Biomarkers for Immunotherapy Efficacy in Advanced Hepatocellular Carcinoma: A Comprehensive Review
Abstract
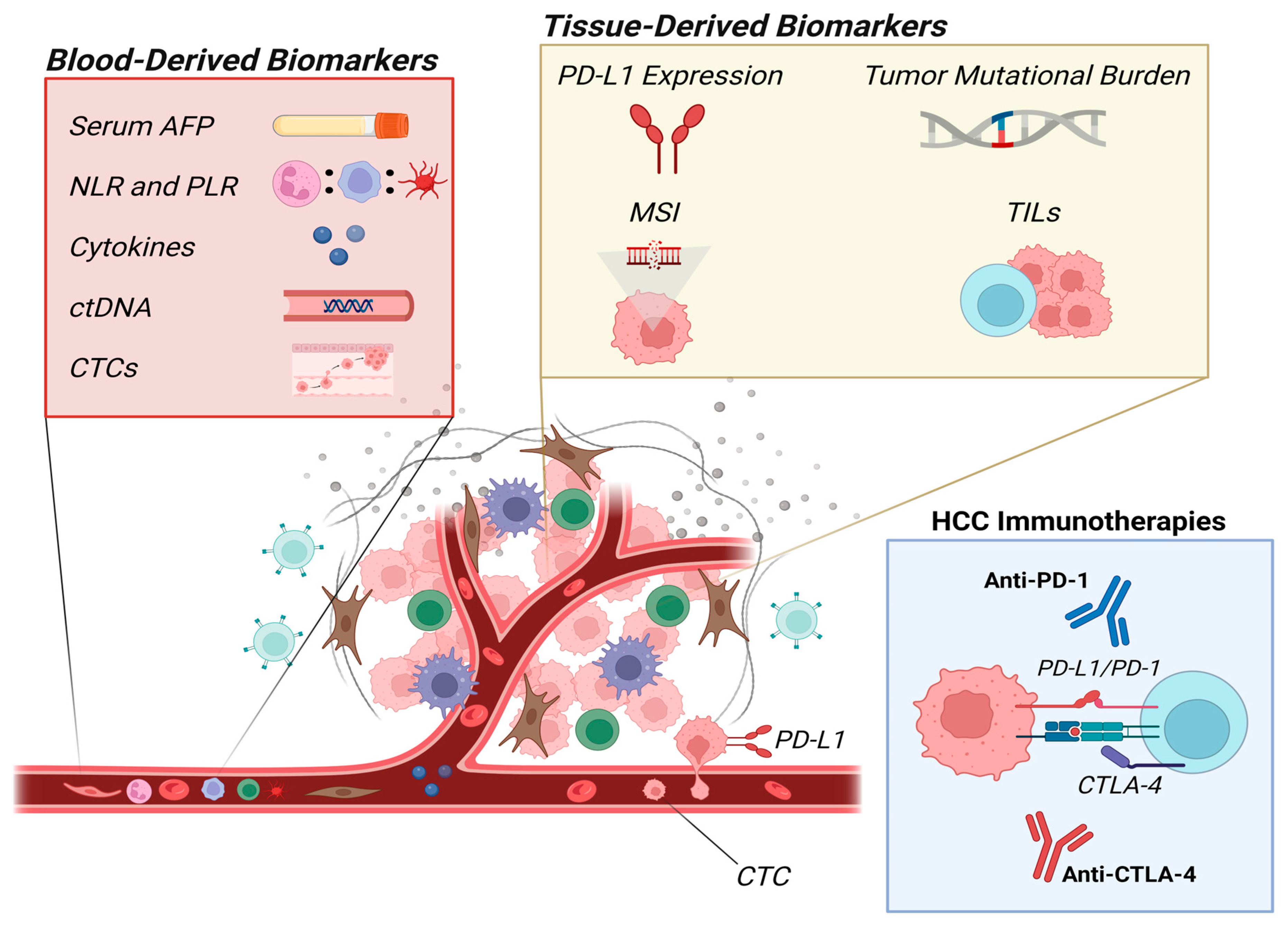
:1. Introduction
2. Biomarkers
2.1. Blood-Derived Biomarkers
2.1.1. Serum Alpha-Fetoprotein
2.1.2. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio
2.1.3. Cytokines
2.1.4. Circulating Tumor DNA
2.1.5. Circulating Tumor Cells
2.2. Tissue-Derived Biomarkers
2.2.1. PD-L1 Expression
2.2.2. Tumor Mutational Burden
2.2.3. Microsatellite Instability
2.2.4. Tumor-Infiltrating Lymphocytes
3. Current Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. Open Access Maced. J. Med. Sci. 2015, 3, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Taherifard, E.; Saeed, A.; Saeed, A. MASLD-Related HCC: A Comprehensive Review of the Trends, Pathophysiology, Tumor Microenvironment, Surveillance, and Treatment Options. Curr. Issues Mol. Biol. 2024, 46, 5965–5983. [Google Scholar] [CrossRef] [PubMed]
- Edoardo, P.; Eleonora, D.M. Progression of liver disease and associated risk of hepatocellular carcinoma. Hepatoma Res. 2024, 10, 15. [Google Scholar]
- Shannon, A.H.; Ruff, S.M.; Pawlik, T.M. Expert Insights on Current Treatments for Hepatocellular Carcinoma: Clinical and Molecular Approaches and Bottlenecks to Progress. J. Hepatocell. Carcinoma 2022, 9, 1247–1261. [Google Scholar] [CrossRef]
- Seif El Dahan, K.; Reczek, A.; Daher, D.; Rich, N.E.; Yang, J.D.; Hsiehchen, D.; Zhu, H.; Patel, M.S.; Bayona Molano, M.d.P.; Sanford, N.; et al. Multidisciplinary care for patients with HCC: A systematic review and meta-analysis. Hepatol. Commun. 2023, 7, e0143. [Google Scholar] [CrossRef]
- Byrd, K.; Alqahtani, S.; Yopp, A.C.; Singal, A.G. Role of Multidisciplinary Care in the Management of Hepatocellular Carcinoma. Semin. Liver Dis. 2021, 41, 1–8. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K.; Choudhary, H.B. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J. Gastroenterol. 2023, 29, 1054–1075. [Google Scholar] [CrossRef]
- Fan, Y.; Xue, H.; Zheng, H. Systemic Therapy for Hepatocellular Carcinoma: Current Updates and Outlook. J. Hepatocell. Carcinoma 2022, 9, 233–263. [Google Scholar] [CrossRef]
- van Bömmel, F.; Berg, T.; Lordick, F. Immune checkpoint inhibition (ICI) in current systemic therapies for hepatocellular carcinoma (HCC). ESMO Gastrointest. Oncol. 2023, 1, 27–39. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Haap, M.; Kopp, H.G.; Lipp, H.P. Tyrosine kinase inhibitors—A review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009, 10, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beal, E.; Finn, R.S.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Hoang, H.T.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 1830–1850. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Hepatocellular Carcinoma. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf (accessed on 23 June 2024).
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Kelly, Z.R.; Davar, D. The financial and physical toxicity of immune checkpoint inhibitors in cancer. ASCO Daily News 2019. [Google Scholar]
- Premnath, S.M.; Zubair, M. Laboratory Evaluation of Tumor Biomarkers; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lazarevich, N.L. Molecular mechanisms of alpha-fetoprotein gene expression. Biochemistry 2000, 65, 117–133. [Google Scholar]
- Ball, D.; Rose, E.; Alpert, E. Alpha-fetoprotein levels in normal adults. Am. J. Med. Sci. 1992, 303, 157–159. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Jezierska, M.; Gawrychowska, A.; Stefanowicz, J. Diagnostic, Prognostic and Predictive Markers in Pediatric Germ Cell Tumors-Past, Present and Future. Diagnostics 2022, 12, 278. [Google Scholar] [CrossRef] [PubMed]
- Terentiev, A.A.; Moldogazieva, N.T. Alpha-fetoprotein: A renaissance. Tumour Biol. 2013, 34, 2075–2091. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhu, M.; Wang, W.; Li, W.; Dong, X.; Chen, Y.; Lu, Y.; Guo, J.; Li, M. Structural basis for alpha fetoprotein-mediated inhibition of caspase-3 activity in hepatocellular carcinoma cells. Int. J. Cancer 2017, 141, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Xu, B. Stimulation of tumor-cell growth by alpha-fetoprotein. Int. J. Cancer 1998, 75, 596–599. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, J.; Kuang, T.; Chai, D.; Qiu, Z.; Deng, W.; Dong, K.; Zhao, K.; Wang, W. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint Inhibitors: A pooled analysis of 44 retrospective sudies. Int. Immunopharmacol. 2023, 118, 110019. [Google Scholar] [CrossRef]
- Qin, S.; Kudo, M.; Meyer, T.; Bai, Y.; Guo, Y.; Meng, Z.; Satoh, T.; Marino, D.; Assenat, E.; Li, S.; et al. Tislelizumab vs Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1651–1659. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- He, C.; Peng, W.; Liu, X.; Li, C.; Li, X.; Wen, T.-F. Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma: A meta-analysis. Medicine 2019, 98, e16557. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Zhu, A.X.; Dayyani, F.; Yen, C.J.; Ren, Z.; Bai, Y.; Meng, Z.; Pan, H.; Dillon, P.; Mhatre, S.K.; Gaillard, V.E.; et al. Alpha-Fetoprotein as a Potential Surrogate Biomarker for Atezolizumab + Bevacizumab Treatment of Hepatocellular Carcinoma. Clin. Cancer Res. 2022, 28, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Tazzyman, S.; Lewis, C.E.; Murdoch, C. Neutrophils: Key mediators of tumour angiogenesis. Int. J. Exp. Pathol. 2009, 90, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Z.; Tian, Y.; Li, Z.; Liu, Z.; Zhu, S. The critical role of platelet in cancer progression and metastasis. Eur. J. Med. Res. 2023, 28, 385. [Google Scholar] [CrossRef] [PubMed]
- Jost-Brinkmann, F.; Demir, M.; Wree, A.; Luedde, T.; Loosen, S.H.; Müller, T.; Tacke, F.; Roderburg, C.; Mohr, R. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma: Results from a German real-world cohort. Aliment. Pharmacol. Ther. 2023, 57, 1313–1325. [Google Scholar] [CrossRef]
- Wu, Y.L.; Fulgenzi, C.A.M.; D’Alessio, A.; Cheon, J.; Nishida, N.; Saeed, A.; Wietharn, B.; Cammarota, A.; Pressiani, T.; Personeni, N.; et al. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Prognostic Biomarkers in Unresectable Hepatocellular Carcinoma Treated with Atezolizumab plus Bevacizumab. Cancers 2022, 14, 5834. [Google Scholar] [CrossRef]
- Dharmapuri, S.; Özbek, U.; Lin, J.-Y.; Sung, M.; Schwartz, M.; Branch, A.D.; Ang, C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti–PD-1 therapy. Cancer Med. 2020, 9, 4962–4970. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.; Li, Y.; Liu, J. Neutrophil to Lymphocyte ratio as a predictor for immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1234142. [Google Scholar] [CrossRef]
- Pocino, K.; Stefanile, A.; Basile, V.; Napodano, C.; D’Ambrosio, F.; Di Santo, R.; Callà, C.A.M.; Gulli, F.; Saporito, R.; Ciasca, G.; et al. Cytokines and Hepatocellular Carcinoma: Biomarkers of a Deadly Embrace. J. Pers. Med. 2022, 13, 5. [Google Scholar] [CrossRef]
- Rico Montanari, N.; Anugwom, C.M.; Boonstra, A.; Debes, J.D. The Role of Cytokines in the Different Stages of Hepatocellular Carcinoma. Cancers 2021, 13, 4876. [Google Scholar] [CrossRef]
- Budhu, A.; Wang, X.W. The role of cytokines in hepatocellular carcinoma. J. Leukoc. Biol. 2006, 80, 1197–1213. [Google Scholar] [CrossRef]
- Liu, H.; Shen, J.; Lu, K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem. Biophys. Res. Commun. 2017, 486, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; Liu, L.F.; Li, Y.; Gupta, V.; Li, C.; et al. CD8(+) T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med. 2023, 4, 100878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, J.; Yan, X.; Jin, K.; Li, W.; Zhang, R. Targeting Interleukin-6 (IL-6) Sensitizes Anti-PD-L1 Treatment in a Colorectal Cancer Preclinical Model. Med. Sci. Monit. 2018, 24, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kang, B.; Ha, Y.; Lee, S.H.; Kim, I.; Kim, H.; Lee, W.S.; Kim, G.; Jung, S.; Rha, S.Y.; et al. High serum IL-6 correlates with reduced clinical benefit of atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. JHEP Rep. 2023, 5, 100672. [Google Scholar] [CrossRef] [PubMed]
- Myojin, Y.; Kodama, T.; Sakamori, R.; Maesaka, K.; Matsumae, T.; Sawai, Y.; Imai, Y.; Ohkawa, K.; Miyazaki, M.; Tanaka, S.; et al. Interleukin-6 Is a Circulating Prognostic Biomarker for Hepatocellular Carcinoma Patients Treated with Combined Immunotherapy. Cancers 2022, 14, 883. [Google Scholar] [CrossRef]
- Suzuki, T.; Matsuura, K.; Suzuki, Y.; Okumura, F.; Nagura, Y.; Sobue, S.; Matoya, S.; Miyaki, T.; Kimura, Y.; Kusakabe, A.; et al. Serum interleukin-6 levels at the start of the second course of atezolizumab plus bevacizumab therapy predict therapeutic efficacy in patients with advanced hepatocellular carcinoma: A multicenter analysis. J. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef]
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef]
- Tobin, R.P.; Jordan, K.R.; Kapoor, P.; Spongberg, E.; Davis, D.; Vorwald, V.M.; Couts, K.L.; Gao, D.; Smith, D.E.; Borgers, J.S.W.; et al. IL-6 and IL-8 Are Linked with Myeloid-Derived Suppressor Cell Accumulation and Correlate with Poor Clinical Outcomes in Melanoma Patients. Front. Oncol. 2019, 9, 1223. [Google Scholar] [CrossRef]
- Rizzo, M.; Varnier, L.; Pezzicoli, G.; Pirovano, M.; Cosmai, L.; Porta, C. IL-8 and its role as a potential biomarker of resistance to anti-angiogenic agents and immune checkpoint inhibitors in metastatic renal cell carcinoma. Front. Oncol. 2022, 12, 990568. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.; Tang, J.; Zhang, Y.; Tian, Y.; Sun, F. Changes in Serum Interleukin-8 Levels Predict Response to Immune Checkpoint Inhibitors Immunotherapy in Unresectable Hepatocellular Carcinoma Patients. J. Inflamm. Res. 2024, 17, 3397–3406. [Google Scholar] [CrossRef]
- Zou, D.; Song, A.; Yong, W. Prognostic role of IL-8 in cancer patients treated with immune checkpoint inhibitors: A system review and meta-analysis. Front. Oncol. 2023, 13, 1176574. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gingold, J.A.; Su, X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol. Med. 2019, 25, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Masugi, Y.; Sakamoto, M. Molecular pathogenesis of hepatocellular carcinoma: Altering transforming growth factor-β signaling in hepatocarcinogenesis. Dig. Dis. 2011, 29, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Meindl-Beinker, N.M.; Matsuzaki, K.; Dooley, S. TGF-β signaling in onset and progression of hepatocellular carcinoma. Dig. Dis. 2012, 30, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.G.; Li, Y.Y.; Wu, C.; Wangpaichitr, M.; Jones, P.D.; Richman, S.P.; Madrazo, B.; Kwon, D.; Garcia-Buitrago, M.; Martin, P.; et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 2019, 125, 3603–3614. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, H.; Lee, W.S.; Cheon, J.; Sang, Y.B.; Kang, B.; Chon, H.J.; Kim, C. High levels of baseline serum IL-10 are associated with reduced clinical benefit from first-line immune checkpoint inhibitor therapy in advanced renal cell carcinoma. J. Cancer 2023, 14, 935–942. [Google Scholar] [CrossRef]
- Babačić, H.; Lehtiö, J.; Pico de Coaña, Y.; Pernemalm, M.; Eriksson, H. In-depth plasma proteomics reveals increase in circulating PD-1 during anti-PD-1 immunotherapy in patients with metastatic cutaneous melanoma. J. Immunother. Cancer 2020, 8, e000204. [Google Scholar] [CrossRef]
- Chehrazi-Raffle, A.; Meza, L.; Alcantara, M.; Dizman, N.; Bergerot, P.; Salgia, N.; Hsu, J.; Ruel, N.; Salgia, S.; Malhotra, J.; et al. Circulating cytokines associated with clinical response to systemic therapy in metastatic renal cell carcinoma. J. Immunother. Cancer 2021, 9, e002009. [Google Scholar] [CrossRef]
- Costantini, A.; Takam Kamga, P.; Julie, C.; Corjon, A.; Dumenil, C.; Dumoulin, J.; Ouaknine, J.; Giraud, V.; Chinet, T.; Rottman, M.; et al. Plasma Biomarkers Screening by Multiplex ELISA Assay in Patients with Advanced Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Cancers 2020, 13, 97. [Google Scholar] [CrossRef]
- Boutsikou, E.; Domvri, K.; Hardavella, G.; Tsiouda, D.; Zarogoulidis, K.; Kontakiotis, T. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: A pragmatic approach in clinical practice. Ther. Adv. Med. Oncol. 2018, 10, 1758835918768238. [Google Scholar] [CrossRef]
- Yamazaki, N.; Kiyohara, Y.; Uhara, H.; Iizuka, H.; Uehara, J.; Otsuka, F.; Fujisawa, Y.; Takenouchi, T.; Isei, T.; Iwatsuki, K.; et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017, 108, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef] [PubMed]
- Sas, Z.; Cendrowicz, E.; Weinhäuser, I.; Rygiel, T.P. Tumor Microenvironment of Hepatocellular Carcinoma: Challenges and Opportunities for New Treatment Options. Int. J. Mol. Sci. 2022, 23, 3778. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef]
- Tran, N.H.; Kisiel, J.; Roberts, L.R. Using cell-free DNA for HCC surveillance and prognosis. JHEP Rep. 2021, 3, 100304. [Google Scholar] [CrossRef]
- Fan, R.; Chen, L.; Zhao, S.; Yang, H.; Li, Z.; Qian, Y.; Ma, H.; Liu, X.; Wang, C.; Liang, X.; et al. Novel, high accuracy models for hepatocellular carcinoma prediction based on longitudinal data and cell-free DNA signatures. J. Hepatol. 2023, 79, 933–944. [Google Scholar] [CrossRef]
- Park, S.; Lee, E.J.; Rim, C.H.; Seong, J. Plasma Cell-Free DNA as a Predictive Marker after Radiotherapy for Hepatocellular Carcinoma. Yonsei Med. J. 2018, 59, 470–479. [Google Scholar] [CrossRef]
- Chen, L.; Wu, T.; Fan, R.; Qian, Y.-S.; Liu, J.-F.; Bai, J.; Zheng, B.; Liu, X.-L.; Zheng, D.; Du, L.-T.; et al. Cell-free DNA testing for early hepatocellular carcinoma surveillance. eBioMedicine 2024, 100, 104962. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Z.; Hu, Z.; Yang, Z.; Pan, Y.; Chen, J.; Wang, J.; Hu, D.; Zhou, Z.; Xu, L.; et al. Preoperative serum ctDNA predicts early hepatocellular carcinoma recurrence and response to systemic therapies. Hepatol. Int. 2022, 16, 868–878. [Google Scholar] [CrossRef]
- Matsumae, T.; Kodama, T.; Myojin, Y.; Maesaka, K.; Sakamori, R.; Takuwa, A.; Oku, K.; Motooka, D.; Sawai, Y.; Oshita, M.; et al. Circulating Cell-Free DNA Profiling Predicts the Therapeutic Outcome in Advanced Hepatocellular Carcinoma Patients Treated with Combination Immunotherapy. Cancers 2022, 14, 3367. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Lu, S.; Abbas, A.; Guan, Y.; Zhu, A.X.; Aleshin, A.; Lee, K.-H.; Lee, M.S.; Mahipal, A.; Ryoo, B.-Y.; et al. Longitudinal and personalized detection of circulating tumor DNA (ctDNA) for monitoring efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2020, 38, 3531. [Google Scholar] [CrossRef]
- Zhan, Q.; Liu, B.; Situ, X.; Luo, Y.; Fu, T.; Wang, Y.; Xie, Z.; Ren, L.; Zhu, Y.; He, W.; et al. New insights into the correlations between circulating tumor cells and target organ metastasis. Signal Transduct. Target. Ther. 2023, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.M.; Lynch, C.C. How circulating tumor cluster biology contributes to the metastatic cascade: From invasion to dissemination and dormancy. Cancer Metastasis Rev. 2023, 42, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef]
- Pantel, K.; Speicher, M.R. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, C.; Peng, B.; Pu, X.; Cai, L.; Liao, H.; Chen, K.; Zhang, C.; Cheng, Y.; Pan, M. Circulating Tumor-Cell-Associated White Blood Cell Clusters in Peripheral Blood Indicate Poor Prognosis in Patients with Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 1758. [Google Scholar] [CrossRef]
- Espejo-Cruz, M.L.; González-Rubio, S.; Zamora-Olaya, J.; Amado-Torres, V.; Alejandre, R.; Sánchez-Frías, M.; Ciria, R.; De la Mata, M.; Rodríguez-Perálvarez, M.; Ferrín, G. Circulating Tumor Cells in Hepatocellular Carcinoma: A Comprehensive Review and Critical Appraisal. Int. J. Mol. Sci. 2021, 22, 13073. [Google Scholar] [CrossRef]
- Chen, J.; Cao, S.W.; Cai, Z.; Zheng, L.; Wang, Q. Epithelial-mesenchymal transition phenotypes of circulating tumor cells correlate with the clinical stages and cancer metastasis in hepatocellular carcinoma patients. Cancer Biomark. 2017, 20, 487–498. [Google Scholar] [CrossRef]
- Chen, V.L.; Xu, D.; Wicha, M.S.; Lok, A.S.; Parikh, N.D. Utility of Liquid Biopsy Analysis in Detection of Hepatocellular Carcinoma, Determination of Prognosis, and Disease Monitoring: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 2879–2902.e2879. [Google Scholar] [CrossRef]
- Winograd, P.; Hou, S.; Court, C.M.; Lee, Y.T.; Chen, P.J.; Zhu, Y.; Sadeghi, S.; Finn, R.S.; Teng, P.C.; Wang, J.J.; et al. Hepatocellular Carcinoma-Circulating Tumor Cells Expressing PD-L1 Are Prognostic and Potentially Associated with Response to Checkpoint Inhibitors. Hepatol. Commun. 2020, 4, 1527–1540. [Google Scholar] [CrossRef]
- Su, K.; Guo, L.; He, K.; Rao, M.; Zhang, J.; Yang, X.; Huang, W.; Gu, T.; Xu, K.; Liu, Y.; et al. PD-L1 expression on circulating tumor cells can be a predictive biomarker to PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma. Front. Oncol. 2022, 12, 873830. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Guo, L.; Wu, Z.Y.; He, K.; Li, H.; Yang, C.; Han, Y.W. Prognostic value of circulating tumor cells combined with neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma. World J. Gastrointest. Oncol. 2024, 16, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, T.; Murata, Y.; Akazawa, Y.; Tanaka, T.; Takahashi, K.; Naito, T.; Matsuda, H.; Ohtani, M.; Imamura, Y.; Nakamoto, Y. Programmed Death Ligand 1 Expression in Circulating Tumor Cells as a Predictor and Monitor of Response to Atezolizumab plus Bevacizumab Treatment in Patients with Hepatocellular Carcinoma. Cancers 2024, 16, 1785. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Edd, J.F.; Mishra, A.; Smith, K.C.; Kapur, R.; Maheswaran, S.; Haber, D.A.; Toner, M. Isolation of circulating tumor cells. iScience 2022, 25, 104696. [Google Scholar] [CrossRef]
- Ahn, J.C.; Teng, P.C.; Chen, P.J.; Posadas, E.; Tseng, H.R.; Lu, S.C.; Yang, J.D. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021, 73, 422–436. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Shao, W.; Li, Z.; Zhao, R.; Ye, Z. Strategies for enrichment of circulating tumor cells. Transl. Cancer Res. 2020, 9, 2012–2025. [Google Scholar] [CrossRef]
- Schöniger, S.; Jasani, B. The PD-1/PD-L1 Pathway: A Perspective on Comparative Immuno-Oncology. Animals 2022, 12, 2661. [Google Scholar] [CrossRef]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef]
- Dermani, F.K.; Samadi, P.; Rahmani, G.; Kohlan, A.K.; Najafi, R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J. Cell Physiol. 2019, 234, 1313–1325. [Google Scholar] [CrossRef]
- Nimmagadda, S. Quantifying PD-L1 Expression to Monitor Immune Checkpoint Therapy: Opportunities and Challenges. Cancers 2020, 12, 3173. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, D.; Zhao, B.; Ren, L.; Huang, R.; Feng, B.; Chen, H. The predictive value of PD-L1 expression in patients with advanced hepatocellular carcinoma treated with PD-1/PD-L1 inhibitors: A systematic review and meta-analysis. Cancer Med. 2023, 12, 9282–9292. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, J.; Topatana, W.; Xie, T.; Chen, T.; Hu, J.; Li, S.; Juengpanic, S.; Lu, Z.; Zhang, B.; et al. Evaluation of PD-L1 as a biomarker for immunotherapy for hepatocellular carcinoma: Systematic review and meta-analysis. Immunotherapy 2023, 15, 353–365. [Google Scholar] [CrossRef]
- Cooper, W.A.; Russell, P.A.; Cherian, M.; Duhig, E.E.; Godbolt, D.; Jessup, P.J.; Khoo, C.; Leslie, C.; Mahar, A.; Moffat, D.F.; et al. Intra- and Interobserver Reproducibility Assessment of PD-L1 Biomarker in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 4569–4577. [Google Scholar] [CrossRef]
- Ilie, M.; Long-Mira, E.; Bence, C.; Butori, C.; Lassalle, S.; Bouhlel, L.; Fazzalari, L.; Zahaf, K.; Lalvée, S.; Washetine, K.; et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti-PD-L1 therapeutic strategies. Ann. Oncol. 2016, 27, 147–153. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, H.J.; Park, S.Y.; Park, E.; Chung, J.H. PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: A comparative study. Oncotarget 2017, 8, 98524–98532. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Trapani, J.A.; Neeson, P.J. Challenges of PD-L1 testing in non-small cell lung cancer and beyond. J. Thorac. Dis. 2020, 12, 4541–4548. [Google Scholar] [CrossRef]
- Lu, L.C.; Lee, Y.H.; Chang, C.J.; Shun, C.T.; Fang, C.Y.; Shao, Y.Y.; Liu, T.H.; Cheng, A.L.; Hsu, C.H. Increased Expression of Programmed Death-Ligand 1 in Infiltrating Immune Cells in Hepatocellular Carcinoma Tissues after Sorafenib Treatment. Liver Cancer 2019, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Wu, S.P.; Liao, R.Q.; Huang, S.M.; Wu, Y.L. Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumour Biol. 2016, 37, 4251–4261. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Klempner, S.J.; Ali, S.M.; Madison, R.; Ross, J.S.; Severson, E.A.; Fabrizio, D.; Goodman, A.; Kurzrock, R.; Suh, J.; et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget 2019, 10, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Liu, Y.; Wang, M.; Li, Y.; Xu, T.; Wei, Y. The Predictive Value of Tumor Mutation Burden on Clinical Efficacy of Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 748674. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non–Small Cell Lung Cancers with Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Jia, R.; Yue, C.; Chang, L.; Liu, R.; Zhang, G.; Zhao, C.; Zhang, Y.; Chen, C.; et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin. Cancer Res. 2019, 25, 515–523. [Google Scholar] [CrossRef]
- Zhu, A.X.; Abbas, A.R.; de Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.-H.; He, A.R.; Ryoo, B.-Y.; et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022, 28, 1599–1611. [Google Scholar] [CrossRef]
- Spahn, S.; Roessler, D.; Pompilia, R.; Gabernet, G.; Gladstone, B.P.; Horger, M.; Biskup, S.; Feldhahn, M.; Nahnsen, S.; Hilke, F.J.; et al. Clinical and Genetic Tumor Characteristics of Responding and Non-Responding Patients to PD-1 Inhibition in Hepatocellular Carcinoma. Cancers 2020, 12, 3830. [Google Scholar] [CrossRef] [PubMed]
- Kavun, A.; Veselovsky, E.; Lebedeva, A.; Belova, E.; Kuznetsova, O.; Yakushina, V.; Grigoreva, T.; Mileyko, V.; Fedyanin, M.; Ivanov, M. Microsatellite Instability: A Review of Molecular Epidemiology and Implications for Immune Checkpoint Inhibitor Therapy. Cancers 2023, 15, 2288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Samstein, R.M.; Lee, K.W.; Havel, J.J.; Wang, H.; Krishna, C.; Sabio, E.Y.; Makarov, V.; Kuo, F.; Blecua, P.; et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science 2019, 364, 485–491. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Wang, G.; Zhang, F.; Zhang, Z.; Zhang, F.; Zhang, Y.; Dong, H.; Zhao, X.; Duan, J.; et al. Comutations in DNA Damage Response Pathways Serve as Potential Biomarkers for Immune Checkpoint Blockade. Cancer Res. 2018, 78, 6486–6496. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, W. Precision medicine becomes reality—Tumor type-agnostic therapy. Cancer Commun. 2018, 38, 6. [Google Scholar] [CrossRef]
- Chang, L.; Chang, M.; Chang, H.M.; Chang, F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, e15–e21. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Food, U.; Administration, D. FDA Approves First Cancer Treatment for Any Solid Tumor with a Specific Genetic Feature; US Food and Drug Administration: Silver Spring, MD, USA, 2017. [Google Scholar]
- Ando, Y.; Yamauchi, M.; Suehiro, Y.; Yamaoka, K.; Kosaka, Y.; Fuji, Y.; Uchikawa, S.; Kodama, K.; Morio, K.; Fujino, H.; et al. Complete response to pembrolizumab in advanced hepatocellular carcinoma with microsatellite instability. Clin. J. Gastroenterol. 2020, 13, 867–872. [Google Scholar] [CrossRef]
- Kawaoka, T.; Ando, Y.; Yamauchi, M.; Suehiro, Y.; Yamaoka, K.; Kosaka, Y.; Fuji, Y.; Uchikawa, S.; Morio, K.; Fujino, H.; et al. Incidence of microsatellite instability-high hepatocellular carcinoma among Japanese patients and response to pembrolizumab. Hepatol. Res. 2020, 50, 885–888. [Google Scholar] [CrossRef]
- Eso, Y.; Shimizu, T.; Takeda, H.; Takai, A.; Marusawa, H. Microsatellite instability and immune checkpoint inhibitors: Toward precision medicine against gastrointestinal and hepatobiliary cancers. J. Gastroenterol. 2020, 55, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jin, W.; Wang, S.; Ding, H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients with Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 729705. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.L.; Yau, T.; Furuse, J.; Park, J.W.; Boyd, Z.; et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2020, 73, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gong, C.; Peng, X.; Bi, X.; Sun, Y.; Zhou, J.; Wu, F.; Zeng, H.; Wang, Y.; Zhou, H.; et al. Serum Concentration of CD137 and Tumor Infiltration by M1 Macrophages Predict the Response to Sintilimab plus Bevacizumab Biosimilar in Advanced Hepatocellular Carcinoma Patients. Clin. Cancer Res. 2022, 28, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, A.; Yada, M.; Miyazaki, Y.; Tanaka, K.; Kurosaka, K.; Ohishi, Y.; Masumoto, A.; Motomura, K. Tumor-infiltrating CD8(+) T cells as a biomarker for chemotherapy efficacy in unresectable hepatocellular carcinoma. Oncol. Lett. 2023, 25, 259. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- Ng, H.H.M.; Lee, R.Y.; Goh, S.; Tay, I.S.Y.; Lim, X.; Lee, B.; Chew, V.; Li, H.; Tan, B.; Lim, S.; et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J. Immunother. Cancer 2020, 8, e000987. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Seah, Y.H.J.; Fang, J.; Orpilla, N.H.C.; Lau, M.C.; Lim, C.J.; Lim, X.; Lee, J.; Lim, J.C.T.; Lim, S.; et al. Immunohistochemical scoring of LAG-3 in conjunction with CD8 in the tumor microenvironment predicts response to immunotherapy in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1150985. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, L.; Yoo, J.-K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e1316. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Z. Understanding tumor-infiltrating lymphocytes by single cell RNA sequencing. Adv. Immunol. 2019, 144, 217–245. [Google Scholar] [CrossRef]
- Peeters, F.; Cappuyns, S.; Piqué-Gili, M.; Phillips, G.; Verslype, C.; Lambrechts, D.; Dekervel, J. Applications of single-cell multi-omics in liver cancer. JHEP Rep. 2024, 6, 101094. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-K.; Tsui, Y.-M.; Ho, D.W.-H.; Ng, I.O.-L. Cellular heterogeneity and plasticity in liver cancer. Semin. Cancer Biol. 2022, 82, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Kalasekar, S.M.; VanSant-Webb, C.H.; Evason, K.J. Intratumor Heterogeneity in Hepatocellular Carcinoma: Challenges and Opportunities. Cancers 2021, 13, 5524. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Losic, B.; Craig, A.J.; Villacorta-Martin, C.; Martins-Filho, S.N.; Akers, N.; Chen, X.; Ahsen, M.E.; von Felden, J.; Labgaa, I.; DʹAvola, D.; et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 2020, 11, 291. [Google Scholar] [CrossRef]
- Kim, M.S.; Xu, A.; Haslam, A.; Prasad, V. Quality of biomarker defined subgroups in FDA approvals of PD-1/PD-L1 inhibitors 2014 to 2020. Int. J. Cancer 2022, 150, 1905–1910. [Google Scholar] [CrossRef]
- Ballman, K.V. Biomarker: Predictive or Prognostic? J. Clin. Oncol. 2015, 33, 3968–3971. [Google Scholar] [CrossRef]
- Oldenhuis, C.N.; Oosting, S.F.; Gietema, J.A.; de Vries, E.G. Prognostic versus predictive value of biomarkers in oncology. Eur. J. Cancer 2008, 44, 946–953. [Google Scholar] [CrossRef]
- Song, B.G.; Goh, M.J.; Kang, W.; Sinn, D.H.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Paik, Y.H. Analysis of Factors Predicting the Real-World Efficacy of Atezolizumab and Bevacizumab in Patients with Advanced Hepatocellular Carcinoma. Gut Liver 2024, 18, 709–718. [Google Scholar] [CrossRef]
- Kunichika, H.; Minamiguchi, K.; Tachiiri, T.; Shimizu, K.; Taiji, R.; Yamada, A.; Nakano, R.; Irizato, M.; Yamauchi, S.; Marugami, A.; et al. Prediction of Efficacy for Atezolizumab/Bevacizumab in Unresectable Hepatocellular Carcinoma with Hepatobiliary-Phase Gadolinium Ethoxybenzyl-Diethylenetriaminepentaacetic Acid MRI. Cancers 2024, 16, 2275. [Google Scholar] [CrossRef]
- Haber, P.K.; Castet, F.; Torres-Martin, M.; Andreu-Oller, C.; Puigvehí, M.; Miho, M.; Radu, P.; Dufour, J.-F.; Verslype, C.; Zimpel, C.; et al. Molecular Markers of Response to Anti-PD1 Therapy in Advanced Hepatocellular Carcinoma. Gastroenterology 2023, 164, 72–88.e18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, L.; Liu, C.; Wang, Z.; Xu, W.; Lu, J.; Wang, C.; Xu, X. Identification and validation of immune-related gene signature models for predicting prognosis and immunotherapy response in hepatocellular carcinoma. Front. Immunol. 2024, 15, 1371829. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhou, K.; Zhang, R.; Li, J.; Guo, M.; Qiao, H.; Wu, M.; Cao, X.; Dong, G.; Zhang, S. Construction and validation of prognostic signature for transcription factors regulating T cell exhaustion in hepatocellular carcinoma. Medicine 2024, 103, e38713. [Google Scholar] [CrossRef] [PubMed]

| Biomarker | Measurement Method | Limitations |
|---|---|---|
| Blood-derived biomarkers | ||
| Serum alpha-fetoprotein | Radio- and fluorescent-immunoassay techniques | Lacks sensitivity; not always indicative of HCC |
| Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio | Complete blood count | Standardized cutoffs needed; varies between studies |
| Cytokines | Immunoassays, multiplex assays | More HCC-specific clinical trials are needed for definitive conclusions |
| Circulating tumor DNA | Next-generation sequencing, digital PCR | Challenges in isolating ctDNA; not consistently reliable as a biomarker |
| Circulating tumor cells | Filtration, electrophoresis, antigen-antibody binding | Low levels in early-stage HCC; conflicting study results |
| Tissue-derived biomarkers | ||
| PD-L1 expression | Immunohistochemistry, cancerous tumor tissue biopsies | Controversial due to mixed results; not effective for all treatments |
| Tumor mutational burden | Next-generation sequencing | Lacks consistency; independent from PD-L1 expression |
| Microsatellite instability | Polymerase chain reaction | MSI-H is rare in HCC, limiting its standalone use |
| Tumor-infiltrating lymphocytes | Immunohistochemistry, single-cell RNA sequencing | Inconsistent data; presence varies among patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taherifard, E.; Tran, K.; Saeed, A.; Yasin, J.A.; Saeed, A. Biomarkers for Immunotherapy Efficacy in Advanced Hepatocellular Carcinoma: A Comprehensive Review. Diagnostics 2024, 14, 2054. https://doi.org/10.3390/diagnostics14182054
Taherifard E, Tran K, Saeed A, Yasin JA, Saeed A. Biomarkers for Immunotherapy Efficacy in Advanced Hepatocellular Carcinoma: A Comprehensive Review. Diagnostics. 2024; 14(18):2054. https://doi.org/10.3390/diagnostics14182054
Chicago/Turabian StyleTaherifard, Erfan, Krystal Tran, Ali Saeed, Jehad Amer Yasin, and Anwaar Saeed. 2024. "Biomarkers for Immunotherapy Efficacy in Advanced Hepatocellular Carcinoma: A Comprehensive Review" Diagnostics 14, no. 18: 2054. https://doi.org/10.3390/diagnostics14182054
APA StyleTaherifard, E., Tran, K., Saeed, A., Yasin, J. A., & Saeed, A. (2024). Biomarkers for Immunotherapy Efficacy in Advanced Hepatocellular Carcinoma: A Comprehensive Review. Diagnostics, 14(18), 2054. https://doi.org/10.3390/diagnostics14182054







