Artificial Intelligence Tools in Pediatric Urology: A Comprehensive Review of Recent Advances
Abstract
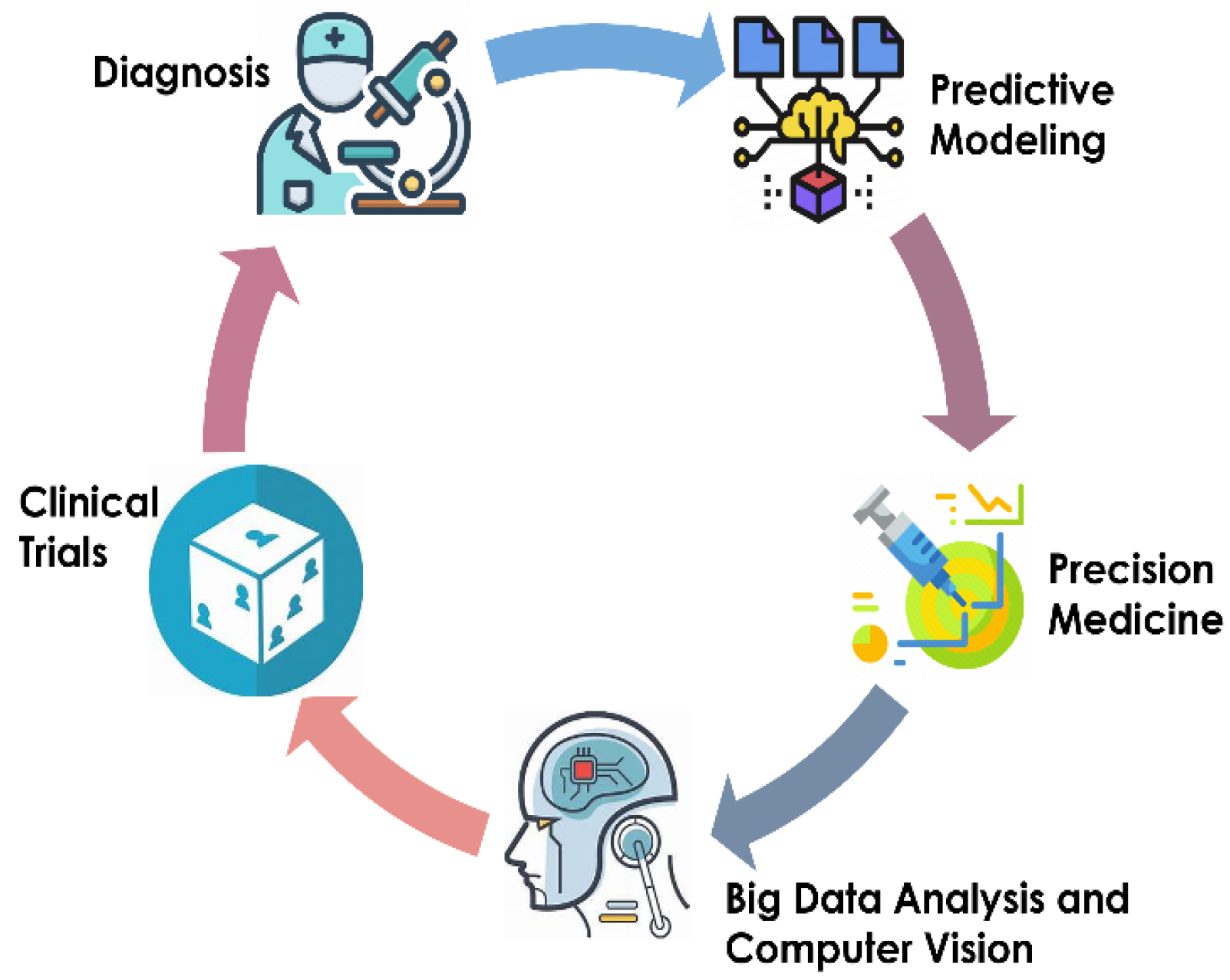
:1. Introduction
1.1. Background on Pediatric Urology
1.2. Importance of AI in Pediatric Urology
| Aspect | Importance of AI in Pediatric Urology |
|---|---|
| Diagnosis | AI can aid in the accurate and early diagnosis of pediatric urological conditions through analysis of medical images and patient data [5]. |
| Treatment Planning | AI algorithms can assist in developing personalized treatment plans based on patient-specific factors, improving outcomes, and reducing risks [6]. |
| Surgical Assistance | AI-enabled surgical tools can enhance precision and safety during pediatric urological procedures, reducing complications and recovery times [7]. |
| Predictive Analytics | AI can help predict progression of certain urological conditions in pediatric patients, allowing for proactive intervention and management [3]. |
| Research and Innovation | AI facilitates the analysis of large datasets to identify trends, patterns, and novel insights, driving advances in pediatric urology [8]. |
| Education and Training | AI-powered simulations and virtual reality environments offer valuable educational resources for training pediatric urologists and residents [8]. |
| Patient Monitoring | AI-driven monitoring systems can continuously track pediatric urology patients, providing real-time alerts for any concerning developments [6]. |
1.3. AI Applications in Other Medical Fields
1.4. Objectives of This Review
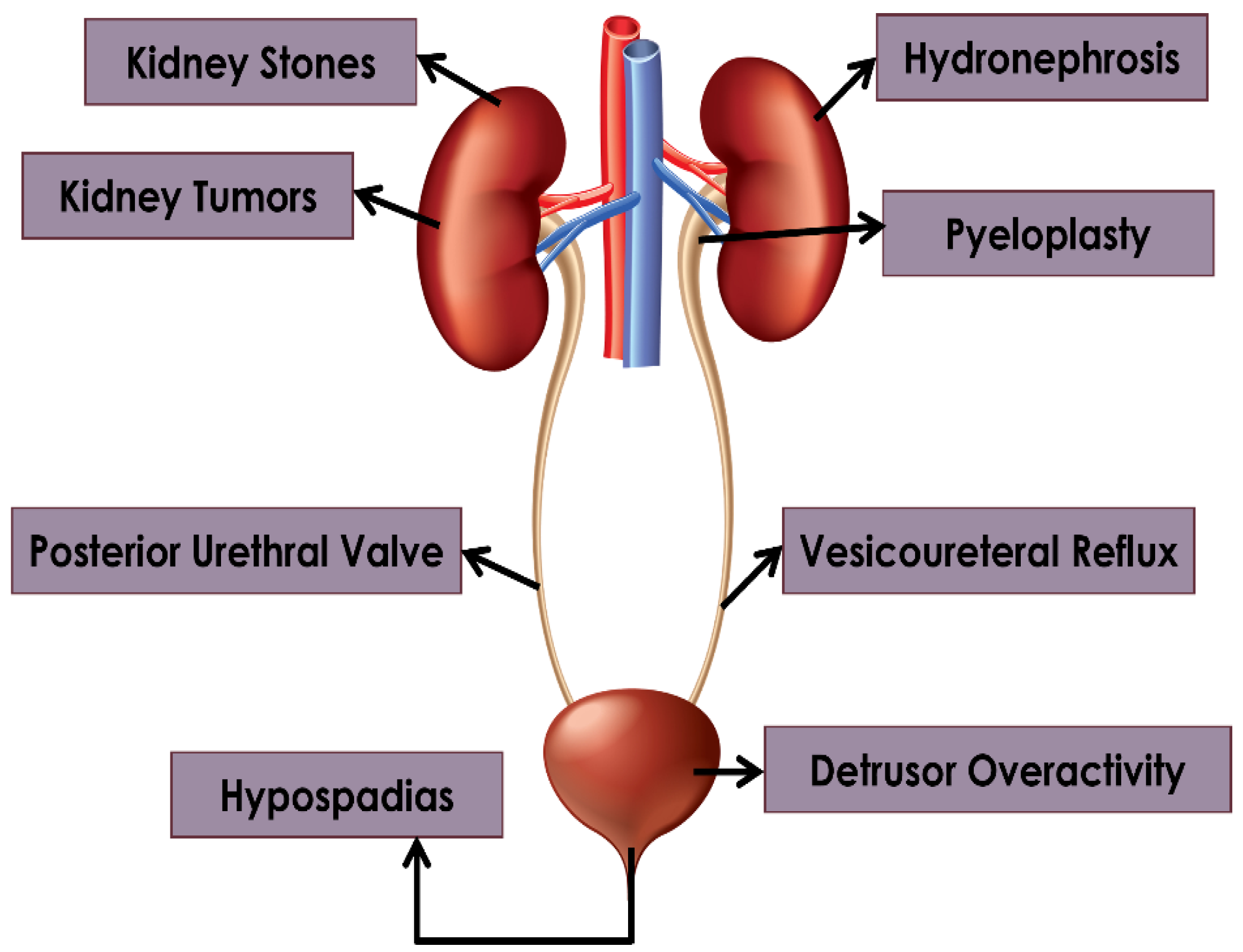
2. Hydronephrosis
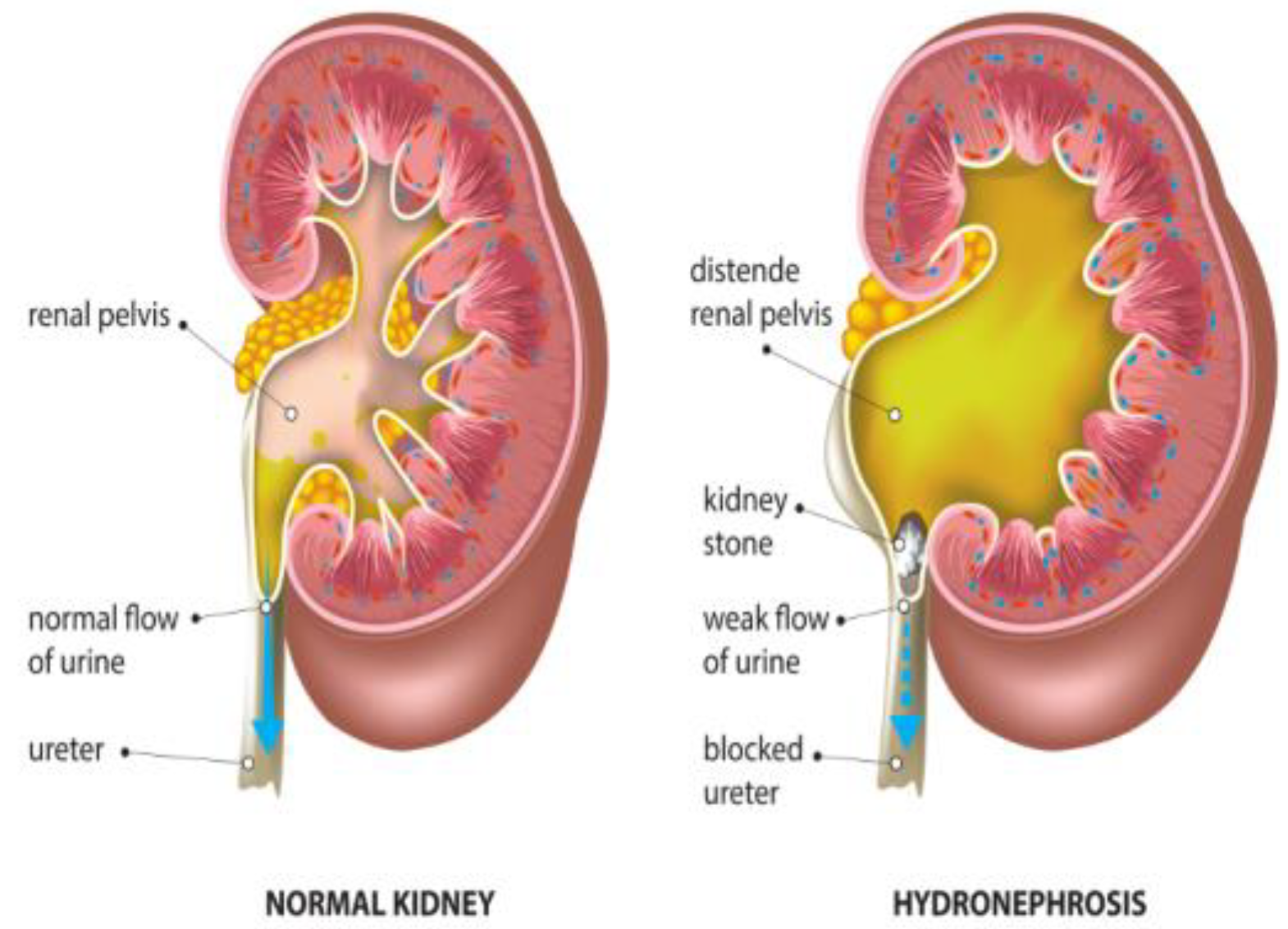
2.1. Diagnostic Challenges in Pediatric Hydronephrosis

| Diagnostic Challenge | Description | Mitigation Strategies |
|---|---|---|
| Subjective nature of ultrasound imaging [24] | Traditional methods for diagnosing pediatric hydronephrosis rely heavily on ultrasound imaging, which is subjective and prone to variability. | Implementing DL models can offer more impartial and replicable diagnostic tools, reducing the subjective and variable nature of ultrasound interpretation. |
| Lack of clear guidelines for intervention [25] | Establishing precise criteria for intervention based on grading systems presents challenges. | Continued research is needed to develop clearer guidelines for intervention, considering factors such as patient age, severity of hydronephrosis, and potential risks. |
| Issues with imaging techniques [20] | Although imaging techniques like abdominal CT are efficient, they pose risks of radiation exposure and higher cost. | Exploring alternative imaging modalities with lower radiation exposure, such as magnetic resonance urography (MRU), can mitigate the risks associated with radiation exposure while maintaining diagnostic efficiency. |
2.2. AL Solutions for Hydronephrosis Management
2.3. Impact on Treatment Planning and Long-Term Monitoring
3. Pyeloplasty
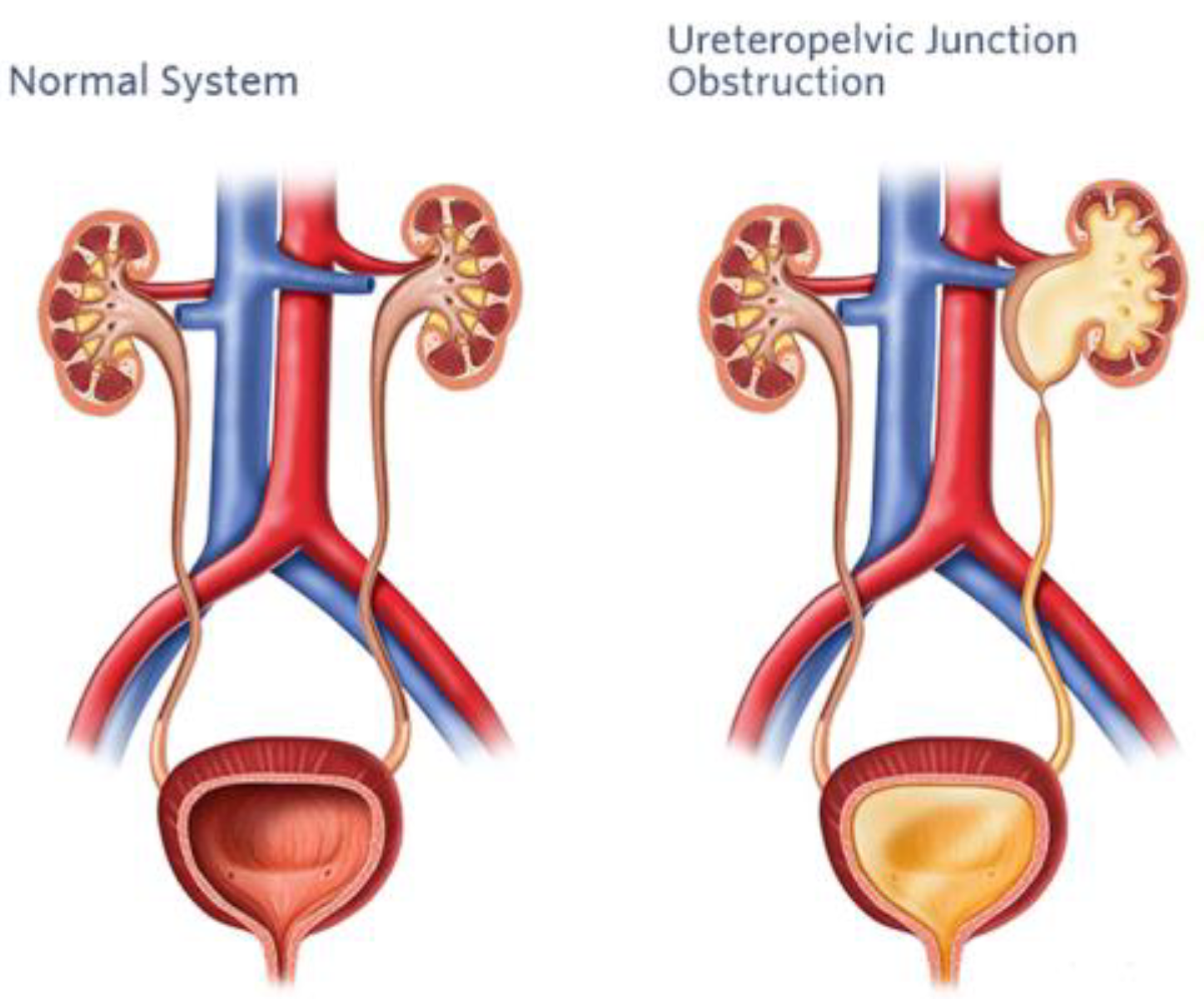
3.1. Surgical Challenges in Pediatric Pyeloplasty
3.2. AI-Assisted Surgical Techniques
3.3. Outcomes and Future Directions
4. Pyeloplasty: Kidney Tumors and Stones
4.1. Outcomes and Future Directions: Diagnostic Challenges and Treatment Options
- Diagnostic difficulties: Children with kidney tumors and stones may exhibit nonspecific symptoms such hematuria, stomach discomfort, or urinary tract infections, which can be mistaken for other common pediatric ailments. Therefore, a combination of clinical evaluation, imaging exams, and laboratory investigations is crucial for accurate diagnosis. However, it can be difficult to differentiate between different forms of stones or distinguish between benign and malignant tumors, requiring a thorough diagnostic approach [39].
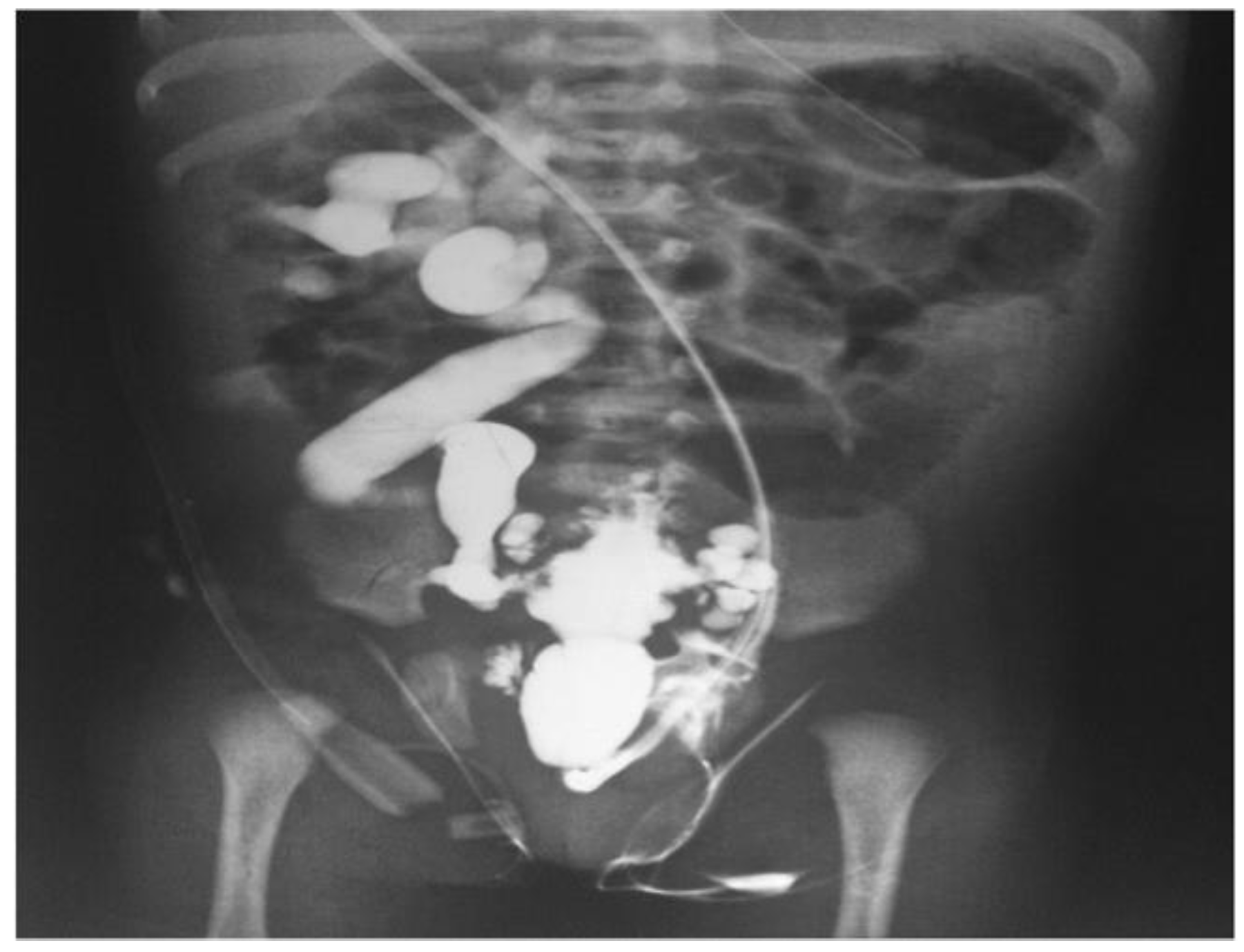
- Imaging modalities: A number of imaging modalities, such as intravenous pyelography (IVP), CT, MRI, and ultrasound, are essential for the diagnosis of juvenile kidney cancers and stones. Ultrasound is noninvasive and emits no ionizing radiation, so this method is used as a first-line imaging modality to examine renal morphology and identify structural problems (Figure 6). CT and MRI are more sensitive and specific for assessing stone load and identifying renal masses but also entail radiation exposure and require patient sedation [40].
- Treatment options: Tumor type, size, and location, and patient age are among the characteristics that influence how juvenile kidney tumors and stones are managed. Options for treatment vary from minimally invasive techniques and surgical intervention to cautious maintenance and attentive waiting. The primary therapy for kidney cancers that are localized is surgical excision; nephron-sparing techniques are recommended whenever possible in order to maintain renal function. Alternatively, depending on the size and composition of the stones, medicinal therapy, dietary changes, and minimally invasive techniques like ureteroscopy or shock wave lithotripsy (SWL) may be employed [41].

4.2. AI Applications in the Management of Kidney Tumors and Stones
4.2.1. Pediatric Kidney Stones
- Improved diagnostics: AI-driven image analysis tools are improving the identification and measurement of juvenile kidney stones, enabling more precise diagnosis and therapy. Clinicians can estimate stone load and composition by applying ML techniques, making it possible to customize treatment and patient management [40].
- Personalized therapy planning: AI systems create individualized therapy suggestions based on the analysis of patient-specific information, including imaging data and medical history. This improves outcomes and lowers the likelihood of recurrence [39].
- Predictive modeling and risk stratification: Physicians can identify juvenile patients with kidney stones who are more likely to experience problems or a recurrence by using AI-powered predictive modeling. ML algorithms can forecast future stone occurrences and direct treatment and preventative actions by evaluating a variety of clinical factors and imaging data to achieve better long-term results [41].
- Ethics and regulations: Careful consideration of ethical and regulatory problems is required when integrating AI into the treatment of juvenile kidney stones, which should employ defined protocols and encourage openness and accountability as standard. For AI to be used ethically and responsibly, cooperation among researchers, regulatory agencies, and healthcare practitioners will be crucial [39].
4.2.2. Pediatric Kidney Tumors
- Improved diagnostics: AI-driven image analysis tools can enhance the identification and description of pediatric kidney cancers and tumors. Radiologists can spot minor abnormalities, characterize renal masses, and estimate tumor burden more accurately using ML algorithms that have been trained on large datasets of pediatric renal images (Figure 7). AI algorithms enable the early identification of kidney cancers and tumors by methods including pattern recognition, masking, segmentation, and quantitative analysis, allowing for confident diagnosis and timely treatment [24].
- Personalized treatment planning: AI systems provide personalized therapy recommendations and perform the prognostic evaluation of patient-specific data, including clinical history, imaging results, and laboratory values. For young patients with kidney cancers, this customized strategy enables medical practitioners to maximize therapeutic efficacy, reduce treatment-related morbidity, and enhance long-term outcomes [43].
- Predictive modeling and risk stratification: AI predictive modeling enables doctors to identify children with kidney tumors who are more likely to experience treatment failure or disease progression. ML algorithms identify prognostic characteristics and biomarkers through the analysis of varied datasets. This improves treatment outcomes and survival rates for children with kidney cancers by empowering doctors to use tailored risk mitigation strategies and start treatment early [44].
- Ethical and regulatory considerations: Incorporating AI into the treatment of juvenile kidney cancers and tumors requires careful consideration to overcome legal, ethical, and practical challenges. Collaboration between developers, healthcare providers, and other stakeholders will be essential for ensuring the ethical and effective use of AI in clinical practice [45].
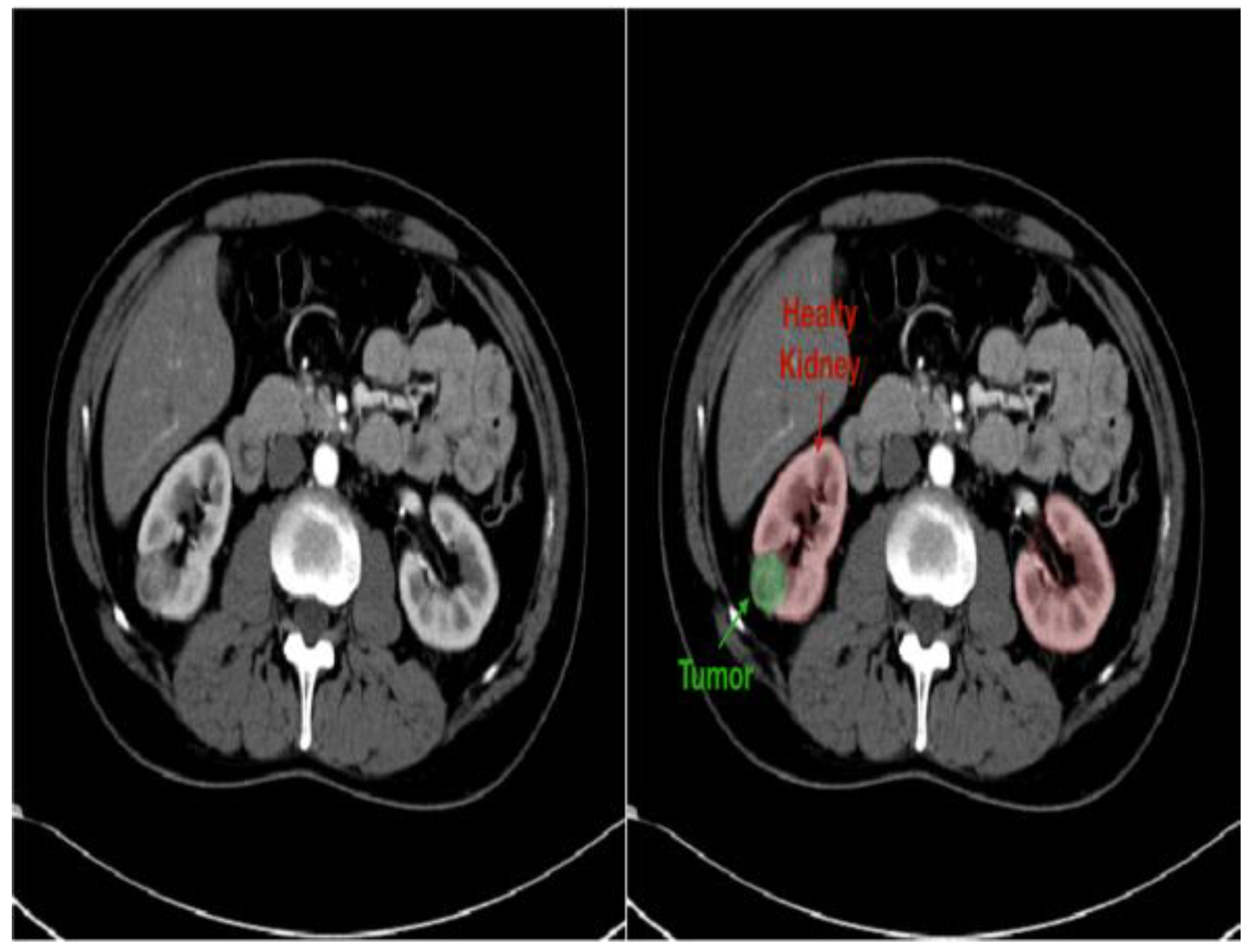
4.3. Outcomes and Future Directions: Impact on Treatment Strategies and Outcomes
| Pediatric Kidney Care | Description | Example | Importance |
|---|---|---|---|
| Optimized treatment planning [44] | AI-driven algorithms optimize treatment plans for kidney tumors and stones in children by evaluating vast databases of patient-specific data. | Individualized treatment regimens tailored to each patient’s requirements based on clinical features, imaging results, and therapy responses. | Maximizes therapeutic efficacy, reduces treatment-related morbidity, and enhances long-term results. |
| Enhanced surgical precision [40] | AI technologies provide greater precision and accuracy in surgical interventions for pediatric kidney tumors and stones. | AI-powered surgical navigation systems offer immediate feedback during operations, facilitating accurate tumor and stone localization, ideal tissue resection margins, and shorter operating times. | Improves surgical outcomes and patient safety by increasing precision and reducing intraoperative complications. |
| Improved prognostic approaches [40] | AI-powered predictive analytics revolutionize prognostication and risk stratification for pediatric kidney tumors and stones. | ML algorithms analyze multidimensional datasets to uncover prognostic biomarkers, treatment response predictors, and disease progression indicators. | Informs decisions about patient care and therapy selection, leading to enhanced treatment outcomes, reduced complications, and improved survival rates. |
| Longitudinal monitoring and follow-up [41] | AI technologies enable longitudinal monitoring and follow-up for pediatric patients with kidney tumors and stones beyond treatment planning and intervention. | AI-powered monitoring systems examine long-term patient data to detect subtle changes in disease state, early indicators of recurrence or progression, and prompt interventions or treatment regimen modifications. | Enhances disease monitoring, treatment adherence, and long-term outcomes by enabling proactive management and personalized follow-up care. |
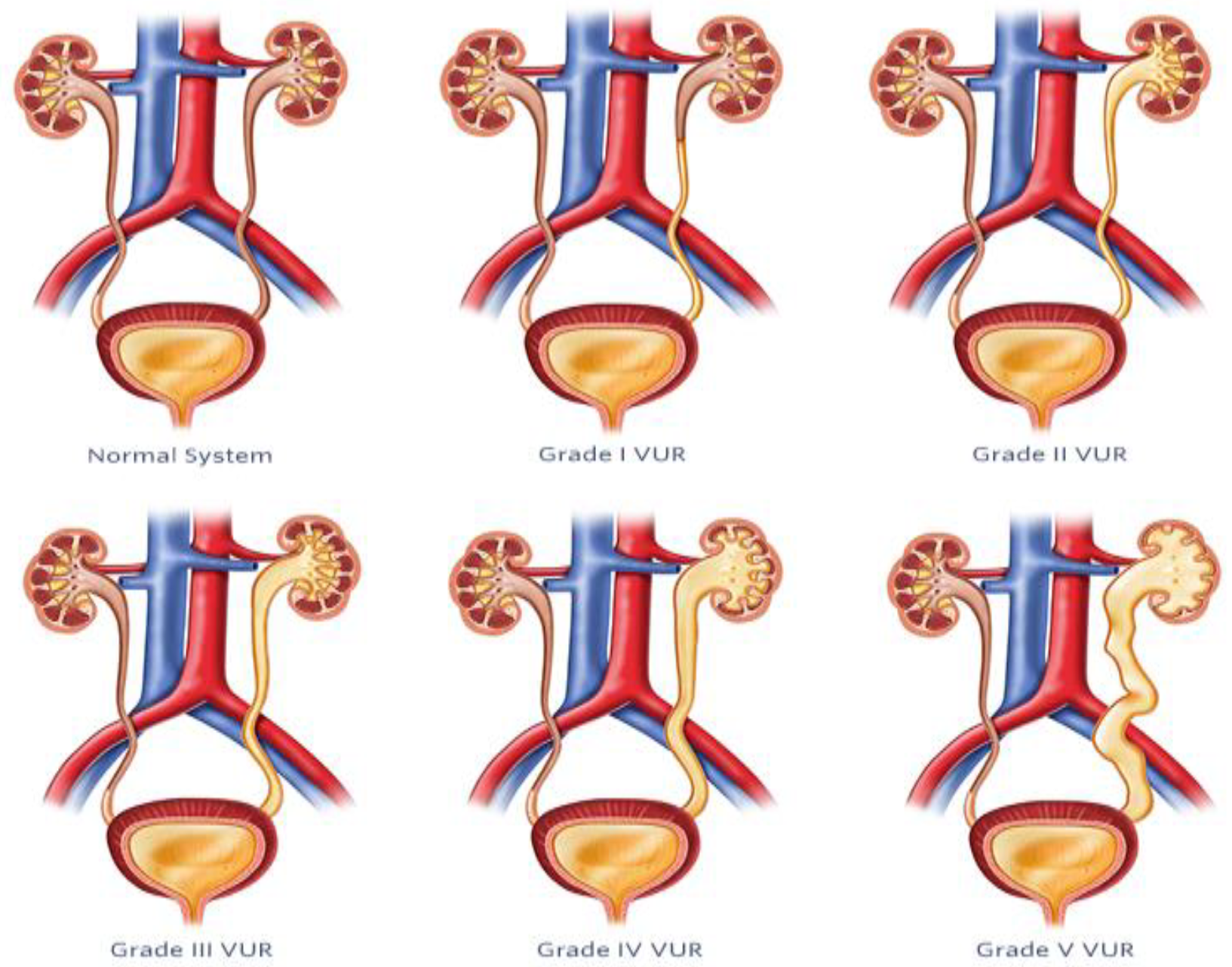
5. Vesicoureteral Reflux
5.1. Diagnosis and Management of VUR
5.2. AI-Based Imaging Techniques
| Approach | Description | Advantages |
|---|---|---|
| Machine learning models for VUR [48] | Utilizes ML to automate VUR severity assessment from VCUG images, aiming to reduce subjectivity and improve reliability of evaluation. | Decreases influence of subjectivity, offers uniform diagnostic procedures. |
| Quantitative vesicoureteral reflux (qVUR) [49] | Employs supervised ML to analyze VCUG images for VUR severity, achieving high accuracy and clarity in grading. | Provides a web program for automated grading, ensures impartial and consistent assessment. |
| Contrast-enhanced voiding ultrasonography (ceVUS) [52] | Uses second-generation ultrasound contrast agents and AI algorithms to diagnose VUR, offering a radiation-free alternative to traditional methods. | Enhances ultrasound imaging, reduces risks associated with ionizing radiation. |
| Vesicoureteral reflux index (VURx) [54] | Predictive tool developed for assessing improvement and resolution rates of VUR in children under two years, aiding in tailored treatment plans. | Accurately predicts reflux improvement and resolution, guides clinical decision making. |
5.3. Prediction Models and Long-Term Follow-Up
6. Detrusor Overactivity
| Study | Study Title | Description of the Study | Key Findings |
|---|---|---|---|
| [55,56] | Machine learning for urodynamic detection of detrusor overactivity | Developed an ML algorithm to identify clinician-detected detrusor overactivity (DO) in urodynamic studies (UDS) for 546 patients with spina bifida. Analyzed data from 805 UDS in time and frequency domains using vesical, abdominal, and detrusor pressure channels. | Generated models included data windowing, dimensionality reduction, and support vector machine. They achieved good performance in detecting DO in agreement with clinicians, with the time-based model having the highest AUC (91.9%), sensitivity (84.2%), and specificity (86.4%). The frequency-based model had the highest specificity (92.9%). |
| [57] | A pilot study: detrusor overactivity diagnosis method based on deep learning | Constructed two convolutional neural network (CNN) models to assist in diagnosing DO based on UDS curves from 92 patients: 44 samples and 48 tests used to develop a threshold screening strategy to filter suspected DO events with 10-fold cross validation. | The CNN models achieved high training and validation accuracies. In testing, the diagnostic accuracy for patients without DO was 78.12%, and for patients with DO, it was 100%. |
| [58] | Pattern recognition algorithm to identify detrusor overactivity urodynamics | Developed a statistical model and applied ML algorithms to identify overactive contractions (OCs) in UDS data from 799 patients. Used dynamic time warping, k-means clustering, and five-fold cross validation. | The model achieved AUC of 0.84, accuracy of 81.27%, sensitivity of 77.77%, and specificity of 81.31% in detecting OC events, showing promising model performance for standardization of UDS interpretation. |
| [59] | Detection and quantification of overactive bladder activity in patients: Can we make it better and automatic? | Developed an algorithm based on time–frequency analysis to analyze bladder pressure and detect patterns in UDS data. Generated a bladder overactivity index (BOI) to quantify nonvoiding activity. Algorithm was tested with three groups: DO group, OAB with DO group and OAB without DO group. | The algorithm successfully identified significant differences in BOI between control and overactive bladder (OAB) groups and could detect detrusor overactivity episodes. It provided quantitative data on nonvoiding bladder activity. |
| [60] | Machine learning for automated bladder event classification from single-channel vesical pressure recordings | Evaluated an ML framework for classifying bladder events (abdominal event, voiding contraction, DO, no event) from single-channel vesical pressure recordings using wavelet analysis, feature extraction and five-fold cross validation. | The k-nearest neighbor, artificial neural network, and support vector machine classifiers achieved overall classification accuracies of 91.5%, 90.8%, and 82.4%, respectively, indicating framework’s ability to automatically classify signal channel UDS data. |
6.1. Current Diagnosis and Management Challenges
6.1.1. Current Diagnosis Challenges in Pediatric Detrusor Overactivity (DO)
6.1.2. Challenges in Managing Pediatric Detrusor Overactivity
6.1.3. Progress and Prospects for the Future
6.2. AI Solutions for Detrusor Overactivity
6.2.1. AI Solutions for the Diagnosis of Detrusor Overactivity in Pediatric Patients
6.2.2. Artificial Intelligence Solutions for Automated Detection of Detrusor Overactivity
6.2.3. AI Solutions for the Management of Detrusor Underactivity (DUA)
6.2.4. Progress in Diagnostic Imaging Techniques
6.3. Case Studies and Clinical Applications
6.3.1. Case Studies and Clinical Applications in Pediatric Patients
6.3.2. Machine Learning Models and Performance
6.3.3. Challenges and Future Directions
7. Posterior Urethral Valves
7.1. Clinical Features and Diagnosis
- Antenatal diagnosis: Deshpande (2018) [63] found that approximately 50% of PUV instances can be diagnosed prenatally using ultrasound. The diagnostic criteria include bilateral hydronephrosis, an enlarged bladder (megacystis), or a keyhole sign (a dilated posterior urethra narrowing at the point of valve obstruction). Unfortunately, the accuracy of prenatal ultrasound is quite low, ranging from 40% to 80%. Fishberg et al. (2018) [64] and Kwong et al. (2022) [65] have also reported that PUV can be indicated by prenatal ultrasound findings including bilateral hydroureteronephrosis (dilation and swelling of the ureters), a distended and thickened bladder, oligohydramnios (low levels of amniotic fluid), the presence of the keyhole sign, and increased renal echogenicity (higher than normal reflection of sound waves in the kidneys).
- Postnatal presentation: Neonates with PUVs may present diverse symptoms such as difficulty breathing, bluish discoloration of the skin, weak or irregular urine flow, lack of energy, difficulty with feeding, and widespread swelling [64,65]. Physical examination may uncover an enlarged abdomen caused by urine ascites, a swollen bladder, or hydronephrosis. Pellegrino et al. (2023) [66] observed that some neonates may exhibit respiratory distress or sepsis, while others show no symptoms. This study also noted that in newborns and older children, symptoms such as urinary tract infections, reduced urine flow, inability to control urination, and bedwetting may also indicate the presence of PUV.
- Bladder and renal function: According to Deshpande (2018) [63], blood creatinine levels are crucial for predicting outcomes after birth. An elevated minimum level of blood creatinine in the first year of life (>0.8–1.0 mg/dL) is linked to an increased likelihood of long-term kidney complications. The velocity of decline in serum creatinine following valve ablation along with the existence of prolonged renal tubular acidosis also serve as indicators for renal outcomes.
- Methods of diagnosis: VCUG is widely recognized as the most reliable method of validating a PUV diagnosis. According to Fishberg et al. (2018) [64] and Kwong et al. (2022) [65], VCUG enables the observation of valvular obstruction, a bladder that is thickened and has trabeculations, diverticuli, and vesicoureteral reflux.
7.2. AI Applications in Prenatal Diagnosis and Risk Stratification
| Application | Description | Advantages | Research |
|---|---|---|---|
| Early PUV detection from prenatal ultrasound | ML algorithms analyze prenatal ultrasound images to identify fetuses at high risk of PUV. |
| Deshpande (2018) developed a DL model achieving high accuracy in PUV detection from first-trimester ultrasounds [63]. |
| PUV diagnosis from postnatal ultrasound | AI analyzes features in postnatal ultrasound images to aid PUV diagnosis. |
| Fishberg et al. (2018) proposed a ML model using ultrasound and clinical data to diagnose PUV in children [64]. |
| Predicting outcomes in PUV patients | ML models analyze patient data to predict outcomes following PUV treatment. |
| Kwong et al. (2022) developed a tool (PUVOP) using ML to predict outcomes in boys with PUV [65]. |
| AI-assisted minimally invasive surgery | Robotics and AI can be integrated into surgical procedures for improved precision and efficiency of PUV correction. |
| This is an emerging field, but some studies explored the potential of robotic-assisted surgery for PUV (Weaver et al., 2023) [68]. |
7.3. Role of Cystoscopy in the Diagnosis and Management of PUV
- Diagnostic role: Cystoscopy is the definitive diagnostic modality for PUV, as highlighted by Pellegrino et al. (2023) [66]. This method enables the direct observation of the membrane of the posterior urethral valve, thereby establishing diagnosis following first suspicion based on prenatal ultrasound or postnatal clinical presentation. Moreover, these studies assert that cystoscopy is essential for assessing the bladder dysfunction resulting from PUV. It can evaluate any remaining valve, stricture caused by medical intervention, or enlargement in the bladder neck, all of which can lead to ongoing dilation of the upper urinary tract and decline in function.
- Therapeutic role: According to Deshpande (2018) [63], the postnatal treatment of choice for PUV is endoscopic ablation of the valve using diathermy, a cold knife, or a holmium-YAG laser, performed under cystoscopic guidance. Cystoscopy is the main technique used for endoscopic valve ablation, which is the ideal initial surgical treatment for PUV in newborns, as stated by Fishberg et al. (2018), Kwong et al. (2022), and Taskinen et al. (2012) [64,65,67]. They have observed that in certain situations, bladder neck incision can be performed cystoscopically in conjunction with valve ablation to enhance emptying. In addition, Fishberg et al. (2018) [64] and Kwong et al. (2022) [65] have examined the application of cystoscopy for the endoscopic administration of botulinum toxin into the bladder. This procedure aims to enhance bladder compliance and reduce detrusor overactivity in patients experiencing ongoing bladder dysfunction following valve ablation.
- Follow-up and management: Following valve ablation, it is advised to repeat VCUG or cystoscopy within 1 to 3 months to verify the full removal of the valves, as indicated by Fishberg et al. (2018) [64]. According to Taskinen et al. (2012) [67], after surgery, it is advisable to perform cystoscopy or a voiding cystourethrogram to eliminate the possibility of partial ablation, scar tissue, or remaining obstruction.
7.4. Importance, Impact, and Application of Cystoscopy in Pediatric Urology
8. Hypospadias
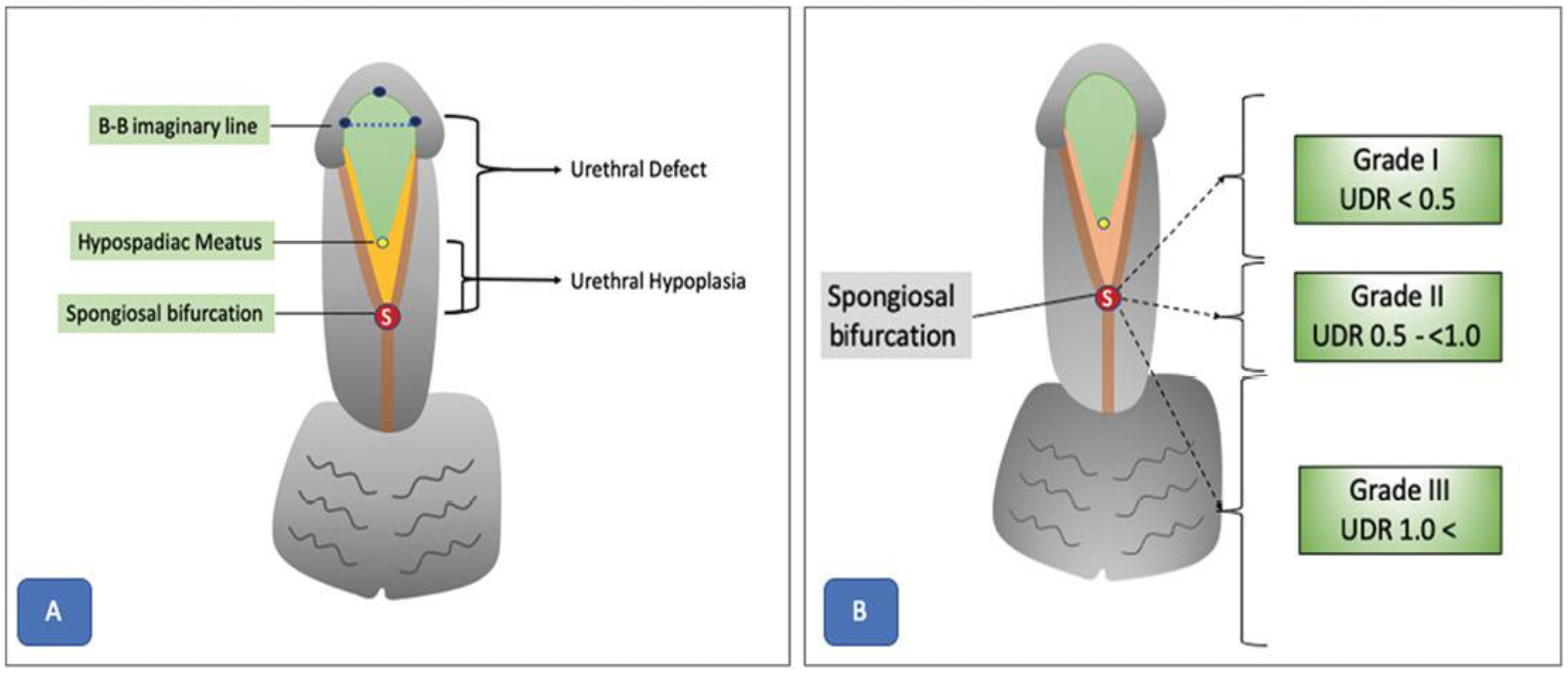
8.1. Surgical Considerations and Complications
- Objective assessment of hypospadias severity: Objective evaluation methods such as the plate objective scoring tool (post) represent major advances in the field of hypospadias surgery [76]. POST provides a systematic way to assess urethral plate quality, which helps surgeons select appropriate repair methods and anticipate postoperative results. However, even standardized methods like POST remain subjective, notably regarding the interpretation of anatomical landmarks from 2D pictures [77]. The need for accurate and reproducible methods to standardize evaluation and reduce variability between surgeons is therefore clear. Objective evaluation methods like POST are an important step towards this aim, but additional refinement and validation are required before they can be used widely in clinical practice.
- Surgical considerations in hypospadias-associated penile curvature (HAPC): The treatment of hypospadias-associated penile curvature (HAPC) is complex, as explained by Fernandez et al. (2021) [78]. Critical aspects are the initial assessment and quantification of HAPC, where variable evaluation techniques highlight the need for standardized, reliable, and repeatable instruments. The lack of agreement on surgical correction thresholds also affects decision making. Surgical method selection can range from dorsal plication to ventral lengthening operations, as described by Abbas et al. (2023) [76]. The complications linked with HAPC correction, such as graft contracture and recurrent curvature, demand careful planning and judicious use of surgical procedures. Overall, treating HAPC requires a multimodal strategy that includes several standardized evaluation tools, evidence-based protocols, and careful surgical method selection.
- Impact of anatomical variables on surgical outcomes: Anatomical characteristics are critical determinants of surgical results and potential problems in hypospadias correction, as highlighted by Fruntelata and Stoica (2021) [79]. Fernandez et al. (2021) [78] reported that the glans size, quality of the urethral plate, and degree of penile curvature all exert a major impact on surgical difficulty and the likelihood of attaining good esthetic and functional outcomes. These studies present information on the many kinds of hypospadias operations performed and their related consequences, emphasizing the need to conduct preoperative screening for concomitant abnormalities and tailor surgical techniques accordingly. Despite acknowledgement that these anatomical features are key predictors of surgical results, there is still substantial subjectivity in their evaluation, making it difficult to standardize categorization criteria and compare outcomes between surgical facilities and individual procedures. Addressing these issues necessitates continued work to provide uniform screening tools, improve categorization criteria, and establish evidence.
8.2. AI Applications in Hypospadias Surgery
- AI-based UP quality assessment: Abbas et al. (2023) [76] explored the potential of AI and DL algorithms to streamline and optimize the assessment of urethral plate (UP) quality from 2D images. The authors proposed a framework that combines glans localization, landmark detection, and plate objective scoring tool (POST) calculation using DL models. The localization step detects the glans area within an image using a YOLOv5 network, which achieved highly accurate results (mean average precision 99.5%, overall sensitivity 99.1%). Isolating the region of interest from image background improves the feature extraction and landmark detection in subsequent stages.
- Landmark detection and benefits: For landmark detection, authors employed a deep convolutional neural network (CNN) architecture called HRNetV2, which maintains high-resolution representations throughout the network [76]. This approach results in spatially precise landmark predictions, which are essential for accurate calculation of the POST score. The proposed model achieved a normalized mean error of 0.07152 in predicting the coordinates of all five POST landmarks. The authors highlighted the potential benefits of this AI-based framework in increasing inter-rater consistency and standardizing UP quality scoring. Less experienced surgeons could benefit from the real-time intraoperative application of the algorithm to aid decision making and potentially improve postoperative outcomes.
- AI for hypospadias parameter recognition: Wahyudi et al. (2022) [80] proposed the development of an artificial neural network (ANN) for the automated recognition of various hypospadias parameters from digital images. The key parameters include hypospadias status, meatal location and shape, urethral plate quality, glans diameter, and glans shape. This type of model needs to be trained on a database of labeled images from hypospadias cases and normal penile anatomy controls. To achieve this, parents or guardians capture standardized photographs using a mobile app, then upload these for analysis by the ANN model. Performance can then be validated against evaluations by pediatric urologists, with measures including accuracy, precision, and inter-rater reliability. Several other AI-based approaches have been proposed to help standardize hypospadias assessment, reduce interobserver variability, and facilitate diagnosis, especially in regions with limited access to specialized healthcare personnel (Table 7).Table 7. Recent AI applications in hypospadias surgery.
Application Description Key Benefits AI-based UP quality assessment [76] Combines glans localization, landmark detection, and POST score calculation using DL models for assessing urethral plate quality from 2D images Increases inter-rater consistency, standardizes UP quality scoring, aids decision making for less experienced surgeons Landmark detection [76] Uses HRNetV2 deep CNN architecture for spatially precise landmark prediction, essential for accurate POST score calculation Enables accurate POST score calculation by precisely localizing anatomical landmarks AI for hypospadias parameter recognition [80] Proposes an AI system using an artificial neural network (ANN) for automated recognition of hypospadias parameters from digital images, including meatal location, urethral plate quality, and glans characteristics Standardizes hypospadias assessment, reduces interobserver variability, facilitates diagnosis in areas with limited access to specialists Deep learning for hypospadias surgery planning [81] Developed a DL model to predict the likelihood of requiring additional surgical procedures based on preoperative patient data and intraoperative findings Helps in preoperative planning and counseling by predicting the need for additional procedures AI-assisted hypospadias classification [82] Developed a convolutional neural network (CNN) model for automated classification of hypospadias severity from clinical photographs Enables objective and consistent classification of hypospadias severity, which can guide surgical decision making Automated glans size measurement [78] Proposed an AI-based method for automated measurement of glans size from digital photographs, a critical parameter in hypospadias surgery planning Provides accurate and reproducible glans size measurements, reducing interobserver variability and facilitating surgical planning
8.3. Impact on Surgical Outcomes
9. Other Emerging AI Applications in Pediatric Urology
9.1. Customized Treatment Planning
| AI Application | Description | Importance |
|---|---|---|
| Personalized treatment planning [8] | AI-driven algorithms analyze patient-specific data, including clinical features, imaging results, and therapy responses, to create personalized treatment plans tailored to each pediatric urology patient’s unique requirements. | Enhances treatment efficacy, reduces treatment-related morbidity, and improves long-term outcomes. |
| Image analysis [5] | AI algorithms analyze medical images such as ultrasound, MRI, and CT scan to assist in the diagnosis and treatment planning of pediatric urological conditions. | Improves accuracy and efficiency in diagnosing and monitoring conditions, aiding in treatment planning. |
| Surgical simulation [83] | AI-powered surgical simulations allow pediatric urologists to practice complex procedures in a virtual environment, enhancing surgical skills and reducing risks during actual surgeries. | Facilitates training and skill development, leading to improved surgical outcomes and patient safety. |
| Longitudinal patient monitoring [2] | AI-enabled monitoring systems track pediatric urology patients over time, analyzing data to detect trends, anticipate complications, and optimize treatment plans. | Provides continuous monitoring and personalized care, enhancing treatment effectiveness and patient outcomes. |
9.2. Virtual Reality Simulation for Real-Time Surgical Training and Guidance
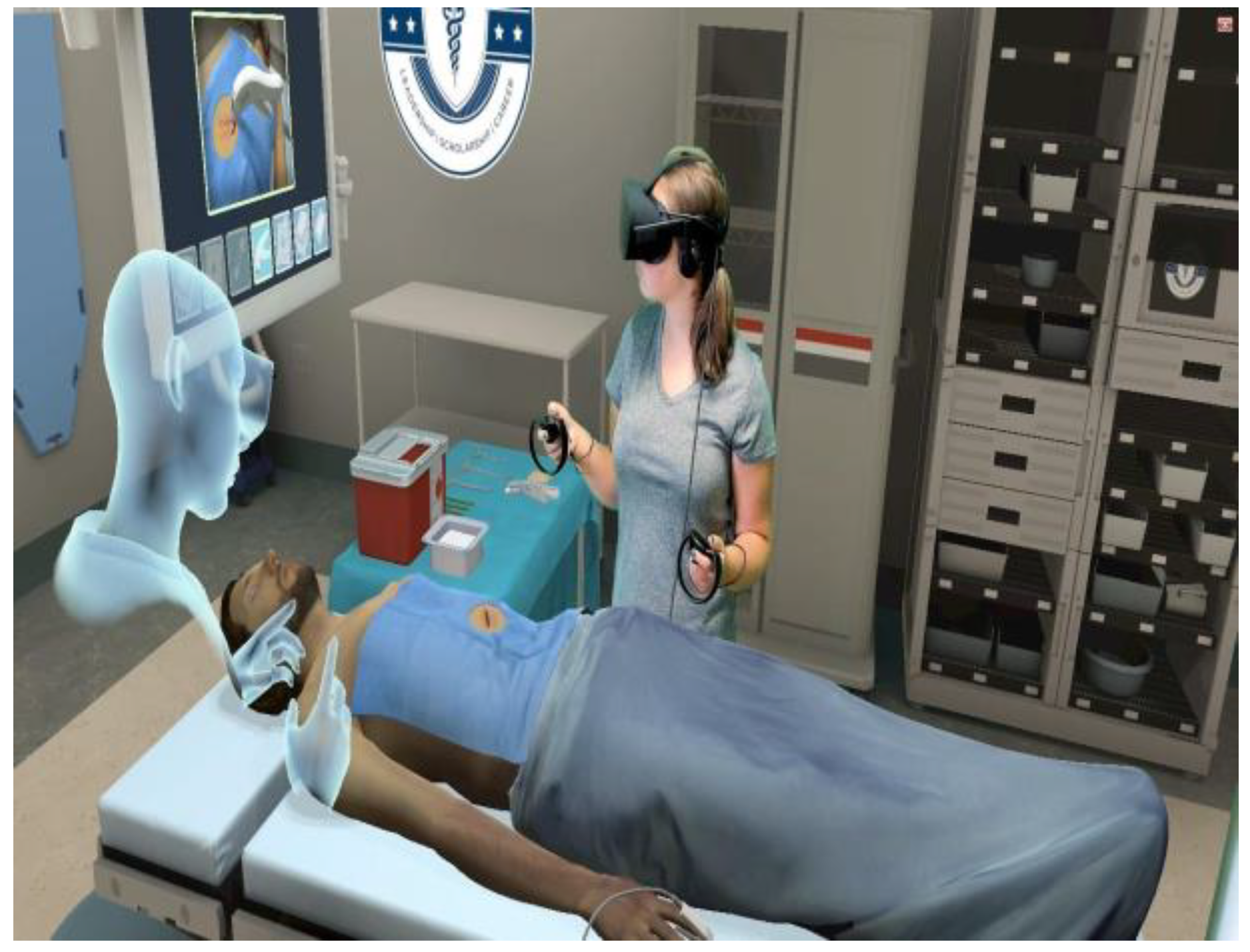
9.3. Integration of Decision Support Mechanisms
- Pediatric image enhancement techniques: Improving picture quality is crucial for precise diagnosis and efficient surgical planning. To meet these needs, a variety of image enhancement techniques have been created to improve clarity, raise contrast, and reduce noise (Table 9).
| Technique | Description | Application |
|---|---|---|
| CLAHE (contrast limited adaptive histogram equalization) [85] | Improves contrast by applying histogram equalization in small regions, limiting noise amplification. | Enhancing image contrast, reducing noise. |
| Histogram equalization [86] | Enhances contrast by spreading out the most frequent intensity values. | Improving image contrast and visibility of features. |
| Unsharp masking [87] | Sharpens images by subtracting a blurred version from the original. | Enhancing edge definition and overall image clarity. |
| Gaussian filtering [88] | Reduces image noise and detail using a Gaussian function. | Smoothing images and reducing noise. |
| Median filtering [88] | Reduces noise while preserving edges by replacing each pixel value with the median of surrounding pixels. | Noise reduction while preserving important image features. |
| Anisotropic diffusion [89] | Smooths images within regions while preserving edges. | Enhancing edge definition and reducing noise. |
| Wavelet transform [85,86] | Decomposes images into multiple scales and reconstructs images with enhanced details. | Enhancing image details and texture. |
| Fourier transform [90] | Manipulates frequency components for noise reduction and image reconstruction. | Enhancing image quality and removing noise. |
| Edge enhancement [91] | Highlights edges in images, improving boundary visibility. | Enhancing edge definition for better visualization. |
| Normalization [92] | Adjusts pixel intensity values to improve feature visibility. | Enhancing visibility of specific features in images. |
| Adaptive filtering [88] | Applies different filters based on local image characteristics. | Enhancing image quality based on local context. |
| Deconvolution [93] | Improves image clarity by reversing the effects of blurring. | Enhancing image resolution and detail. |
| Noise reduction [91] | Utilizes algorithms like nonlocal means and bilateral filtering to reduce noise while preserving features. | Reducing noise while maintaining important image information. |
| Super-resolution [94] | Enhances image resolution by combining multiple images or using DL techniques. | Improving image resolution for better visualization. |
| Deep-learning-based enhancement [95] | Utilizes neural networks to improve image quality, denoise, and enhance features. | Enhancing image quality and feature extraction using DL. |
- Ground-truth-building techniques for segmentation: High-quality image segmentation is standard for certifying and training AI systems. Ground truth construction by combining multiple inputs to provide a consensus segmentation is essential to obtain precise and reliable decision support systems. A summary of the current segmentation ground truth methods in medical imaging is shown in Table 10.
| Technique Name | Description | Key Features |
|---|---|---|
| STAPLE (simultaneous truth and performance level estimation) [96] | Combines multiple segmentations to estimate the most probable ground truth and the performance level of each input segmentation. | Simultaneously estimates true segmentation and performance levels. |
| Majority voting [96] | Labels each pixel or voxel according to the majority label from multiple segmentations. | Simplest approach, based on majority rule. |
| Label fusion [96] | Combines multiple segmentation results, typically through weighted averaging, to produce a consensus segmentation. | Uses weighted averaging to create a consensus. |
| iSTAPLE (intensity-based STAPLE) [97] | An extension of STAPLE that incorporates intensity information to improve the accuracy of ground truth estimation. | Incorporates intensity information for more accurate estimation. |
| Bayesian fusion [17] | Uses Bayesian statistics to combine multiple segmentations, considering the uncertainty and reliability of each segmentation. | Incorporates uncertainty and reliability in the fusion process. |
| Truth and performance level estimation with iterative fusion (T-PLEIF) [98] | Iteratively refines the ground truth estimate and the performance level of each segmentation source. | Iterative refinement process. |
| Simultaneous truth and performance level estimation with relaxation labeling (STAPLER) [99] | Combines relaxation labeling techniques with STAPLE for better performance in some scenarios. | Integrates relaxation labeling with STAPLE. |
| Weighted voting [100] | Similar to majority voting but assigns different weights to each segmentation based on their perceived accuracy or reliability. | Weights segmentations based on accuracy or reliability. |
| Expectation-maximization (EM) Label fusion [101] | Uses the EM algorithm to iteratively estimate the true segmentation and the reliability of each segmentation input. | Utilizes the EM algorithm for iterative estimation. |
| Random walker with priors [102] | Utilizes random walker algorithms with prior knowledge from multiple segmentations to determine the final ground truth. | Incorporates prior knowledge in the random walker algorithm. |
| Markov random field (MRF) label fusion [101] | Uses MRF models to combine segmentations, leveraging spatial context and dependencies between labels. | Leverages spatial context and dependencies with MRF models. |
| Machine-learning-based fusion [101] | Employs ML algorithms to learn the optimal way to combine multiple segmentations based on training data. | Utilizes ML for optimal combination of segmentations. |
10. Conclusions
10.1. Summary of Key Findings
- Improved diagnostic accuracy: Image-enhancing techniques such as ceVUS and quantitative analysis software have greatly advanced diagnostic precision in the field of pediatric urology. These techniques offer crisper and more detailed images that enhance doctors’ ability to identify tiny abnormalities that may have been overlooked by traditional imaging methods. These advanced technologies enable the early identification and treatment of medical conditions, leading to improved results for patients [52].
- Personalized treatment planning: AI-driven preoperative planning technologies analyze imaging data (CT, MRI, and ultrasound) to create three-dimensional reconstructions of the urinary system. This allows pediatric urologists to tailor treatment approaches to the distinct anatomy and pathology of individual patients, thereby maximizing results and reducing problems [49].
- Enhanced surgical guidance: AI-assisted surgical navigation systems improve accuracy and precision by offering real-time guidance during critical procedures. These devices employ AI algorithms to apply anatomical landmarks and trajectory planning onto the surgical field, enabling accurate dissection, tissue manipulation, and suture placement. These instruments enhance outcomes and patient safety by minimizing surgical errors [103].
- Efficient productivity: Image-enhancing technologies equipped with automated functions and user-friendly interfaces can optimize productivity. These tools facilitate the rapid processing of complex data, enabling doctors to prioritize patient care over manual duties, thereby improving satisfaction through increased efficiency [94].
- Enhanced education and training: AI-powered virtual reality simulations provide a secure and regulated learning environment that greatly improves the education and training of pediatric urologists. These tools offer interactive environments to engage in practice operations, enhance techniques, and simulate intricate surgical scenarios, leading to increased proficiency and confidence in trainees [103].
10.2. Ethical and Regulatory Considerations
10.3. Recommendations for Future Research and Clinical Practice
| Recommendations | Focus Area | Action Item |
|---|---|---|
| Conduct prospective studies [83] | Long-term outcomes and cost effectiveness of AI-assisted surgical techniques in pediatric pyeloplasty | Evaluate the long-term outcomes and cost effectiveness of AI-assisted surgical techniques in pediatric pyeloplasty. This includes assessing factors such as postoperative complications, recurrence rates, patient satisfaction, and economic implications over an extended follow-up period. |
| Develop standardized protocols and guidelines [106] | Ethical and responsible use of AI in pediatric urology, with a focus on patient privacy, data security, and algorithmic transparency | Develop standardized protocols and guidelines for the ethical and responsible use of AI in pediatric urology. Emphasis should be placed on safeguarding patient privacy, ensuring robust data security measures, and promoting algorithmic transparency to maintain trust and integrity in AI-driven healthcare applications. |
| Investigate the impact of AI-driven decision support systems [4] | Impact of AI-driven decision support systems on clinical workflow, patient outcomes, and healthcare resource utilization in pediatric urology practice | Investigate the impact of AI-driven decision support systems on various aspects of pediatric urology practice. This includes evaluating their influence on clinical workflow efficiency, patient outcomes such as surgical results and complication rates, as well as healthcare resource utilization including operative times, hospital length of stay, and overall healthcare costs. Additionally, explore user satisfaction and acceptance of these systems among healthcare providers. |
| Explore applications of AI in preoperative planning and simulation [3] | Optimization of surgical planning and training in pediatric urology using AI-driven simulation and modeling techniques | Investigate the potential applications of AI in enhancing preoperative planning and simulation in pediatric urology, using AI algorithms to analyze patient-specific data and anatomical variations, thereby optimizing surgical strategies and facilitating personalized treatment plans. Additionally, explore the use of AI-driven virtual reality simulations for surgical training and skill enhancement among pediatric urologists. |
| Assess the role of AI in predictive analytics and personalized medicine [5] | Integration of AI-based predictive analytics for prognostication and personalized treatment approaches in pediatric urological conditions | Assess the utility of AI-based predictive analytics in pediatric urology for predicting disease progression, treatment response, and individualized risk stratification. Explore the potential of AI algorithms in analyzing multidimensional datasets and biomarker profiles to tailor treatment strategies and optimize patient outcomes in pediatric urological conditions such as vesicoureteral reflux, hydronephrosis, and urinary tract infection. |
| Investigate the application of AI for enhancing patient communication and engagement [2] | Development of AI-powered tools to facilitate patient education, communication, and shared decision making in pediatric urology | Investigate the use of AI-driven technologies, such as chatbots and virtual assistants, to enhance patient communication, education, and engagement in pediatric urology. Explore the potential of these tools to provide personalized information, support treatment adherence, and facilitate shared decision making among healthcare providers, patients, and their families. |
| Evaluate the impact of AI on disparities in pediatric urological care [105] | Examination of the potential influence of AI technologies on healthcare disparities and access to pediatric urological services | Evaluate the impact of AI-driven technologies on disparities in pediatric urological care, including access to specialized services, diagnostic accuracy, and treatment outcomes among underserved populations. Identify potential barriers to and challenges facing equitable implementation of AI in pediatric urology and develop strategies to mitigate disparities and promote inclusive healthcare delivery. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, M.-C.; Lu, H.H.-S.; Chang, Y.-C.; Huang, Y.-C.; Fu, L.-S. Automatic Screening of Pediatric Renal Ultrasound Abnormalities: Deep Learning and Transfer Learning Approach. JMIR Public Health Surveill. 2022, 10, e40878. [Google Scholar] [CrossRef]
- Bar-Sever, Z.; Shammas, A.; Gheisari, F.; Vali, R. Pediatric Nephro-Urology: Overview and Updates in Diuretic Renal Scans and Renal Cortical Scintigraphy. Semin. Nucl. Med. 2022, 52, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, E.; De Cillis, S.; Granato, S.; Chang, P.; Afyouni, A.S.; Okhunov, Z. Applications of neural networks in urology: A systematic review. Curr. Opin. Urol. 2020, 30, 788–807. [Google Scholar] [CrossRef] [PubMed]
- Khondker, A.; Kwong, J.C.; Malik, S.; Erdman, L.; Keefe, D.T.; Fernandez, N.; Tasian, G.E.; Wang, H.-H.S.; Estrada, C.R.; Nelson, C.P.; et al. The state of artificial intelligence in pediatric urology. Front. Urol. 2022, 2, 1024662. [Google Scholar] [CrossRef]
- Hameed, B.; Dhavileswarapu, A.S.; Raza, S.; Karimi, H.; Khanuja, H.; Shetty, D.; Ibrahim, S.; Shah, M.; Naik, N.; Paul, R.; et al. Artificial Intelligence and Its Impact on Urological Diseases and Management: A Comprehensive Review of the Literature. J. Clin. Med. 2021, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, F.Y.; Krebs, V.L.J.; de Carvalho, W.B. Artificial intelligence and machine learning in pediatrics and neonatology healthcare. Front. Public Health 2022, 68, 745–750. [Google Scholar] [CrossRef]
- Wang, H.-H.S.; Vasdev, R.; Nelson, C.P. Artificial Intelligence in Pediatric Urology. Urol. Clin. N. Am. 2024, 51, 91–103. [Google Scholar] [CrossRef]
- Matta, R.; Schaeffer, A.J. The top 100 cited articles in pediatric urology: A bibliometric analysis. J. Pediatr. Urol. 2021, 17, 709.e1–709.e12. [Google Scholar] [CrossRef]
- Ashrafi, N.; Liu, Y.; Xu, X.; Wang, Y.; Zhao, Z.; Pishgar, M. Deep learning model utilization for mortality prediction in mechanically ventilated ICU patients. Inform. Med. Unlocked 2024, 49, 101562. [Google Scholar] [CrossRef]
- Pishgar, M.; Razo, M.; Theis, J.; Darabi, H. Process Mining Model to Predict Mortality in Paralytic Ileus Patients. In Proceedings of the 2021 International Conference on Cyber-Physical Social Intelligence (ICCSI), Beijing, China, 18–20 December 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Theis, J.; Galanter, W.L.; Boyd, A.D.; Darabi, H. Improving the In-Hospital Mortality Prediction of Diabetes ICU Patients Using a Process Mining/Deep Learning Architecture. IEEE J. Biomed. Health Inform. 2021, 26, 388–399. [Google Scholar] [CrossRef]
- Bodaghi, M.; Hosseini, M.; Gottumukkala, R. A Multimodal Intermediate Fusion Network with Manifold Learning for Stress Detection. arXiv 2024, arXiv:2403.08077. [Google Scholar] [CrossRef]
- Duff, L.M.; Scarsbrook, A.F.; Ravikumar, N.; Frood, R.; van Praagh, G.D.; Mackie, S.L.; Bailey, M.A.; Tarkin, J.M.; Mason, J.C.; van der Geest, K.S.M.; et al. An Automated Method for Artifical Intelligence Assisted Diagnosis of Active Aortitis Using Radiomic Analysis of FDG PET-CT Images. Biomolecules 2023, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Maini, A. “Hydronephrosis,” SAI Nephrology. Available online: https://sainephrology.com/hydronephrosis/ (accessed on 9 May 2024).
- Cerrolaza, J.J.; Peters, C.A.; Martin, A.D.; Myers, E.; Safdar, N.; Linguraru, M.G. Quantitative Ultrasound for Measuring Obstructive Severity in Children with Hydronephrosis. J. Urol. 2015, 195, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Jung, I.; Kim, Y.H.; Kwon, J.-Y. Reliability of society of fetal urology and Onen grading system in fetal hydronephrosis. Obstet. Gynecol. Sci. 2019, 62, 87–92. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, P.H.; Yoon, H.M.; Song, S.H.; Jung, A.Y.; Lee, J.S.; Cho, Y.A. Application of the postnatal urinary tract dilation classification system to predict the need for surgical intervention among neonates and young infants. Ultrasonography 2023, 42, 136–146. [Google Scholar] [CrossRef]
- Erdman, L.; Skreta, M.; Rickard, M.; McLean, C.; Mezlini, A.; Keefe, D.T.; Blais, A.-S.; Brudno, M.; Lorenzo, A.; Goldenberg, A. Predicting Obstructive Hydronephrosis Based on Ultrasound Alone. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2020; Martel, A.L., Abolmaesumi, P., Stoyanov, D., Mateus, D., Zuluaga, M.A., Zhou, S.K., Racoceanu, D., Joskowicz, L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 12263, pp. 493–503. [Google Scholar] [CrossRef]
- Alshoabi, S.A.; Alhamodi, D.S.; Alhammadi, M.A.; Alshamrani, A.F. Etiology of Hydronephrosis in adults and children: Ultrasonographic Assessment in 233 patients. Pak. J. Med. Sci. 2021, 37, 1326–1330. [Google Scholar] [CrossRef]
- Lien, W.-C.; Chang, Y.-C.; Chou, H.-H.; Lin, L.-C.; Liu, Y.-P.; Liu, L.; Chan, Y.-T.; Kuan, F.-S. Detecting Hydronephrosis through Ultrasound Images Using State-of-the-Art Deep Learning Models. Ultrasound Med. Biol. 2022, 49, 723–733. [Google Scholar] [CrossRef]
- Sloan, M.; Li, H.; Lescay, H.A.; Judge, C.; Lan, L.; Hajiyev, P.; Giger, M.L.; Gundeti, M.S. Pilot study of machine learning in the task of distinguishing high and low-grade pediatric hydronephrosis on ultrasound. Investig. Clin. Urol. 2023, 64, 588–596. [Google Scholar] [CrossRef]
- Kazlauskas, V.; Bilius, V.; Jakutis, V.; Komiagiene, R.; Burnyte, B.; Verkauskas, G. Urine Biomarkers Combined With Ultrasound for the Diagnosis of Obstruction in Pediatric Hydronephrosis. Front. Pediatr. 2022, 9, 762417. [Google Scholar] [CrossRef]
- Song, S.H.; Han, J.H.; Kim, K.S.; Cho, Y.A.; Youn, H.J.; Kim, Y.I.; Kweon, J. Deep-learning segmentation of ultrasound images for automated calculation of the hydronephrosis area to renal parenchyma ratio. Investig. Clin. Urol. 2022, 63, 455–463. [Google Scholar] [CrossRef]
- Roshanitabrizi, P.; Zember, J.; Sprague, B.M.; Hoefer, S.; Sanchez-Jacob, R.; Jago, J.; Bulas, D.; Pohl, H.G.; Linguraru, M.G. Standardized Analysis of Kidney Ultrasound Images for the Prediction of Pediatric Hydronephrosis Severity. In Machine Learning in Medical Imaging: 12th International Workshop, MLMI 2021, Held in Conjunction with MICCAI 2021, Strasbourg, France, 27 September 2021, Proceedings; Springer: Berlin/Heidelberg, Germany, 2021; pp. 366–375. [Google Scholar] [CrossRef]
- Smail, L.C.; Dhindsa, K.; Braga, L.H.; Becker, S.; Sonnadara, R.R. Using Deep Learning Algorithms to Grade Hydronephrosis Severity: Toward a Clinical Adjunct. Front. Pediatr. 2020, 8, 1. [Google Scholar] [CrossRef]
- Onen, A. Grading of Hydronephrosis: An Ongoing Challenge. Front. Pediatr. 2020, 8, 458. [Google Scholar] [CrossRef]
- Ostrowski, D.A.; Logan, J.R.; Antony, M.; Broms, R.; Weiss, D.A.; Van Batavia, J.; Long, C.J.; Smith, A.L.; Zderic, S.A.; Edwins, R.C.; et al. Automated Society of Fetal Urology (SFU) grading of hydronephrosis on ultrasound imaging using a convolutional neural network. J. Pediatr. Urol. 2023, 19, 566.e1–566.e8. [Google Scholar] [CrossRef]
- Kelley, J.C.; White, J.T.; Goetz, J.T.; Romero, E.; Leslie, J.A.; Prieto, J.C. Sonographic Renal Parenchymal Measurements for the Evaluation and Management of Ureteropelvic Junction Obstruction in Children. Front. Pediatr. 2016, 4, 42. [Google Scholar] [CrossRef]
- Ariyanagam, M. “A Guide to Laparoscopic Pyeloplasty and How It Works,” Urology Specialist. Available online: https://urologyspecialist.com.au/enhancing-kidney-health-comprehensive-guide-laparoscopic-pyeloplasty-robotic-surgery/ (accessed on 8 May 2024).
- Vauth, F.; Zöhrer, P.; Girtner, F.; Rösch, W.H.; Hofmann, A. Open Pyeloplasty in Infants under 1 Year—Proven or Meaningless? Children 2023, 10, 257. [Google Scholar] [CrossRef]
- Abbas, T.; Elifranji, M.; Al-Salihi, M.; Ahmad, J.; Vallasciani, S.; Elkadhi, A.; Özcan, C.; Burgu, B.; Akinci, A.; Alnaimi, A.; et al. Functional recoverability post-pyeloplasty in children with ureteropelvic junction obstruction and poorly functioning kidneys: Systematic review. J. Pediatr. Urol. 2022, 18, 616–628. [Google Scholar] [CrossRef]
- Abbas, T.O.; Ali, M.; Moog, R. “Double-Lumen Valve-Controlled Intra-Operative Pyeloplasty Stent (VIPs)”: A New Technology for Post-Pyeloplasty Stenting—Proof of Concept Study in a Preclinical Large Animal Model. Res. Rep. Urol. 2020, 12, 61–74. [Google Scholar] [CrossRef]
- Helmy, T.E.; Harraz, A.; Sharaf, D.E.; El Demerdash, Y.; Hafez, A.T.; Gad, H.; Dawaba, M. Can Renal Ultrasonography Predict Early Success after Pyeloplasty in Children? A Prospective Study. Urol. Int. 2014, 93, 406–410. [Google Scholar] [CrossRef]
- Esposito, C.; Cerulo, M.; Lepore, B.; Coppola, V.; D’auria, D.; Esposito, G.; Carulli, R.; Del Conte, F.; Escolino, M. Robotic-assisted pyeloplasty in children: A systematic review of the literature. J. Robot. Surg. 2023, 17, 1239–1246. [Google Scholar] [CrossRef]
- Masieri, L.; Sforza, S.; Grosso, A.A.; Valastro, F.; Tellini, R.; Cini, C.; Landi, L.; Taverna, M.; Elia, A.; Mantovani, A.; et al. Robot-assisted laparoscopic pyeloplasty in children: A systematic review. Minerva Urol. Nefrol. 2020, 72, 673–690. [Google Scholar] [CrossRef]
- Cheung, C.L.; Looi, T.; Lendvay, T.S.; Drake, J.M.; Farhat, W.A. Use of 3-dimensional printing technology and silicone modeling in surgical simulation: Development and face validation in pediatric laparoscopic pyeloplasty. J. Surg. Educ. 2014, 71, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Burgart, A.; Daneshjou, R.; Rose, S. Recommendations for the use of pediatric data in artificial intelligence and machine learning ACCEPT-AI. npj Digit. Med. 2023, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Avery, D.I.; Herbst, K.W.; Lendvay, T.S.; Noh, P.H.; Dangle, P.; Gundeti, M.S.; Steele, M.C.; Corbett, S.T.; Peters, C.A.; Kim, C. Robot-assisted laparoscopic pyeloplasty: Multi-institutional experience in infants. J. Pediatr. Urol. 2015, 11, 139.e1–139.e5. [Google Scholar] [CrossRef]
- Ahmed, F.; Abbas, S.; Athar, A.; Shahzad, T.; Khan, W.A.; Alharbi, M.; Khan, M.A.; Ahmed, A. Identification of kidney stones in KUB X-ray images using VGG16 empowered with explainable artificial intelligence. Sci. Rep. 2024, 14, 6173. [Google Scholar] [CrossRef]
- Babajide, R.; Lembrikova, K.; Ziemba, J.; Ding, J.; Li, Y.; Fermin, A.S.; Fan, Y.; Tasian, G.E. Automated Machine Learning Segmentation and Measurement of Urinary Stones on CT Scan. Urology 2022, 169, 41–46. [Google Scholar] [CrossRef]
- Islam, N.; Hasan, M.; Hossain, K.; Alam, G.R.; Uddin, Z.; Soylu, A. Vision transformer and explainable transfer learning models for auto detection of kidney cyst, stone and tumor from CT-radiography. Sci. Rep. 2022, 12, 11440. [Google Scholar] [CrossRef]
- Li, D.; Xiao, C.; Liu, Y.; Chen, Z.; Hassan, H.; Su, L.; Liu, J.; Li, H.; Xie, W.; Zhong, W.; et al. Deep Segmentation Networks for Segmenting Kidneys and Detecting Kidney Stones in Unenhanced Abdominal CT Images. Diagnostics 2022, 12, 1788. [Google Scholar] [CrossRef]
- Lee, H.; Nguyen, N.H.; Hwang, S.I.; Lee, H.J.; Hong, S.K.; Byun, S.-S. Personalized 3D kidney model produced by rapid prototyping method and its usefulness in clinical applications. Int. Braz. J. Urol. 2018, 44, 952–957. [Google Scholar] [CrossRef]
- Raina, R.; Nada, A.; Shah, R.; Aly, H.; Kadatane, S.; Abitbol, C.; Aggarwal, M.; Koyner, J.; Neyra, J.; Sethi, S.K. Artificial intelligence in early detection and prediction of pediatric/neonatal acute kidney injury: Current status and future directions. Pediatr. Nephrol. 2023, 39, 2309–2324. [Google Scholar] [CrossRef]
- Filler, G.; Gipson, D.S.; Iyamuremye, D.; de Ferris, M.E.D.G. Artificial Intelligence in Pediatric Nephrology—A Call for Action. Adv. Kidney Dis. Health 2023, 30, 17–24. [Google Scholar] [CrossRef]
- Santini, G.S. AI in Medical Imaging: The Kidney Tumor Segmentation Challenge. Available online: https://blog.keosys.com/ai-in-medical-imaging-the-kidney-tumor-segmentation-challenge (accessed on 9 May 2024).
- Jacobsen, S.; Klemetsen; Birkelund, Y. Vesicoureteral reflux in young children: A study of radiometric thermometry as detection modality using an ex vivo porcine model. Phys. Med. Biol. 2012, 57, 5557–5573. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.; Salle, J.P.; Chowdhury, M.E.; Abbas, T.O. Quantification of vesicoureteral reflux using machine learning. J. Pediatr. Urol. 2024, 20, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Khondker, A.; Kwong, J.C.; Rickard, M.; Skreta, M.; Keefe, D.T.; Lorenzo, A.J.; Erdman, L. A machine learning-based approach for quantitative grading of vesicoureteral reflux from voiding cystourethrograms: Methods and proof of concept. J. Pediatr. Urol. 2021, 18, 78.e1–78.e7. [Google Scholar] [CrossRef]
- Vesicoureteral Reflux (VUR). The Children’s Hospital of Philadelphia. Available online: https://www.chop.edu/conditions-diseases/vesicoureteral-reflux-vur (accessed on 9 May 2024).
- Koçyiğit, A.; Yüksel, S.; Bayram, R.; Yılmaz, I.; Karabulut, N. Efficacy of magnetic resonance urography in detecting renal scars in children with vesicoureteral reflux. Pediatr. Nephrol. 2014, 29, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanovska, D.; Risteski, A.; Kambovska, M.; Trpcevski, T.; Sahpazova, E.; Petrovski, M. Voiding Urosonography with Second-Generation Ultrasound Contrast Agent for Diagnosis of Vesicoureteric Reflux: First Local Pilot Study. Open Access Maced. J. Med. Sci. 2017, 5, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Toloui, A.; Alavi, S.N.R.; Neishaboori, A.M.; Ahmadzadeh, K.; Ghelichkhani, P.; Safari, S.; Abbasi, A.; Ataei, N.; Hosseini, M. Contrast-enhanced voiding urosonography, a possible candidate for the diagnosis of vesicoureteral reflux in children and adolescents; a systematic review and meta-analysis. J. Pediatr. Urol. 2021, 18, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, A.J.; Arlen, A.M.; Leong, T.; Merriman, L.S.; Herrel, L.A.; Scherz, H.C.; Smith, E.A.; Srinivasan, A.K. Vesicoureteral reflux index (VURx): A novel tool to predict primary reflux improvement and resolution in children less than 2 years of age. J. Pediatr. Urol. 2014, 10, 1249–1254. [Google Scholar] [CrossRef]
- Hobbs, K.T.; Choe, N.; Aksenov, L.I.; Reyes, L.; Aquino, W.; Routh, J.C.; Hokanson, J.A. Machine Learning for Urodynamic Detection of Detrusor Overactivity. Urology 2021, 159, 247–254. [Google Scholar] [CrossRef]
- Wang, J.; Ren, L.; Liu, X.; Liu, J.; Ling, Q. Underactive Bladder and Detrusor Underactivity: New Advances and Prospectives. Int. J. Mol. Sci. 2023, 24, 15517. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Z.; Wu, B.; Lin, D.; Hu, Y.; Zhang, X.; Liu, J. A Pilot Study: Detrusor Overactivity Diagnosis Method Based on Deep Learning. Urology 2023, 179, 188–195. [Google Scholar] [CrossRef]
- Wang, H.S.; Cahill, D.; Panagides, J.; Nelson, C.P.; Wu, H.; Estrada, C. Pattern recognition algorithm to identify detrusor overactivity on urodynamics. Neurourol. Urodyn. 2020, 40, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Niederhauser, T.; Gafner, E.S.; Cantieni, T.; Grämiger, M.; Haeberlin, A.; Obrist, D.; Burkhard, F.; Clavica, F. Detection and quantification of overactive bladder activity in patients: Can we make it better and automatic? Neurourol. Urodyn. 2017, 37, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Abbaraju, V.; Lewis, K.; Majerus, S. Machine Learning for Automated Bladder Event Classification from Single-Channel Vesical Pressure Recordings. In Proceedings of the 2022 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 3 December 2022; IEEE: New York, NY, USA, 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Roic, A.C.; Milošević, D.; Turudić, D.; Roic, G. An innovative diagnostic procedure in children: Videourodynamics with contrast-enhanced voiding urosonography. J. Ultrasound 2022, 26, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Esperto, F.; Chapple, C.R. Detrusor Underactivity and the Underactive Bladder: A Systematic Review of Preclinical and Clinical Studies. Eur. Urol. 2018, 74, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.V. Current strategies to predict and manage sequelae of posterior urethral valves in children. Pediatr. Nephrol. 2018, 33, 1651–1661. [Google Scholar] [CrossRef]
- Fishberg, S.E.; Landau, E.H.; Duvdevani, M.; Gofrit, O.N.; Friedman, S.E.; Hidas, G. Posterior Urethral Valves: Prenatal, Neonatal, and Long-Term Management. NeoReviews 2018, 19, e753–e761. [Google Scholar] [CrossRef]
- Kwong, J.C.; Khondker, A.; Kim, J.K.; Chua, M.; Keefe, D.T.; Dos Santos, J.; Skreta, M.; Erdman, L.; D’souza, N.; Selman, A.F.; et al. Posterior Urethral Valves Outcomes Prediction (PUVOP): A machine learning tool to predict clinically relevant outcomes in boys with posterior urethral valves. Pediatr. Nephrol. 2021, 37, 1067–1074. [Google Scholar] [CrossRef]
- Pellegrino, C.; Capitanucci, M.L.; Forlini, V.; Zaccara, A.; Lena, F.; Sollini, M.L.; Castelli, E.; Mosiello, G. Posterior urethral valves: Role of prenatal diagnosis and long-term management of bladder function; a single center point of view and review of literature. Front. Pediatr. 2023, 10, 1057092. [Google Scholar] [CrossRef]
- Taskinen, S.; Heikkilä, J.; Rintala, R. Effects of posterior urethral valves on long-term bladder and sexual function. Nat. Rev. Urol. 2012, 9, 699–706. [Google Scholar] [CrossRef]
- Weaver, J.K.; Milford, K.; Rickard, M.; Logan, J.; Erdman, L.; Viteri, B.; D’souza, N.; Cucchiara, A.; Skreta, M.; Keefe, D.; et al. Deep learning imaging features derived from kidney ultrasounds predict chronic kidney disease progression in children with posterior urethral valves. Pediatr. Nephrol. 2022, 38, 839–846. [Google Scholar] [CrossRef]
- Cohen, H.L.; Zinn, H.L.; Patel, A.; Zinn, D.L.; Haller, J.O. Prenatal sonographic diagnosis of posterior urethral valves: Identification of valves and thickening of the posterior urethral wall. J. Clin. Ultrasound 1998, 26, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Buffin-Meyer, B.; Klein, J.; van der Zanden, L.F.M.; Levtchenko, E.; Moulos, P.; Lounis, N.; Conte-Auriol, F.; Hindryckx, A.; Wühl, E.; Persico, N.; et al. The ANTENATAL multicentre study to predict postnatal renal outcome in fetuses with posterior urethral valves: Objectives and design. Clin. Kidney J. 2019, 13, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Krzemień, G.; Szmigielska, A.; Wawer, Z.; Roszkowska-Blaim, M. Significance of early diagnosis of posterior urethral valves in fetus for further development—Own experience. Med. Wieku Rozw. 2013, 17, 301–305. [Google Scholar]
- Ikeda, A.; Nosato, H. Overview of current applications and trends in artificial intelligence for cystoscopy and transurethral resection of bladder tumours. Curr. Opin. Urol. 2023, 34, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, M.; Elkousy, M.M.; Emran, A.; Zamel, S.; Nabil, A.E.W. The importance of second look cystoscopy in children with PUV and its impact on future bladder, kidney functions and fertility. Int. J. Health Sci. 2022, Vol. 6, 3189–3195. [Google Scholar] [CrossRef]
- Hakan, N.; Karakus, S.C.; Aydin, M.; Suzen, A.; Cengiz, N. An Anterior Urethral Valve without Urethral Dilatation Diagnosed by Cystoscopy in a Neonate. Pediatr. Oncall 2019, 16, 122–123. [Google Scholar] [CrossRef]
- Topuz, B.; Ebiloğlu, T.; Kaya, E.; Çoğuplugil, A.E.; Gürdal, M.; Bedir, S.; Yalçın, S. Comparison of Ultrasonography and Cystoscopy in the Evaluation of Hematuria. J. Urol. Surg. 2019, 6, 27–31. [Google Scholar] [CrossRef]
- Abbas, T.O.; AbdelMoniem, M.; Khalil, I.A.; Hossain, S.A.; Chowdhury, M.E. Deep learning based automated quantification of urethral plate characteristics using the plate objective scoring tool (POST). J. Pediatr. Urol. 2023, 19, 373.e1–373.e9. [Google Scholar] [CrossRef]
- Abbas, T.O.; Sennert, M.; Tiryaki, S.; Fernandez, N.; Fawzy, M.; Hadidi, A. Hypospadias-associated penile curvature assessment and management: A global survey of current practice. J. Pediatr. Urol. 2024, 20, 440.e1–440.e10. [Google Scholar] [CrossRef]
- Fernandez, N.; Lorenzo, A.J.; Rickard, M.; Chua, M.; Pippi-Salle, J.L.; Perez, J.; Braga, L.H.; Matava, C. Digital Pattern Recognition for the Identification and Classification of Hypospadias Using Artificial Intelligence vs. Experienced Pediatric Urologist. Urology 2020, 147, 264–269. [Google Scholar] [CrossRef]
- Fruntelată, R.; Stoica, G.A.; Ciobanu, M.O.; Ciurea, M.E.; Nica, O.; Stoica, M. Retrospective Study Over the Hypospadias Surgery in a Single Tertiary Center. Curr. Health Sci. J. 2021, 47, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wahyudi, I.; Utomo, C.P.; Djauzi, S.; Fathurahman, M.; Situmorang, G.R.; Rodjani, A.; Yonathan, K.; Santoso, B. Digital Pattern Recognition for the Identification of Various Hypospadias Parameters via an Artificial Neural Network: Protocol for the Development and Validation of a System and Mobile App. JMIR Res. Protoc. 2022, 11, e42853. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, B.; Tang, Y.; Wang, X. Development and verification of machine learning model based on anogenital distance, penoscrotal distance, and 2D:4D finger ratio before puberty to predict hypospadias classification. Front. Pediatr. 2024, 12, 1297642. [Google Scholar] [CrossRef]
- Baray, S.B.; Abdelmoniem, M.; Mahmud, S.; Kabir, S.; Faisal, A.A.; Chowdhury, M.E.H.; Abbas, T.O. Automated measurement of penile curvature using deep learning-based novel quantification method. Front. Pediatr. 2023, 11, 1149318. [Google Scholar] [CrossRef]
- Diamond, D.A.; Chan, I.H.Y.; Holland, A.J.A.; Kurtz, M.P.; Nelson, C.; Estrada, C.R.; Bauer, S.; Tam, P.K.H. Advances in paediatric urology. Lancet 2017, 390, 1061–1071. [Google Scholar] [CrossRef]
- Envision a Surgery. Virtually with Immersive Healthcare Simulation | Arch Virtual VR Training and Simulation for Education and Enterprise. Available online: https://archvirtual.com/project/envision-a-surgery-virtually-with-immersive-healthcare-simulation/ (accessed on 9 May 2024).
- Bhan, B.; Patel, S. Efficient Medical Image Enhancement using CLAHE Enhancement and Wavelet Fusion. Int. J. Comput. Appl. 2017, 167, 1–5. [Google Scholar] [CrossRef]
- Lidong, H.; Wei, Z.; Jun, W.; Zebin, S. Combination of contrast limited adaptive histogram equalisation and discrete wavelet transform for image enhancement. IET Image Process. 2015, 9, 908–915. [Google Scholar] [CrossRef]
- Song, Y.; Li, C.; Xiao, S.; Xiao, H.; Guo, B. Unsharp masking image enhancement the parallel algorithm based on cross-platform. Sci. Rep. 2022, 12, 20175. [Google Scholar] [CrossRef]
- Ali, H.M. MRI Medical Image Denoising by Fundamental Filters. In High-Resolution Neuroimaging—Basic Physical Principles and Clinical Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Kachouie, N. Anisotropic Diffusion for Medical Image Enhancement. Kachouie Int. J. Image Process. 2010, 4, 436–443. [Google Scholar]
- Radhika, R.; Mahajan, R. Medical Image Enhancement: A Review. In Proceedings of the International Conference on Data Science and Applications, Muğla, Turkey, 8–11 September 2022; Saraswat, M., Roy, S., Chowdhury, C., Gandomi, A.H., Eds.; Springer: Singapore, 2022; pp. 105–118. [Google Scholar] [CrossRef]
- Dabass, J.; Vig, R. Biomedical Image Enhancement Using Different Techniques—A Comparative Study. In Data Science and Analytics; Panda, B., Sharma, S., Roy, N.R., Eds.; Springer: Singapore, 2018; pp. 260–286. [Google Scholar] [CrossRef]
- Delisle, P.-L.; Anctil-Robitaille, B.; Desrosiers, C.; Lombaert, H. Realistic image normalization for multi-Domain segmentation. Med. Image Anal. 2021, 74, 102191. [Google Scholar] [CrossRef]
- Guo, M.; Li, Y.; Su, Y.; Lambert, T.; Nogare, D.D.; Moyle, M.W.; Duncan, L.H.; Ikegami, R.; Santella, A.; Rey-Suarez, I.; et al. Rapid image deconvolution and multiview fusion for optical microscopy. Nat. Biotechnol. 2020, 38, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Markov, K. Medical Image Enhancement Using Super Resolution Methods. In Computational Science—ICCS 2020, Lecture Notes in Computer Science; Krzhizhanovskaya, V.V., Závodszky, G., Lees, M.H., Dongarra, G.G., Sloot, P.M.A., Brissos, S., Teixeira, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 12141. [Google Scholar] [CrossRef]
- Nazir, N.; Sarwar, A.; Saini, B.S. Recent developments in denoising medical images using deep learning: An overview of models, techniques, and challenges. Micron 2024, 180, 103615. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, G.; Arcos, J.L.; Cerquides, J. Enhancing Medical Image Segmentation: Ground Truth Optimization through Evaluating Uncertainty in Expert Annotations. Mathematics 2023, 11, 3771. [Google Scholar] [CrossRef]
- Liu, X.; Montillo, A.; Tan, E.T.; Schenck, J.F. iSTAPLE: Improved label fusion for segmentation by combining STAPLE with image intensity. In Presented at the SPIE Medical Imaging; Ourselin, S., Haynor, D.R., Eds.; SPIE: Lake Buena Vista (Orlando Area), FL, USA, 2013; p. 86692O. [Google Scholar] [CrossRef]
- Akhondi-Asl, A.; Warfield, S.K. Simultaneous Truth and Performance Level Estimation through Fusion of Probabilistic Segmentations. IEEE Trans. Med. Imaging 2013, 32, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Landman, B.A.; Bogovic, J.A.; Prince, J.L. Simultaneous Truth and Performance Level Estimation with Incomplete, Over-complete, and Ancillary Data. Proc. SPIE Int. Soc. Opt. Eng. 2010, 7623, 76231N. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Zhang, R.; Li, S.; Hong, J.; Zhang, Y.-D.; Chu, W.C.W.; Shi, L. Voting-Based Contour-Aware Framework for Medical Image Segmentation. Appl. Sci. 2022, 13, 84. [Google Scholar] [CrossRef]
- Zhou, T.; Ruan, S.; Canu, S. A review: Deep learning for medical image segmentation using multi-modality fusion. Array 2020, 3–4, 100004. [Google Scholar] [CrossRef]
- Andrews, S.; Hamarneh, G.; Saad, A. Fast Random Walker with Priors Using Precomputation for Interactive Medical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2010: 13th International Conference, Beijing, China, 20–24 September 2010; Volume 13 Pt 3, pp. 9–16. [Google Scholar]
- Keskinoğlu, A.; Özgür, S. The Use of Artificial Neural Networks for Differential Diagnosis between Vesicoureteral Reflux and Urinary Tract Infection in Children. J. Pediatr. Res. 2020, 7, 230–235. [Google Scholar] [CrossRef]
- Blum, E.S.; Porras, A.R.; Biggs, E.; Tabrizi, P.R.; Sussman, R.D.; Sprague, B.M.; Shalaby-Rana, E.; Majd, M.; Pohl, H.G.; Linguraru, M.G. Early Detection of Ureteropelvic Junction Obstruction Using Signal Analysis and Machine Learning: A Dynamic Solution to a Dynamic Problem. J. Urol. 2018, 199, 847–852. [Google Scholar] [CrossRef]
- Liang, X.; Du, M.; Chen, Z. Artificial intelligence-aided ultrasound in renal diseases: A systematic review. Quant. Imaging Med. Surg. 2023, 13, 3988–4001. [Google Scholar] [CrossRef]
- Logvinenko, T.; Chow, J.S.; Nelson, C.P. Predictive value of specific ultrasound findings when used as a screening test for abnormalities on VCUG. J. Pediatr. Urol. 2015, 11, 176.e1–176.e7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, A.T.; Salam, A.; Naznine, M.; Abdalla, D.; Erdman, L.; Chowdhury, M.E.H.; Abbas, T.O. Artificial Intelligence Tools in Pediatric Urology: A Comprehensive Review of Recent Advances. Diagnostics 2024, 14, 2059. https://doi.org/10.3390/diagnostics14182059
Chowdhury AT, Salam A, Naznine M, Abdalla D, Erdman L, Chowdhury MEH, Abbas TO. Artificial Intelligence Tools in Pediatric Urology: A Comprehensive Review of Recent Advances. Diagnostics. 2024; 14(18):2059. https://doi.org/10.3390/diagnostics14182059
Chicago/Turabian StyleChowdhury, Adiba Tabassum, Abdus Salam, Mansura Naznine, Da’ad Abdalla, Lauren Erdman, Muhammad E. H. Chowdhury, and Tariq O. Abbas. 2024. "Artificial Intelligence Tools in Pediatric Urology: A Comprehensive Review of Recent Advances" Diagnostics 14, no. 18: 2059. https://doi.org/10.3390/diagnostics14182059
APA StyleChowdhury, A. T., Salam, A., Naznine, M., Abdalla, D., Erdman, L., Chowdhury, M. E. H., & Abbas, T. O. (2024). Artificial Intelligence Tools in Pediatric Urology: A Comprehensive Review of Recent Advances. Diagnostics, 14(18), 2059. https://doi.org/10.3390/diagnostics14182059







