Criteria and Definitions of Artificial Intelligence, Deep Learning, and Convolutional Neural Network
3.4. Types of Studies
3.5. Non-Diagnostic Studies
3.6. Diagnostic Studies
3.6.1. Screening
3.6.2. Diagnosis
3.6.3. Prediction
3.7. Benefits of Utilizing AI/DL in Ultrasound Evaluation
3.8. Limitations of AI/DL-Assisted Ultrasound
3.8.1. Image Quality Dependency
3.8.2. Region of Interest (ROI) Sensitivity
4. Utility of Artificial Intelligence in Ultrasound-Guided Surgery
4.1. Literature Search
4.2. AI in Ultrasound-Guided Surgery
4.3. Applications in Spine Surgery
4.4. Current State and Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McDonald, D.G.; Leopold, G.R. Ultrasound B-scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br. J. Radiol. 1972, 45, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Mayer, V. Ultrasonography of the rotator cuff. J. Ultrasound Med. 1985, 4, 608. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, P.L.; Tsang, I.; Truelove, L.; Knickerbocker, W.J. Gray scale ultrasound in the evaluation of rheumatoid arthritis of the knee. Radiology 1978, 126, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Finnof, J.T.; Hall, M.M.; Adams, E.; Berkoff, D.; Concoff, A.L.; Dexter, W.; Smith, J. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. PM R 2015, 7, 151–168. [Google Scholar] [CrossRef]
- Hattori, S.; Onishi, K. Ultrasound-guided surgery in Musculoskeletal medicine. J. Med. Ultrasound 2022, 49, 513–515. [Google Scholar] [CrossRef]
- Masoumi, N.; Rivaz, H.; Hacihaliloglu, I.; Ahmad Reinertsen, I.; Xiao, Y. The Big Bang of Deep Learning in Ultrasound-Guided Surgery: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 909–919. [Google Scholar] [CrossRef]
- Jacobson, J.A. Introduction. In Fundamentals of Musculoskeletal Ultrasound, 3rd ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2017; Volume 1, pp. 1–15. [Google Scholar]
- Lee, J.-G.; Jun, S.; Cho, Y.-W.; Lee, H.; Kim, G.B.; Seo, J.B.; Kim, N. Deep Learning in Medical Imaging: General overview. Korean J. Radiol. 2017, 18, 570–584. [Google Scholar] [CrossRef]
- Samy, A.M. Intra-operative ultrasound: Does it improve the results of percutaneous repair of acute Achilles tendon rupture? Eur. J. Trauma. Emerg. Surg. 2022, 48, 4061–4068. [Google Scholar] [CrossRef]
- Paczesny, Ł.; Zabrzyński, J.; Domżalski, M.; Gagat, M.; Termanowski, M.; Szwedowski, D.; Łapaj, Ł.; Kruczyński, J. Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture-A Pilot Study. J. Clin. Med. 2021, 10, 2370. [Google Scholar] [CrossRef]
- Rowe, N.M.; Joseph Michaels, V.; Soltanian, H.; Dobryansky, M.; Peimer, C.A.; Gurtner, G.C. Sonographically guided percutaneous carpal tunnel release: An anatomic and cadaveric study. Ann. Plast. Surg. 2005, 55, 52–56. [Google Scholar] [CrossRef]
- Knörr, J.; Soldado, F.; Menendez, M.E.; Domenech, P.; Sanchez, M.; Sales de Gauzy, J. Arthroscopic Talocalcaneal Coalition Resection in Children. Arthroscopy 2015, 31, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Moses, V.; Daniel, R.T.; Chacko, A.G. The value of intraoperative ultrasound in oblique corpectomy for cervical spondylotic myelopathy and ossified posterior longitudinal ligament. Br. J. Neurosurg. 2010, 24, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Torres, R.J.; Shinga, K.; Ichikawa, K.; Kato, Y.; Hattori, S.; Yamada, S. Ultrasound-assisted posteromedial portal placement of the elbow joint to prevent ulnar nerve injury. Arthrosc. Tech. 2017, 6, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Koga, H.; Nakamura, T.; Nakagawa, Y.; Ohara, T.; An, J.S.; Sekiya, I. Ultrasound-assisted arthroscopic all-inside repair technique for posterior lateral meniscus tear. Arthrosc. Tech. 2022, 11, e929–e935. [Google Scholar] [CrossRef] [PubMed]
- Chryssikos, T.; Wessell, A.; Pratt, N.; Cannarsa, G.; Sharma, A.; Olexa, J.; Han, N.; Schwartzbauer, G.; Sansur, C.; Crandall, K. Enhanced safety of pedicle subtraction osteotomy using intraoperative ultrasound. World Neurosurg. 2021, 152, 523–531. [Google Scholar] [CrossRef]
- Ge, X.; Ge, X.; Wang, C.; Liu, Q.; Wang, B.; Chen, L.; Cheng, K.; Qin, M. Application of ultrasound in avoiding radial nerve injury during elbow arthroscopy: A retrospective follow-up study. BMC Musculoskelet. Disord. 2022, 23, 1126. [Google Scholar] [CrossRef]
- Farfalli, G.L.; Aponte-Tinao, L.A.; Rasumoff, A.; Ayerza, M.A.; Muscolo, D.L. Intraoperative ultrasound assistance for excision of impalpable musculoskeletal soft tissue tumors. Orthopedics 2011, 34, 570–573. [Google Scholar] [CrossRef]
- Levin, R.S.; Vasiliev, S.A.; Aslanukov, M.N.; Zuev, A.A.; Oshchepkov, S.K. Intraoperative ultrasound-assisted surgery of spinal tumors. Burdenko’s J. Neurosurg. Zhurnal Voprosy Neirokhirurgii Imeni N.N. Burdenko 2022, 86, 56–65. [Google Scholar] [CrossRef]
- Martinel, V.; Bonnevialle, N.; Maltes Fermandois, P. Does intraoperative ultrasound help the surgeon in arthroscopic excision of rotator cuff tendon calcifications? Eur. J. Orthop. Surg. Traumatol. 2022, 32, 939–944. [Google Scholar] [CrossRef]
- Tat, J.; Tat, J.; Yoon, S.; Yee, A.J.; Larouche, J. Intraoperative ultrasound in spine decompression surgery: A systematic review. Spine 2022, 47, 73–85. [Google Scholar] [CrossRef]
- Hattori, S.; Onishi, K.; Chan, C.K.; Yamakawa, S.; Yano, Y.; Winkler, P.W.; Hogan, M.V.; Debski, R.E. Ultrasound-Guided Anterior Talofibular Ligament Repair with Augmentation Can Restore Ankle Kinematics: A Cadaveric Biomechanical Study. Orthop. J. Sports Med. 2022, 10, 23259671221111397. [Google Scholar] [CrossRef] [PubMed]
- Zappia, M.; Berritto, D.; Oliva, F.; Maffulli, N. High resolution real time ultrasonography of the sural nerve after percutaneous repair of the Achilles tendon. Foot Ankle Surg. 2018, 24, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wu, T.C.; Yang, K.C.; Li, Y.C.; Wang, C.C. Ultrasonography-Guided Minimally Invasive Surgery for Achilles Sleeve Avulsions. Foot Ankle Int. 2021, 42, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, S.; Patricola, A.A.; Stancati, A.; Santucci, A. Intraoperative ultrasound assistance for percutaneous repair of the acute Achilles tendon rupture. Orthopedics 2014, 37, 820–824. [Google Scholar] [CrossRef]
- White, R.Z.; Rezaian, P.; Parasuramar, A.; Sampson, M.J. Ultrasound-assisted foreign body extraction (U-SAFE): Review of technique and technical pearls. J. Med. Imaging Radiat. Oncol. 2022, 66, 362–369. [Google Scholar] [CrossRef]
- Shiels, W.E.; Babcock, D.S.; Wilson, J.L.; Burch, R.A. Localization and guided removal of soft-tissue foreign bodies with sonography. AJR Am. J. Roentgenol. 1990, 155, 1277–1281. [Google Scholar] [CrossRef]
- Quiñones, P.K.; Hattori, S.; Yamada, S.; Kato, Y.; Ohuchi, H. Ultrasonography-Guided Muscle Hematoma Evacuation. Arthrosc. Tech. 2019, 8, 721–725. [Google Scholar] [CrossRef]
- Michalski, Ł.; Paczesny, Ł.; Zabrzyński, J.; Kruczyński, J. Ultrasound-assisted Endoscopic Evacuation of Recurrent Calf Hematoma Following Anterior Cruciate Ligament Reconstruction. Case Study. Ortop. Traumatol. Rehabil. 2019, 21, 357–365. [Google Scholar] [CrossRef]
- Rakovac, I.; Madarevic, T.; Tudor, A.; Prpic, T.; Sestan, B.; Mihelic, R.; Santic, V.; Jurkovic, H.; Ruzic, L. The “cello technique”: A new technique for ultrasound-assisted calcaneoplasty. Arthrosc. Tech. 2012, 1, 91. [Google Scholar] [CrossRef]
- Wang, C.L.; Chen, P.Y.; Yang, K.C.; Wu, H.C.; Wang, C.C. Ultrasound-Guided Minimally Invasive Surgical Resection of Retrocalcaneal Bursitis: A Preliminary Comparison with Traditional Open Surgery. J. Foot Ankle Surg. 2019, 58, 855–860. [Google Scholar] [CrossRef]
- Madarevic, T.; Rakovac, I.; Ruzic, L.; Tudor, A.; Gudac Madarevic, D.; Prpic, T.; Sestan, B. Ultrasound-assisted calcaneoplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2250–2253. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Guo, J.; Malone, D.G.; Wei, N.; McCool, L.C. A Cadaveric Study for the Improvement of Thread Carpal Tunnel Release. J. Hand Surg. Am. 2016, 41, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, P.D.; Hebbard, A.I.; Tomka, J.; Appleyard, R. Ultrasound-guided microinvasive carpal tunnel release using a novel retractable needle-mounted blade: A cadaveric study. J. Ultrasound Med. 2018, 37, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Dekimpe, C.; Andreani, O.; Camuzard, O.; Raffaelli, C.; Petrover, D.; Foti, P.; Amoretti, N. Ultrasound-guided percutaneous release of the carpal tunnel: Comparison of the learning curves of a senior versus a junior operator. A cadaveric study. Skeletal Radiol. 2019, 48, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Sangha, H.; Flannery, J.; Robinson, L.R.; Agur, A. Ultrasound-Guided Incisionless Carpal Tunnel Release Using a Hook Knife: A Cadaveric Study. PM R 2019, 11, 1101–1106. [Google Scholar] [CrossRef]
- Guo, D.; Guo, J.; Schmidt, S.C.; Lytie, R.M. A Clinical Study of the Modified Thread Carpal Tunnel Release. Hand 2017, 12, 453–460. [Google Scholar] [CrossRef]
- McShane, J.M.; Slaff, S.; Gold, J.E.; Nazarian, L.N. Sonographically guided percutaneous needle release of the carpal tunnel for treatment of carpal tunnel syndrome: Preliminary report. J. Ultrasound Med. 2012, 31, 1341–1349. [Google Scholar] [CrossRef]
- Henning, P.T.; Yang, L.; Awan, T.; Lueders, D.; Pourcho, A.M. Minimally Invasive Ultrasound-Guided Carpal Tunnel Release: Preliminary Clinical Results. J. Ultrasound Med. 2018, 37, 2699–2706. [Google Scholar] [CrossRef]
- Joseph, A.E.; Leiby, B.M.; Beckman, J.P. Clinical Results of Ultrasound-Guided Carpal Tunnel Release Performed by a Primary Care Sports Medicine Physician. J. Ultrasound Med. 2020, 39, 441–452. [Google Scholar] [CrossRef]
- Leiby, B.M.; Beckman, J.P.; Joseph, A.E. Long-term Clinical Results of Carpal Tunnel Release Using Ultrasound Guidance. Hand 2021, 17, 1558944720988080. [Google Scholar] [CrossRef]
- David, I. Sonography-Guided Carpal Tunnel Release. Hand Clin. 2022, 38, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Krogh, T.P.; Isaksen, C.; Rojo-Manaute, J.; Damkier, H.H.; Jensen, P.; Fredberg, U.; Brix, L. Implementation of ultrasound-guided carpal tunnel release. Dan. Med. J. 2023, 70, A11220689. [Google Scholar] [PubMed]
- Capa-Grasa, A.; Rojo-Manaute, J.; Rodríguez, F.C.; Martín, J.V. Ultra minimally invasive sonographically guided carpal tunnel release: An external pilot study. Orthop. Traumatol. Surg. Res. 2014, 100, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Manaute, J.; Capa-Grasa, A.; Chana-Rodríguez, F.; Perez-Mañanes, R.; Rodriguez-Maruri, G.; Sanz-Ruiz, P.; Muñoz-Ledesma, J.; Aburto-Bernardo, M.; Esparragoza-Cabrera, L.; Cerro-Gutiérrez, M.D.; et al. Ultra-Minimally Invasive Ultrasound-Guided Carpal Tunnel Release: A Randomized Clinical Trial. J. Ultrasound Med. 2016, 35, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Aramendi, J.F.; Ibañez, J.M.; Blasi, M.; Vazquez, A.; Aurrekoetxea, J.J.; Dávila, F. Minimally invasive ultrasound-guided vs open release for carpal tunnel syndrome in working population: A randomized controlled trial. J. Clin. Ultrasound 2021, 49, 693–703. [Google Scholar] [CrossRef]
- Fernández-Gibello, A.; Moroni, S.; Camuñas, G.; Montes, R.; Zwierzina, M.; Tasch, C.; Starke, V.; Sañudo, J.; Vazquez, T.; Konschake, M. Ultrasound-guided decompression surgery of the tarsal tunnel: A novel technique for the proximal tarsal tunnel syndrome-Part II. Surg. Radiol. Anat. 2019, 41, 43–51. [Google Scholar] [CrossRef]
- Iborra, Á.; Villanueva-Martínez, M.; Barrett, S.L.; Rodríguez-Collazo, E.R.; Sanz-Ruiz, P. Ultrasound-Guided Release of the Tibial Nerve and Its Distal Branches: A Cadaveric Study. J. Ultrasound Med. 2019, 38, 2067–2079. [Google Scholar] [CrossRef]
- Iborra, A.; Villanueva, M.; Sanz-Ruiz, P. Results of ultrasound-guided release of tarsal tunnel syndrome: A review of 81 cases with a minimum follow-up of 18 months. J. Orthop. Surg. Res. 2020, 15, 30. [Google Scholar] [CrossRef]
- Iborra Marcos, A.; Villanueva Martinez, M.; Sanz-Ruiz, P.; Barrett, S.L.; Zislis, G. Ultrasound-Guided Proximal and Distal Tarsal Decompression: An Analysis of Pressures in the Tarsal, Medial Plantar, and Lateral Plantar Tunnels. Foot Ankle Spec. 2021, 14, 133–139. [Google Scholar] [CrossRef]
- Wahezi, S.; Yerra, S.; Rivelis, Y.; Sitapara, K.; Gonzalez, D.; Downie, S.; Jain, R.; Deer, T.; Abd-Elsayed, A.; Gulati, A. Sonographically guided percutaneous sectioning of the coracohumeral ligament for the treatment of refractory adhesive capsulitis: Proof of concept. Pain Med. 2020, 21, 3314–3319. [Google Scholar] [CrossRef]
- Ahn, K.; Jhun, H.J.; Choi, K.M.; Lee, Y.S. Ultrasound-guided interventional release of rotator interval and posteroinferior capsule for adhesive capsulitis of the shoulder using a specially designed needle. Pain Physician 2011, 14, 531–537. [Google Scholar] [PubMed]
- Yukata, K.; Goto, T.; Sakai, T.; Fujii, H.; Hamawaki, J.; Yasui, N. Ultrasound-guided coracohumeral ligament release. Orthop. Traumatol. Surg. Res. 2018, 104, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Wahezi, S.E.; Naeimi, T.; Yerra, S.; Gruson, K.; Hossack, M.; Alvarez, E.T.; Vydyanathan, A.; Voleti, P.; Malhotra, R.; Martinez, E. Percutaneous ultrasound-guided coracohumeral ligament release for refractory adhesive capsulitis: A prospective, randomized, controlled, crossover trial demonstrating one-year efficacy. Pain Physician 2023, 26, 509–516. [Google Scholar]
- Moroni, S.; Fernández-Gibello, A.; Nieves, G.C.; Montes, R.; Zwierzina, M.; Vazquez, T.; Garcia-Escudero, M.; Duparc, F.; Moriggl, B.; Konschake, M. Anatomical basis of a safe mini-invasive technique for lengthening of the anterior gastrocnemius aponeurosis. Surg. Radiol. Anat. 2021, 43, 53–61. [Google Scholar] [CrossRef]
- Iborra Marcos, Á.; Villanueva Martínez, M.; Fahandezh-Saddi Díaz, H. Needle-based gastrocnemius lengthening: A novel ultrasound-guided noninvasive technique. J. Orthop. Surg. Res. 2022, 17, 435. [Google Scholar] [CrossRef]
- Villanueva, M.; Iborra, Á.; Ruiz, M.D.M.; Sanz-Ruiz, P. Proximal Ultrasound-Guided Gastrocnemius Recession: A New Ultra-Minimally Invasive Surgical Technique. J. Foot Ankle Surg. 2019, 58, 870–876. [Google Scholar] [CrossRef]
- Chern, T.; Jou, I.; Yen, S.; Lai, K.; Shao, C. Cadaveric study of sonographically assisted percutaneous release of the A1 pulley. Plast. Reconstr. Surg. 2005, 115, 811–822. [Google Scholar] [CrossRef]
- Kuo, L.C.; Su, F.C.; Tung, W.L.; Lai, K.Y.; Jou, I.M. Kinematical and functional improvements of trigger digits after sonographically assisted percutaneous release of the A1 pulley. J. Orthop. Res. 2009, 27, 891–896. [Google Scholar] [CrossRef]
- Rojo-Manaute, J.M.; Rodríguez-Maruri, G.; Capa-Grasa, A.; Chana-Rodríguez, F.; Soto Mdel, V.; Martín, J.V. Sonographically guided intrasheath percutaneous release of the first annular pulley for trigger digits, part 1: Clinical efficacy and safety. J. Ultrasound Med. 2012, 31, 417–424. [Google Scholar] [CrossRef]
- Lapègue, F.; André, A.; Meyrignac, O.; Pasquier-Bernachot, E.; Dupré, P.; Brun, C.; Bakouche, S.; Chiavassa-Gandois, H.; Sans, N.; Faruch, M. US-guided Percutaneous Release of the Trigger Finger by Using a 21-gauge Needle: A Prospective Study of 60 Cases. Radiology 2016, 280, 493–499. [Google Scholar] [CrossRef]
- Pan, M.; Sheng, S.; Fan, Z.; Lu, H.; Yang, H.; Yan, F.; E, Z. Ultrasound-Guided Percutaneous Release of A1 Pulley by Using a Needle Knife: A Prospective Study of 41 Cases. Front. Pharmacol. 2019, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Colberg, R.E.; Jurado Vélez, J.A.; Garrett, W.H.; Hart, K.; Fleisig, G.S. Ultrasound-guided microinvasive trigger finger release technique using an 18-gauge needle with a blade at the tip: A prospective study. PM R 2022, 14, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.S.; Malahias, M.A.; Kaseta, M.K.; Sourlas, I.; Babis, G.C. Comparative clinical study of ultrasound-guided A1 pulley release. World J. Orthop. 2017, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Colberg, R.E.; Henderson, R.G. Ultrasound-Guided First Dorsal Compartment Release for Refractory de Quervain Tenosynovitis: A Case Report. PM R 2019, 11, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Lueders, D.R.; Sellon, J.L.; Smith, J.; Finnoff, J.T. Ultrasound-Guided Fasciotomy for Chronic Exertional Compartment Syndrome: A Cadaveric Investigation. PM R 2017, 9, 683–690. [Google Scholar] [CrossRef]
- Balius, R.; Bong, D.A.; Ardèvol, J.; Pedret, C.; Codina, D.; Dalmau, A. Ultrasound-Guided Fasciotomy for Anterior Chronic Exertional Compartment Syndrome of the Leg. J. Ultrasound Med. 2016, 35, 823–829. [Google Scholar] [CrossRef]
- Finnoff, J.T.; Johnson, W. Ultrasound-Guided Fasciotomy for Chronic Exertional Compartment Syndrome: A Case Report. Clin. J. Sports Med. 2020, 30, 231–233. [Google Scholar] [CrossRef]
- Sakellariou, V.I.; Brault, J.; Rizzo, M. Ultrasound-assisted percutaneous needle fasciotomy for Dupuytren’s contracture. Orthopedics 2015, 38, 299–303. [Google Scholar] [CrossRef]
- Villanueva, M.; Iborra, Á.; Fahandezh-Saddi, H.; Sanz-Ruiz, P.; Noriega, C. Ultrasound-guided aponeurotomy and interphalangeal joint capsular release for treatment of Dupuytren’s disease. J. Hand Surg. Eur. Vol. 2022, 47, 742–749. [Google Scholar] [CrossRef]
- Patel, M.M. A novel treatment for refractory plantar fasciitis. Am. J. Orthop. (Belle Mead NJ) 2015, 44, 107–110. [Google Scholar]
- McShane, J.M.; Nazarian, L.N.; Harwood, M.I. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J. Ultrasound Med. 2006, 25, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Testa, V.; Capasso, G.; Benazzo, F.; Maffulli, N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med. Sci. Sports Exerc. 2002, 34, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.; Ellis, M.B.; Johnson, K.; Haddon, T.B. Fasciotomy and Surgical Tenotomy for Chronic Achilles Insertional Tendinopathy. J. Am. Podiatr. Med. Assoc. 2019, 109, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Oliva, F.; Testa, V.; Capasso, G.; Del Buono, A. Multiple percutaneous longitudinal tenotomies for chronic Achilles tendinopathy in runners: A long-term study. Am. J. Sports Med. 2013, 41, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Vohra, P.K.; Japour, C.J. Ultrasound-guided plantar fascia release technique: A retrospective study of 46 feet. J. Am. Podiatr. Med. Assoc. 2009, 99, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Iborra, A.; Villanueva, M.; Sanz-Ruiz, P.; Martin, A.; Noriega, C. A novel closed technique for ultrasound-guided plantar fascia release with a needle: Review of 107 cases with a minimum follow-up of 24 months. J. Orthop. Surg. Res. 2021, 16, 153. [Google Scholar] [CrossRef]
- Malahias, M.; Roumeliotis, L.; Tyrpenou, E.; Kazas, S.; Sourlas, I.; Kaseta, M. Ultrasound-Guided Partial Plantar Fascia Release with the Use of a Fine Cutting Device for the Treatment of Persistent Plantar Fasciitis: A Case Series. J. Am. Podiatr. Med. Assoc. 2022, 112. [Google Scholar] [CrossRef]
- Lévy, B.; Ducat, A.; Gaudin, P.; Maqdés, A.; Brasseur, J.L.; Klouche, S.; Hardy, P. Ultrasound-guided percutaneous tenotomy of the long head of the biceps tendon: A non-reliable technique. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1027–1030. [Google Scholar] [CrossRef]
- Aly, A.R.; Rajasekaran, S.; Mohamed, A.; Beavis, C.; Obaid, H. Feasibility of ultrasound-guided percutaneous tenotomy of the long head of the biceps tendon--A pilot cadaveric study. J. Clin. Ultrasound 2015, 43, 361–366. [Google Scholar] [CrossRef]
- Atlan, F.; Werthel, J.D. Ultrasound-guided intra-articular tenotomy of the long head of the biceps: A cadaveric feasibility study. Int. Orthop. 2016, 40, 2567–2573. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Mauri, G.; Messina, C.; Aliprandi, A.; Secchi, F.; Sardanelli, F.; Randelli, P.S. Ultrasound-Guided Percutaneous Tenotomy of Biceps Tendon: Technical Feasibility on Cadavers. Ultrasound Med. Biol. 2016, 42, 2513–2517. [Google Scholar] [CrossRef] [PubMed]
- Greditzer, H.G.; Kaplan, L.D.; Lesniak, B.P.; Jose, J. Ultrasound-guided percutaneous long head of the biceps tenotomy: A novel technique with case report. HSS J. 2014, 10, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, J.S.; Cheng, J.; Hurwitz, N.; Santiago, K.; Lin, E.; Beatty, N.; Kingsbury, D.; Wendel, I.; Milani, C. Ultrasound-guided percutaneous needle tenotomy (PNT) alone versus PNT plus platelet-rich plasma injection for the treatment of chronic tendinosis: A randomized controlled trial. PM R 2021, 13, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hickey, B.; Lee, J.; Stephen, J.; Antflick, J.; Calder, J. It is possible to release the plantaris tendon under ultrasound guidance: A technical description of ultrasound guided plantaris tendon release (UPTR) in the treatment of non-insertional Achilles tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2858–2862. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Alfredson, H.; Masci, L.; Sellon, J.L.; Woods, C.D. Sonographically Guided Plantaris Tendon Release: A Cadaveric Validation Study. PM R 2019, 11, 56–63. [Google Scholar] [CrossRef]
- Koh, J.S.; Mohan, P.C.; Howe, T.S.; Lee, B.P.; Chia, S.L.; Yang, Z.; Morrey, B.F. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: Early clinical experience with a novel device for minimally invasive percutaneous microresection. Am. J. Sports Med. 2013, 41, 636–644. [Google Scholar] [CrossRef]
- Seng, C.; Mohan, P.C.; Koh, S.B.; Howe, T.S.; Lim, Y.G.; Lee, B.P.; Morrey, B.F. Ultrasonic Percutaneous Tenotomy for Recalcitrant Lateral Elbow Tendinopathy: Sustainability and Sonographic Progression at 3 Years. Am. J. Sports Med. 2016, 44, 504–510. [Google Scholar] [CrossRef]
- Stover, D.; Fick, B.; Chimenti, R.L.; Hall, M.M. Ultrasound-guided tenotomy improves physical function and decreases pain for tendinopathies of the elbow: A retrospective review. J. Shoulder Elbow Surg. 2019, 28, 2386–2393. [Google Scholar] [CrossRef]
- Sanchez, P.J.; Grady, J.F.; Saxena, A. Percutaneous Ultrasonic Tenotomy for Achilles Tendinopathy Is a Surgical Procedure with Similar Complications. J. Foot Ankle Surg. 2017, 56, 982–984. [Google Scholar] [CrossRef]
- Pourcho, A.M.; Hall, M.M. Percutaneous Ultrasonic Fasciotomy for Refractory Plantar Fasciopathy After Failure of a Partial Endoscopic Release Procedure. PM R 2015, 7, 1194–1197. [Google Scholar] [CrossRef]
- Turner, A.; Wang, J.; Liu, G.; Wukich, D.; VanPelt, M. Retrospective Evaluation of Ultrasound Guided Percutaneous Plantar Fasciotomy with and without Platelet Rich Plasma. J. Foot Ankle Surg. 2024, 63, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Boden, A.L.; Scott, M.T.; Dalwadi, P.P.; Mautner, K.; Mason, R.A.; Gottschalk, M.B. Platelet-rich plasma versus Tenex in the treatment of medial and lateral epicondylitis. J. Shoulder Elbow Surg. 2019, 28, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Fick, B.; Stover, D.W.; Chimenti, R.L.; Hall, M.M. The Safety of Ultrasound Guided Tenotomy and Debridement for Upper and Lower Extremity Tendinopathies: A Retrospective Study. Iowa Orthop. J. 2021, 41, 82–90. [Google Scholar]
- Chimenti, R.L.; Stover, D.W.; Fick, B.S.; Hall, M.M. Percutaneous Ultrasonic Tenotomy Reduces Insertional Achilles Tendinopathy Pain with High Patient Satisfaction and a Low Complication Rate. J. Ultrasound Med. 2019, 38, 1629–1635. [Google Scholar] [CrossRef]
- Hattori, S.; Alvarez, C.A.D.; Canton, S.; Hogan, M.V.; Onishi, K. Ultrasound-Guided Ankle Lateral Ligament Stabilization. Curr. Rev. Musculoskelet. Med. 2019, 12, 497–508. [Google Scholar] [CrossRef]
- Hattori, S.; Onishi, K.; Yano, Y.; Kato, Y.; Ohuchi, H.; Hogan, M.V.; Kumai, T. Sonographically Guided Anchor Placement in Anterior Talofibular Ligament Repair Is Anatomic and Accurate. Orthop. J. Sports Med. 2020, 8, 2325967120967322. [Google Scholar] [CrossRef]
- Chavez, J.; Hattori, S.; Kato, Y.; Takazawa, S.; Yamada, S.; Ohuchi, H. The use of ultrasonography during minimally invasive Achilles tendon repair to avoid sural nerve injury. J. Med. Ultrason. 2019, 46, 513–514. [Google Scholar] [CrossRef]
- Lee, J.K.; Kang, C.; Hwang, D.S.; Kang, D.H.; Lee, G.S.; Hwang, J.M.; Song, J.H.; Lee, C.W. A comparative study of innovative percutaneous repair and open repair for acute Achilles tendon rupture: Innovative usage of intraoperative ultrasonography. J. Orthop. Surg. 2020, 28, 2309499020910274. [Google Scholar] [CrossRef]
- Hirahara, A.M.; Mackay, G.; Andersen, W.J. Ultrasound-Guided Suture Tape Augmentation and Stabilization of the Medial Collateral Ligament. Arthrosc. Tech. 2018, 7, 205–210. [Google Scholar] [CrossRef]
- Hirahara, A.M.; Andersen, W.J. Ultrasound-Guided Percutaneous Repair of Medial Patellofemoral Ligament: Surgical Technique and Outcomes. Am. J. Orthop. 2017, 46, 152–157. [Google Scholar]
- Wright, J.G.; Swiontkowski, M.F.; Heckman, J.D. Introducing Levels of Evidence to The Journal. J. Bone Jt. Surg. 2003, 85, 1–3. [Google Scholar] [CrossRef]
- Altahawi, F.; Li, X.; Demarest, B.; Forney, M.C. Percutaneous ultrasonic tenotomy with the TX-1 device versus surgical tenotomy for the treatment of common extensor tendinosis. Skeletal Radiol. 2021, 50, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, H. Ultrasound and Doppler-guided mini-surgery to treat midportion Achilles tendinosis: Results of a large material and a randomised study comparing two scraping techniques. Br. J. Sports Med. 2011, 45, 407–410. [Google Scholar] [CrossRef]
- Shomal Zadeh, F.; Shafiei, M.; Shomalzadeh, M.; Pierce, J.; Thurlow, P.C.; Chalian, M. Percutaneous ultrasound-guided needle tenotomy for treatment of chronic tendinopathy and fasciopathy: A meta-analysis. Eur. Radiol. 2023, 33, 7303–7320, Erratum in Eur. Radiol. 2023, 33, 7353–7354. [Google Scholar] [CrossRef]
- Jacobson, J.A.; Yablon, C.M.; Henning, P.T.; Kazmers, I.S.; Urquhart, A.; Hallstrom, B.; Bedi, A.; Parameswaran, A. Greater Trochanteric Pain Syndrome: Percutaneous Tendon Fenestration Versus Platelet-Rich Plasma Injection for Treatment of Gluteal Tendinosis. J. Ultrasound Med. 2016, 35, 2413–2420. [Google Scholar] [CrossRef]
- Chuang, B.I.; Hsu, J.H.; Kuo, L.C.; Jou, I.M.; Su, F.C.; Sun, Y.N. Tendon-motion tracking in an ultrasound image sequence using optical-flow-based block matching. Biomed. Eng. Online 2017, 16, 47. [Google Scholar] [CrossRef]
- Dunnhofer, M.; Antico, M.; Sasazawa, F.; Takeda, Y.; Camps, S.; Martinel, N.; Micheloni, C.; Carneiro, G.; Fontanarosa, D. Siam-U-Net: Encoder-decoder siamese network for knee cartilage tracking in ultrasound images. Med. Image Anal. 2020, 60, 101631. [Google Scholar] [CrossRef]
- Alsinan, A.Z.; Patel, V.M.; Hacihaliloglu, I. Automatic segmentation of bone surfaces from ultrasound using a filter-layer-guided CNN. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 775–783. [Google Scholar] [CrossRef]
- Alsinan, A.Z.; Patel, V.M.; Hacihaliloglu, I. Bone shadow segmentation from ultrasound data for orthopedic surgery using GAN. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1477–1485. [Google Scholar] [CrossRef]
- Quader, N.; Hodgson, A.J.; Mulpuri, K.; Schaeffer, E.; Abugharbieh, R. Automatic Evaluation of Scan Adequacy and Dysplasia Metrics in 2-D Ultrasound Images of the Neonatal Hip. Ultrasound Med. Biol. 2017, 43, 1252–1262. [Google Scholar] [CrossRef]
- Rosa, L.G.; Zia, J.S.; Inan, O.T.; Sawicki, G.S. Machine learning to extract muscle fascicle length changes from dynamic ultrasound images in real-time. PLoS ONE 2021, 16, e0246611. [Google Scholar] [CrossRef] [PubMed]
- Kuok, C.P.; Yang, T.H.; Tsai, B.S.; Jou, I.M.; Horng, M.H.; Su, F.C.; Sun, Y.N. Segmentation of finger tendon and synovial sheath in ultrasound image using deep convolutional neural network. Biomed. Eng. Online 2020, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Li, B.; Wang, D.; Chen, W.; Yue, S.; Meng, D.; Qiao, X.; Zhang, Y. Deep learning for the rapid automatic segmentation of forearm muscle boundaries from ultrasound datasets. Front. Physiol. 2023, 14, 1166061. [Google Scholar] [CrossRef] [PubMed]
- Cronin, N.J.; Finni, T.; Seynnes, O. Using deep learning to generate synthetic B-mode musculoskeletal ultrasound images. Comput. Methods Programs Biomed. 2020, 196, 105583. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.J.; Loram, I.D. Estimation of absolute states of human skeletal muscle via standard B-mode ultrasound imaging and deep convolutional neural networks. J. R. Soc. Interface 2020, 17, 20190715. [Google Scholar] [CrossRef]
- Lee, K.S.; Jung, S.H.; Kim, D.H.; Chung, S.W.; Yoon, J.P. Artificial intelligence- and computer-assisted navigation for shoulder surgery. J. Orthop. Surg. 2024, 32, 10225536241243166. [Google Scholar] [CrossRef]
- Marzola, F.; van Alfen, N.; Doorduin, J.; Meiburger, K.M. Deep learning segmentation of transverse musculoskeletal ultrasound images for neuromuscular disease assessment. Comput. Biol. Med. 2021, 135, 104623. [Google Scholar] [CrossRef]
- Faeghi, F.; Ardakani, A.A.; Acharya, U.R.; Mirza-Aghazadeh-Attari, M.; Abolghasemi, J.; Ejtehadifar, S.; Mohammadi, A. Accurate automated diagnosis of carpal tunnel syndrome using radiomics features with ultrasound images: A comparison with radiologists’ assessment. Eur. J. Radiol. 2021, 136, 109518. [Google Scholar] [CrossRef]
- Lee, S.W.; Ye, H.U.; Lee, K.J.; Jang, W.Y.; Lee, J.H.; Hwang, S.M.; Heo, Y.R. Accuracy of New Deep Learning Model-Based Segmentation and Key-Point Multi-Detection Method for Ultrasonographic Developmental Dysplasia of the Hip (DDH) Screening. Diagnostics 2021, 11, 1174. [Google Scholar] [CrossRef]
- Chiu, P.H.; Boudier-Revéret, M.; Chang, S.W.; Wu, C.H.; Chen, W.S.; Özçakar, L. Deep Learning for Detecting Supraspinatus Calcific Tendinopathy on Ultrasound Images. J. Med. Ultrasound 2022, 30, 196–202. [Google Scholar] [CrossRef]
- Droppelmann, G.; Tello, M.; García, N.; Greene, C.; Jorquera, C.; Feijoo, F. Lateral elbow tendinopathy and artificial intelligence: Binary and multilabel findings detection using machine learning algorithms. Front. Med. 2022, 9, 945698. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, L.; Chen, T.; Lyu, X.; Yu, J.; Guo, W.; Wang, D.; Qin, X.; Zhao, Y.; Zhang, S. Study on multiplanar measurements of infant hips with three-dimensional ultrasonography. J. Clin. Ultrasound 2022, 50, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Chen, Z.; Zhang, Q.; Lei, B.; Chen, Z.; Fu, Y.; Guo, P.; Li, C.; Ma, T.; Liu, J.; et al. Osteoporosis Diagnostic Model Using a Multichannel Convolutional Neural Network Based on Quantitative Ultrasound Radiofrequency Signal. Ultrasound Med. Biol. 2022, 48, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Kamada, M.; Imamura, A.; Shimizu, M.; Inagaki, M.; Tsuji, Y.; Hashimoto, M.; Tanaka, M.; Ito, H.; Fujii, Y. Machine learning-based prediction of relapse in rheumatoid arthritis patients using data on ultrasound examination and blood test. Sci. Rep. 2022, 12, 7224. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, I.; Inui, A.; Mifune, Y.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Yoshikawa, T.; Kato, T.; Furukawa, T.; Hoshino, Y.; et al. Using deep learning for ultrasound images to diagnose carpal tunnel syndrome with high accuracy. Ultrasound Med. Biol. 2022, 48, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, I.; Inui, A.; Mifune, Y.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Yoshikawa, T.; Kato, T.; Furukawa, T.; Hoshino, Y.; et al. Diagnosis of cubital tunnel syndrome using deep learning on ultrasonographic images. Diagnostics 2022, 12, 632. [Google Scholar] [CrossRef]
- Shinohara, I.; Yoshikawa, T.; Inui, A.; Mifune, Y.; Nishimoto, H.; Mukohara, S.; Yoshikawa, T.; Furukawa, T.; Tanaka, S.; Kusunose, M.; et al. Ultrasound with artificial intelligence models predicted Palmer 1B triangular fibrocartilage complex injuries. Arthroscopy 2022, 38, 2417–2424. [Google Scholar] [CrossRef]
- Tiulpin, A.; Saarakkala, S.; Mathiessen, A.; Hammer, H.B.; Furnes, O.; Nordsletten, L.; Englund, M.; Magnusson, K. Predicting total knee arthroplasty from ultrasonography using machine learning. Osteoarthr. Cartil. Open 2022, 4, 100319. [Google Scholar] [CrossRef]
- Atalar, H.; Üreten, K.; Tokdemir, G.; Tolunay, T.; Çiçeklidağ, M.; Atik, O. The diagnosis of developmental dysplasia of the hip from hip ultrasonography images with deep learning methods. J. Pediatr. Orthop. 2023, 43, e132–e137. [Google Scholar] [CrossRef]
- Jaremko, J.L.; Hareendranathan, A.; Bolouri, S.E.S.; Frey, R.F.; Dulai, S.; Bailey, A.L. AI aided workflow for hip dysplasia screening using ultrasound in primary care clinics. Sci. Rep. 2023, 13, 9224. [Google Scholar] [CrossRef]
- Kinugasa, M.; Inui, A.; Satsuma, S.; Kobayashi, D.; Sakata, R.; Morishita, M.; Komoto, I.; Kuroda, R. Diagnosis of Developmental Dysplasia of the Hip by Ultrasound Imaging Using Deep Learning. J. Pediatr. Orthop. 2023, 43, e538–e544. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Zhang, Y.; Zhang, M.; Jiang, M.; Yu, J.; Zhu, J.; Zhang, B. Ultrasound-based radiomics in the diagnosis of carpal tunnel syndrome: The influence of regions of interest delineation method on mode. J. Clin. Ultrasound 2023, 51, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Lin, C.Y.; Shu, Y.C.; Shen, P.C.; Lin, T.Y.; Chang, K.V.; Ozcakar, L. The Potential of Ultrasound Radiomics in Carpal Tunnel Syndrome Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 3280. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, I.; Yoshikawa, T.; Inui, A.; Mifune, Y.; Nishimoto, H.; Mukohara, S.; Yoshikawa, T.; Kato, T.; Furukawa, T.; Tanaka, S.; et al. Degree of accuracy with which deep learning for ultrasound images identifies osteochondritis dissecans of the humeral capitellum. Am. J. Sports Med. 2023, 51, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, D.; Yin, Y.; Zhang, P.; Wen, W.; Gao, J.; Jiang, Z. Musculoskeletal Ultrasound Image-Based Radiomics for the Diagnosis of Achilles Tendinopathy in Skiers. J. Ultrasound Med. 2023, 42, 363–371. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Wang, X.F.; Zhang, Z.Q. Analysis of the value of artificial intelligence combined with musculoskeletal ultrasound in the differential diagnosis of pain rehabilitation of scapulohumeral periarthritis. Medicine 2023, 102, e33125. [Google Scholar] [CrossRef]
- Sasaki, K.; Fujita, D.; Takatsuji, K.; Kotoura, Y.; Minami, M.; Kobayashi, Y.; Sukenari, T.; Kida, Y.; Takahashi, K.; Kobashi, S. Deep learning-based osteochondritis dissecans detection in ultrasound images with humeral capitellum localization. Int. J. Comput. Assist. Radiol. Surg. 2024, 51, 1–10. [Google Scholar] [CrossRef]
- Takatsuji, K.; Kida, Y.; Sasaki, K.; Fujita, D.; Kobayashi, Y.; Sukenari, T.; Kotoura, Y.; Minami, M.; Kobashi, S.; Takahashi, K. Deep Learning-Based Computer-Aided Diagnosis of Osteochondritis Dissecans of the Humeral Capitellum Using Ultrasound Images. J. Bone Joint Surg. Am. 2024. [Google Scholar] [CrossRef]
- Baka, N.; Leenstra, S.; van Walsum, T. Ultrasound Aided Vertebral Level Localization for Lumbar Surgery. IEEE Trans. Med. Imaging 2017, 36, 2138–2147. [Google Scholar] [CrossRef]


| Primary Author | Year | Sample | Design | Pathology | Target Tissue | Key Findings |
|---|---|---|---|---|---|---|
| Wahezi [54] | 2023 | 26 vs. 13 | RCT | Adhesive capsulitis | Shoulder capsule | Ultrasound-guided PCHLR significantly improved post-procedure ROM compared to control. |
| Nikolaou [64] | 2017 | 16 vs. 16 | RCT | Trigger finger | Tendon | US-guided release resulted in shortened days off work, better cosmetic results, and no major complications compared to open surgery. |
| De la Fuente [46] | 2021 | 47 vs. 42 | RCT | CTS | Nerve | Hand functionality improved for ultrasound group; pain decreased significantly at 3-month follow-up. |
| Rojo-Manaute [45] | 2016 | 46 vs. 46 | RCT | CTS | Nerve | US guidance resulted in earlier functional recovery and significantly better pain scores without complications. |
| Kirschner [84] | 2021 | 19 vs. 21 | RCT | Chronic tendinopathy | Tendon | Pain scores in the PNT group were significantly lower than those in the PNT + LR-PRP group at 6 weeks, but no significant outcome differences. |
| Altahawi [103] | 2020 | 23 vs. 10 | RCT | CET tendinopathy | Tendon | Percutaneous tenotomy had similar outcomes to traditional surgery. |
| Alfredson [104] | 2011 | 19 vs. 18 | RCT | Achilles tendinopathy | Tendon | US-guided scraping shows good results with minimal complications, no significant difference between percutaneous and mini-open scraping. |
| Shomal [105] | 2023 | 35 | Meta-analysis | Tendinopathy fasciopathy | Tendon | PUNT (including dry needling) alleviated pain, improved function, and has low rate of complications and failures. |
| Boden [93] | 2019 | 30 vs. 32 | Comparative | Lateral and medial epicondylitis | Tendon | Both Tenex® and PRP were successful in improving pain, function, and quality of life, but no significant difference between treatments. |
| Jacobson [106] | 2016 | 15 vs. 15 | Comparative | Gluteal tendinopathy | Tendon | US-guided tendon fenestration and PRP injection are effective for treatment of gluteal tendinosis with no significant difference between treatments. |
| Turner [92] | 2024 | 17 vs. 13 | Comparative | Plantar fascia | Tendon | PUT and PRP significantly decreased pain levels compared to only PUT. |
| Capa-Grasa [44] | 2014 | 20 vs. 20 | Comparative | CTS | Nerve | Ultra-MIS resulted in better recovery of functionality and symptoms in less post-operative time than mini-OCTR. |
| Hattori [97] | 2020 | 11 vs. 15 | Comparative | Chronic ankle instability | Ligament | US-guided anchor placement is accurate and anatomic for ATFL repair. |
| Wang [31] | 2019 | 10 vs. 12 | Comparative | Haglund deformity | Bone bursa | US-guided group had less pain and better function at 2 months compared to open surgery group. |
| Paczesny [10] | 2021 | 20 vs. 15 | Comparative | AT rupture | Tendon | US performed intraoperatively can minimize risk of sural nerve injury during percutaneous AT repair. |
| Lee [99] | 2020 | 12 vs. 18 | Comparative | AT rupture | Tendon | Percutaneous repair provides similar clinical outcomes, greater overall and aesthetic satisfaction levels, and minimal complications compared to open repair surgeries. |
| Primary Author | Year | Pathology | Evidence Level | Reference Standard | Key Findings |
|---|---|---|---|---|---|
| Faeghi [119] | 2021 | CTS | 2 | Nerve conduction test | Computer system performed better than two radiologists. |
| Lee [120] | 2021 | Hip dysplasia | 4 | Medical experts | DL model tested performed similarly to medical experts in dysplasia detection. |
| Chiu [121] | 2022 | RC (calcific tendinopathy) | 2 | Two experts | DL algorithm was accurate for the diagnosis of supraspinatus calcific tendinopathy. |
| Droppelmann [122] | 2022 | LET | 4 | Diagnosis by specialists | AI (specifically random forest model) detected LET with high diagnostic accuracy. |
| He [123] | 2022 | Hip dysplasia | 4 | Graf method | AI and 3D US-based automatic evaluation technology showed good agreement with the Graf method. |
| Luo [124] | 2022 | Osteoporosis | 2 | DEXA | Multichannel CNN could be more accurate than the conventional speed of sound model using quantitative US. |
| Matsuo [125] | 2022 | RA | 2 | Relapse | Excellent performance of ML at predicting RA relapse. |
| Shinohara [126] | 2022 | CTS | 2 | EPS | DL could detect carpal tunnel syndrome with high precision and accuracy. |
| Shinohara [127] | 2022 | Cubital tunnel syndrome | 4 | Electromyography | DL provided accurate prediction of cubital tunnel syndrome. |
| Shinohara [128] | 2022 | TFCC injury | 4 | MRI, CT arthrogram, and arthroscopy | DL detected TFCC with high accuracy comparable to MRI and CT arthrogram. |
| Tiulpin [129] | 2022 | Knee OA | 2 | TKR | DL-guided US was effective at predicting TKR. |
| Atalar [130] | 2023 | Hip dysplasia | 4 | Graf method | Successful differentiation of diseased and healthy hips. |
| Jaremko [131] | 2023 | Hip dysplasia | 2 | Orthopedic surgeons | All infants identified by AI-supported portable US were treated for hip dysplasia with 100% specificity. |
| Kinugasa [132] | 2023 | Hip dysplasia | 4 | Graf method | US with DL could assess hip dysplasia with high accuracy. |
| Lin [128] | 2023 | Gout (tophi) | 4 | Rheumatologist | It was possible to re-train deep CNN to identify the patterns of tophi in US images with accuracy. |
| Lyu [133] | 2023 | CTS | 4 | Not specified | Model performed best when median nerve epineurium was included in ROI. |
| Wu [134] | 2023 | CTS | 3 | Not applicable (systematic review) | In contrast to assessments by radiologists, US radiomics exhibited superior diagnostic performance in detecting CTS. |
| Shinohara [135] | 2023 | OCD of elbow | 2 | Radiographs and MRI | DL on US images identified OCD with high accuracy. |
| Wang [136] | 2023 | Achilles tendinopathy | 2 | Clinical diagnosis | US image-based radiomics achieved high diagnostic performance. |
| Yu [137] | 2023 | Scapulohumeral periarthritis | 4 | Diagnostic criteria | US combined with AI algorithm for scapulohumeral periarthritis is a simple method with high diagnostic efficiency. |
| Sasaki [138] | 2024 | OCD of elbow | 4 | Radiographs and orthopedic surgeon | Using DL with ROI focused on the humeral capitellum was effective at classification of OCD. |
| Takatsuji [139] | 2024 | OCD of elbow | 2 | Radiographs and orthopedic surgeon | Computer-assisted diagnosis system with DL achieved high accuracy using US images. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, S.; Saggar, R.; Heidinger, E.; Qi, A.; Mullen, J.; Fee, B.; Brown, C.L.; Canton, S.P.; Scott, D.; Hogan, M.V. Advances in Ultrasound-Guided Surgery and Artificial Intelligence Applications in Musculoskeletal Diseases. Diagnostics 2024, 14, 2008. https://doi.org/10.3390/diagnostics14182008
Hattori S, Saggar R, Heidinger E, Qi A, Mullen J, Fee B, Brown CL, Canton SP, Scott D, Hogan MV. Advances in Ultrasound-Guided Surgery and Artificial Intelligence Applications in Musculoskeletal Diseases. Diagnostics. 2024; 14(18):2008. https://doi.org/10.3390/diagnostics14182008
Chicago/Turabian StyleHattori, Soichi, Rachit Saggar, Eva Heidinger, Andrew Qi, Joseph Mullen, Brianna Fee, Cortez L. Brown, Stephen P. Canton, Devon Scott, and MaCalus V. Hogan. 2024. "Advances in Ultrasound-Guided Surgery and Artificial Intelligence Applications in Musculoskeletal Diseases" Diagnostics 14, no. 18: 2008. https://doi.org/10.3390/diagnostics14182008






