Assessing the Predictive Utility of the C-Reactive Protein-to-Lymphocyte Ratio for Mortality in Isolated Traumatic Brain Injury: A Single-Center Retrospective Analysis
Abstract
:1. Introduction
2. Methods
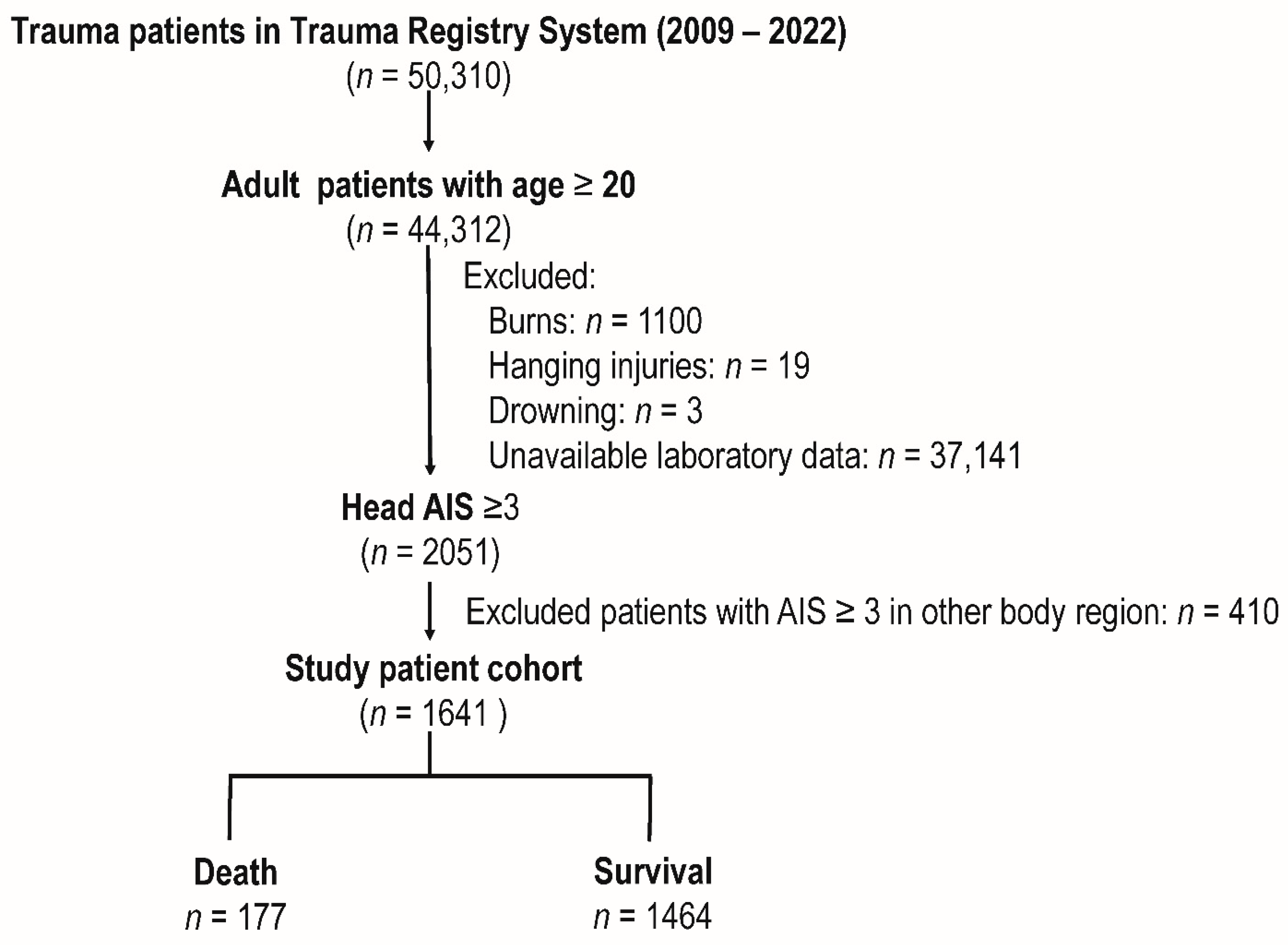
2.1. Patient Enrollment and Study Design
2.2. Statistical Analysis
3. Results
3.1. Patients in the Study Cohort
3.2. Demographic and Clinical Characteristics of Patients Stratified by Outcomes
3.3. Univariate and Multivariate Analysis of Factors Associated with Mortality
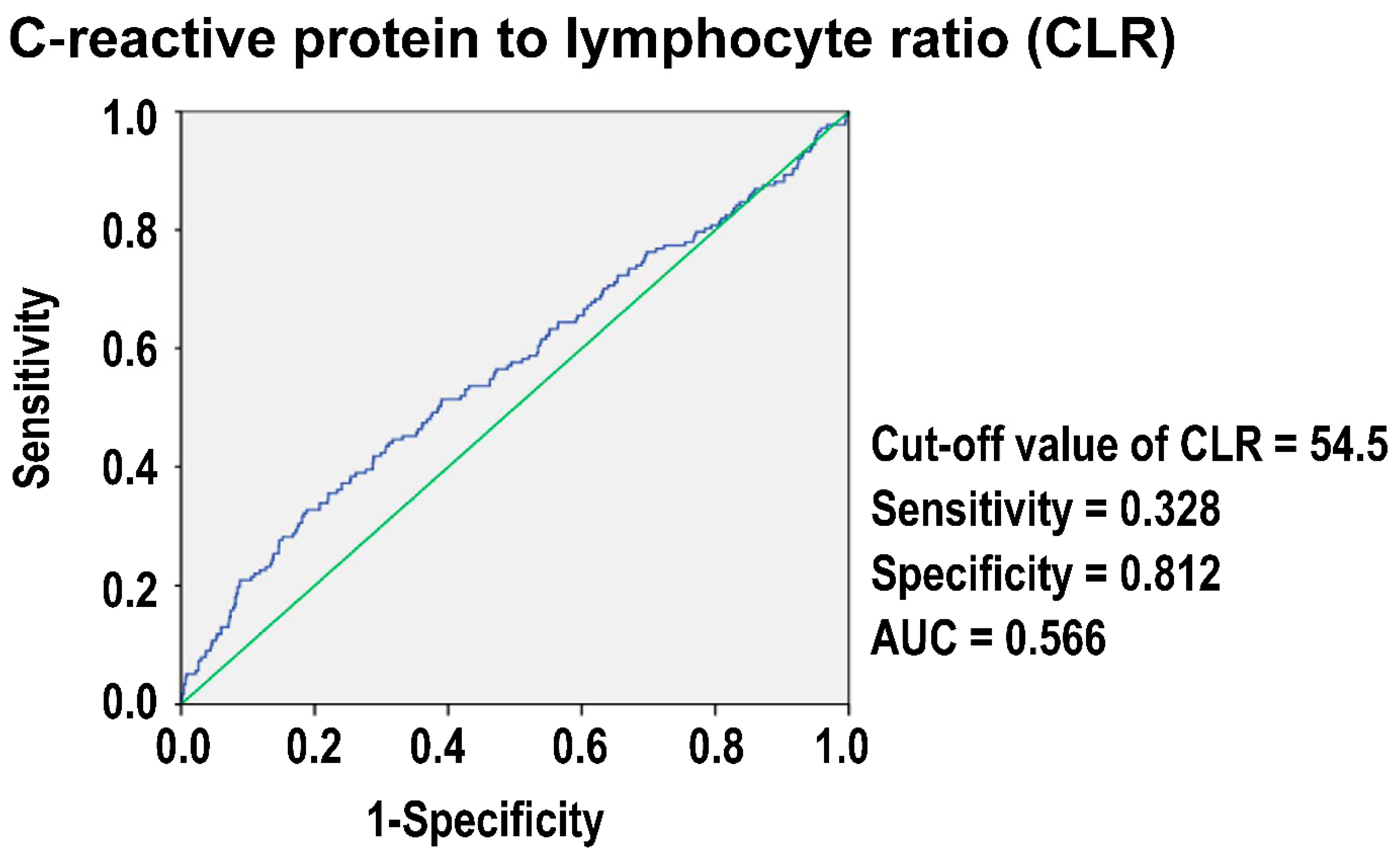
3.4. The Optimal Cut-Off Value of CLR in Predicting Mortality
3.5. Comparative Demographics and Outcomes Based on Grouping by CLR
3.6. Propensity-Score-Matched Analysis of Patients Grouped by CLR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwald, B.D.; Hammond, F.M.; Harrison-Felix, C.; Nakase-Richardson, R.; Howe, L.L.; Kreider, S. Mortality following Traumatic Brain Injury among Individuals Unable to Follow Commands at the Time of Rehabilitation Admission: A National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems Study. J. Neurotrauma 2015, 32, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; Mchugh, G.S.; Murray, G.D.; Marmarou, A.; Roberts, I.; Habbema, D.J.F.; et al. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Med. 2008, 5, e165. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, B.; Chiu, Y.L.; Lingsma, H.F.; Gerber, L.M.; Steyerberg, E.W.; Ghajar, J.; Maas, A.I. Predicting 14-day mortality after severe traumatic brain injury: Application of the IMPACT models in the brain trauma foundation TBI-trac® New York State database. J. Neurotrauma 2012, 29, 1306–1312. [Google Scholar] [CrossRef]
- Utomo, W.K.; Gabbe, B.J.; Simpson, P.M.; Cameron, P.A. Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury 2009, 40, 973–977. [Google Scholar] [CrossRef]
- Nagasawa, H.; Omori, K.; Takeuchi, I.; Jitsuiki, K.E.I.; Ohsaka, H.; Yanagawa, Y. Clinical Significance of C-Reactive Protein in Patients with Trauma on Arrival. Juntendo Med. J. 2019, 65, 451–455. [Google Scholar] [CrossRef]
- Manson, J.; Hoffman, R.; Chen, S.; Ramadan, M.H.; Billiar, T.R. Innate-Like Lymphocytes Are Immediate Participants in the Hyper-Acute Immune Response to Trauma and Hemorrhagic Shock. Front. Immunol. 2019, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Rainer, T.H.; Chan, T.Y.; Cocks, R.A. Do peripheral blood counts have any prognostic value following trauma? Injury 1999, 30, 179–185. [Google Scholar] [CrossRef]
- Mouliou, D.S. C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians. Diseases 2023, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Wu, S.C.; Tsai, C.H.; Chou, S.E.; Su, W.T.; Hsu, S.Y.; Hsieh, C.H. Association of White Blood Cell Subtypes and Derived Ratios with a Mortality Outcome in Adult Patients with Polytrauma. Healthcare 2022, 10, 1384. [Google Scholar] [CrossRef]
- Ke, R.T.; Rau, C.S.; Hsieh, T.M.; Chou, S.E.; Su, W.T.; Hsu, S.Y.; Hsieh, C.H.; Liu, H.T. Association of Platelets and White Blood Cells Subtypes with Trauma Patients’ Mortality Outcome in the Intensive Care Unit. Healthcare 2021, 9, 942. [Google Scholar] [CrossRef]
- Tsai, C.H.; Liu, H.T.; Hsieh, T.M.; Huang, C.Y.; Chou, S.E.; Su, W.T.; Li, C.; Hsu, S.Y.; Hsieh, C.H. Change of neutrophil-to-monocyte ratio to stratify the mortality risk of adult patients with trauma in the intensive care units. Formos. J. Surg. 2022, 55, 177–183. [Google Scholar] [CrossRef]
- Tonduangu, N.; Le Borgne, P.; Lefebvre, F.; Alame, K.; Bérard, L.; Gottwalles, Y.; Cipolat, L.; Gennai, S.; Bilbault, P.; Lavoignet, C.E.; et al. Prognostic Value of C-Reactive Protein to Lymphocyte Ratio (CLR) in Emergency Department Patients with SARS-CoV-2 Infection. J. Pers. Med. 2021, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Hur, J.Y.; Eo, W.; An, S.; Kim, D.H.; Lee, S. Clinical significance of C-Reactive Protein to Lymphocyte Count Ratio as a prognostic factor for Survival in Non-small Cell Lung Cancer Patients undergoing Curative Surgical Resection. J. Cancer 2021, 12, 4497–4504. [Google Scholar] [CrossRef] [PubMed]
- Taniai, T.; Haruki, K.; Hamura, R.; Fujiwara, Y.; Furukawa, K.; Gocho, T.; Shiba, H.; Yanaga, K. The Prognostic Significance of C-reactive Protein-To-Lymphocyte Ratio in Colorectal Liver Metastases. J. Surg. Res. 2021, 258, 414–421. [Google Scholar] [CrossRef]
- Qi, B.; Yang, Z.J.; Huang, N.; Zheng, W.B.; Gui, C. Exploring the diagnostic and prognostic value of the C-reactive protein/lymphocyte ratio for dilated cardiomyopathy based on a real-world study. Sci. Rep. 2023, 13, 18889. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Z.; Chen, Y.; Lin, C. C-reactive protein/lymphocyte ratio as a prognostic biomarker in acute pancreatitis: A cross-sectional study assessing disease severity. Int. J. Surg. 2024, 110, 3223–3229. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The Youden Index and the optimal cut-point corrected for measurement error. Biom. J. 2005, 47, 428–441. [Google Scholar] [CrossRef]
- Balta, O.; Altınayak, H.; Gürler Balta, M.; Astan, S.; Uçar, C.; Kurnaz, R.; Zengin, E.C.; Eren, M.B. Can C-reactive protein-based biomarkers be used as predictive of 30-day mortality in elderly hip fractures?A retrospective study. Ulus. Trauma Acil Cerrahi Derg. 2022, 28, 849–856. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, Y.; Liang, H.; Xiao, J.; Han, Y.; Yang, Z.; Bi, Y. C-reactive protein to lymphocyte ratio is a significant predictive factor for poor short-term clinical outcomes of SARS-CoV-2 BA.2.2 patients. Front. Public. Health 2023, 11, 1168375. [Google Scholar] [CrossRef]
- Koyuncu, S.; Ismail, O. The role of C-reactive protein to lymphocyte ratio in the differentiation of acute and perforated appendicitis. Ulus. Trauma Acil Cerrahi Derg. 2020, 26, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ma, X.; Zhu, J.; Chen, C. C-reactive protein to lymphocyte ratio as a new biomarker in predicting surgical site infection after posterior lumbar interbody fusion and instrumentation. Front. Surg. 2022, 9, 910222. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, C.; Rusin, C.G.; Robertson, C.S. Secondary brain injury: Predicting and preventing insults. Neuropharmacology 2019, 145, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Coplin, W.M. Intracranial pressure and surgical decompression for traumatic brain injury: Biological rationale and protocol for a randomized clinical trial. Neurol. Res. 2001, 23, 277–290. [Google Scholar] [CrossRef]
- Vella, M.A.; Crandall, M.L.; Patel, M.B. Acute Management of Traumatic Brain Injury. Surg. Clin. N. Am. 2017, 97, 1015–1030. [Google Scholar] [CrossRef]
- Strnad, M.; Borovnik Lesjak, V.; Vujanović, V.; Križmarić, M. Predictors of mortality in patients with isolated severe traumatic brain injury. Wien. Klin. Wochenschr. 2017, 129, 110–114. [Google Scholar] [CrossRef]
- Saadat, S.; Akbari, H.; Khorramirouz, R.; Mofid, R.; Rahimi-Movaghar, V. Determinants of mortality in patients with traumatic brain injury. Ulus. Trauma Acil Cerrahi Derg. 2012, 18, 219–224. [Google Scholar] [CrossRef]
- Ostermann, R.C.; Joestl, J.; Tiefenboeck, T.M.; Lang, N.; Platzer, P.; Hofbauer, M. Risk factors predicting prognosis and outcome of elderly patients with isolated traumatic brain injury. J. Orthop. Surg. Res. 2018, 13, 277. [Google Scholar] [CrossRef]
- Gritti, P.; Zangari, R.; Carobbio, A.; Zucchi, A.; Lorini, F.L.; Ferri, F.; Agostinis, C.; Lanterna, L.A.; Brembilla, C.; Foresti, C.; et al. Acute and Subacute Outcome Predictors in Moderate and Severe Traumatic Brain Injury: A Retrospective Monocentric Study. World Neurosurg. 2019, 128, e531–e540. [Google Scholar] [CrossRef]
- Majdan, M.; Brazinova, A.; Rusnak, M.; Leitgeb, J. Outcome Prediction after Traumatic Brain Injury: Comparison of the Performance of Routinely Used Severity Scores and Multivariable Prognostic Models. J. Neurosci. Rural. Pract. 2017, 8, 20–29. [Google Scholar] [CrossRef]
- Xu, L.B.; Yue, J.K.; Korley, F.; Puccio, A.M.; Yuh, E.L.; Sun, X.; Rabinowitz, M.; Vassar, M.J.; Taylor, S.R.; Winkler, E.A.; et al. High-Sensitivity C-Reactive Protein is a Prognostic Biomarker of Six-Month Disability after Traumatic Brain Injury: Results from the TRACK-TBI Study. J. Neurotrauma 2021, 38, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-G.; Lee, K.-S.; Shim, J.-J.; Yoon, S.-M.; Bae, H.G. Prognostic Value of the C-reactive Protein Levels in the Head Injury. J. Korean Neurotraumatol. Soc. 2016, 1, 57–60. [Google Scholar] [CrossRef]
- Hosseininejad, S.M.; Bozorgi, F.; Jahanian, F.; Mohammadian Amiri, M.; Mohammadpour, R.A.; Hajiaghaei, G. C-Reactive Protein and D-dimer as Prognostic Markers for Clinical Outcomes in Patients with Mild Traumatic Brain Injury: A Cross-Sectional Study. Bull. Emerg. Trauma 2023, 11, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Khan, D.; Fischer, I.; Hänggi, D.; Cornelius, J.F.; Muhammad, S. CLR (C-Reactive Protein to Lymphocyte Ratio) Served as a Promising Predictive Biomarker for Cerebral Vasospasm in Aneurysmal Subarachnoid Hemorrhage (aSAH): A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 940. [Google Scholar] [CrossRef]
| Variables | Death n = 177 | Survival n = 1464 | OR (95%CI) | p |
|---|---|---|---|---|
| Sex | 0.109 | |||
| Male, n (%) | 125(70.6) | 945(64.5) | 1.32(0.94–1.86) | |
| Female, n (%) | 52(29.4) | 519(35.5) | 0.76(0.54–1.07) | |
| Age, years (mean ± SD) | 67.0 ± 17.2 | 60.5 ± 19.1 | - | <0.001 |
| CLR | 60.1 ± 111.5 | 33.9 ± 55.1 | - | <0.001 |
| CRP (mg/L) | 66.6 ± 84.4 | 41.8 ± 52.5 | - | <0.001 |
| Lymphocyte (109/L) | 1.9 ± 1.4 | 1.8 ± 1.3 | - | 0.390 |
| Comorbidities | ||||
| CVA, n (%) | 14(7.9) | 108(7.4) | 1.08(0.60–1.93) | 0.799 |
| HTN, n (%) | 84(47.5) | 586(40.0) | 1.35(0.99–1.85) | 0.057 |
| CAD, n (%) | 32(18.1) | 129(8.8) | 2.28(1.50–3.49) | <0.001 |
| CHF, n (%) | 0(0.0) | 11(0.8) | - | 0.247 |
| DM, n (%) | 42(23.7) | 334(22.8) | 1.05(0.73–1.52) | 0.784 |
| ESRD, n (%) | 20(11.3) | 42(2.9) | 4.31(2.47–7.53) | <0.001 |
| GCS, median (IQR) | 7(3–14) | 15(10–15) | - | <0.001 |
| 3–8, n (%) | 97(54.8) | 292(19.9) | 4.87(3.53–6.72) | <0.001 |
| 9–12, n (%) | 21(11.9) | 188(12.8) | 0.91(0.57–1.48) | 0.713 |
| 13–15, n (%) | 59(33.3) | 984(67.2) | 0.24(0.18–0.34) | <0.001 |
| ISS, median (IQR) | 25(16–25) | 16(16–20) | - | <0.001 |
| 1–15, n (%) | 11(6.2) | 327(22.3) | 0.23(0.12–0.43) | <0.001 |
| 16–24, n (%) | 57(32.2) | 914(62.4) | 0.29(0.21–0.40) | <0.001 |
| ≥25, n (%) | 109(61.6) | 223(15.2) | 8.92(6.38–12.47) | <0.001 |
| Hospital stay (days) | 11.8 ± 17.0 | 16.5 ± 14.6 | - | <0.001 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p | OR | (95% CI) | p | |
| Age (years) | 1.02 | (1.01–1.03) | <0.001 | 1.03 | (1.02–1.04) | <0.001 |
| CLR | 1.04 | (1.02–1.06) | <0.001 | 1.03 | (1.00–1.07) | 0.051 |
| CRP (mg/L) | 1.06 | (1.04–1.08) | <0.001 | 1.00 | (0.97–1.04) | 0.944 |
| Lymphocyte (109/L) | 1.05 | (0.94–1.17) | 0.390 | 1.00 | (0.87–1.15) | 0.984 |
| CAD, yes | 2.28 | (1.50–3.49) | <0.001 | 2.05 | (1.24–3.38) | 0.005 |
| ESRD, yes | 4.31 | (2.47–7.53) | <0.001 | 4.69 | (2.37–9.27) | <0.001 |
| GCS, yes | 0.83 | (0.80–0.85) | <0.001 | 0.86 | (0.83–0.90) | <0.001 |
| ISS | 1.18 | (1.14–1.22) | <0.001 | 1.14 | (1.10–1.18) | <0.001 |
| CLR | ||||
|---|---|---|---|---|
| ≥54.5 n = 334 | <54.5 n = 1307 | OR (95%CI) | p | |
| Sex | 0.009 | |||
| Male, n (%) | 238(71.3) | 832(63.7) | 1.42(1.09–1.84) | |
| Female, n (%) | 96(28.7) | 475(36.3) | 0.71(0.54–0.92) | |
| Age, years (mean ± SD) | 66.6 ± 17.2 | 59.8 ± 19.2 | - | <0.001 |
| Comorbidities | ||||
| CVA, n (%) | 31(9.3) | 91(7.0) | 1.37(0.89–2.09) | 0.149 |
| HTN, n (%) | 170(50.9) | 500(38.3) | 1.67(1.31–2.13) | <0.001 |
| CAD, n (%) | 42(12.6) | 119(9.1) | 1.44(0.99–2.09) | 0.057 |
| CHF, n (%) | 2(0.6) | 9(0.7) | 0.87(0.19–4.04) | 0.858 |
| DM, n (%) | 91(27.2) | 285(21.8) | 1.34(1.02–1.77) | 0.035 |
| ESRD, n (%) | 19(5.7) | 43(3.3) | 1.77(1.02–3.09) | 0.040 |
| GCS, median (IQR) | 13(8–15) | 15(9–15) | - | 0.003 |
| 3–8, n (%) | 92(27.5) | 297(22.7) | 1.29(0.98–1.70) | 0.064 |
| 9–12, n (%) | 55(16.5) | 154(11.8) | 1.48(1.06–2.06) | 0.022 |
| 13–15, n (%) | 187(56.0) | 856(65.5) | 0.67(0.53–0.86) | 0.001 |
| ISS, median (IQR) | 16(16–25) | 16(16–20) | - | 0.008 |
| 1–15, n (%) | 51(15.3) | 287(22.0) | 0.64(0.46–0.89) | 0.007 |
| 16–24, n (%) | 195(58.4) | 776(59.4) | 0.96(0.75–1.23) | 0.743 |
| ≥25, n (%) | 88(26.3) | 244(18.7) | 1.56(1.18–2.06) | 0.002 |
| Mortality, n (%) | 58(17.4) | 119(9.1) | 2.10(1.49–2.95) | <0.001 |
| Hospital stay (days) | 19.0 ± 16.5 | 15.2 ± 14.5 | - | <0.001 |
| CLR | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≥54.5 n = 298 | <54.5 n = 298 | OR (95%CI) | p | SD | ||||
| Sex | ||||||||
| Male, n (%) | 216 | (72.5) | 216 | (72.5) | 1.00 | (0.70–1.43) | 1.000 | 0.00% |
| Age, years | 65.2 | ±17.3 | 65.0 | ±17.3 | - | 0.898 | 1.05% | |
| Comorbidities | ||||||||
| CVA, n (%) | 19 | (6.4) | 19 | (6.4) | 1.00 | (0.52–1.93) | 1.000 | 0.00% |
| HTN, n (%) | 147 | (49.3) | 147 | (49.3) | 1.00 | (0.73–1.38) | 1.000 | 0.00% |
| CAD, n (%) | 26 | (8.7) | 26 | (8.7) | 1.00 | (0.57–1.77) | 1.000 | 0.00% |
| CHF, n (%) | 1 | (0.3) | 1 | (0.3) | 1.00 | (0.06–16.06) | 1.000 | 0.00% |
| DM, n (%) | 71 | (23.8) | 71 | (23.8) | 1.00 | (0.69–1.46) | 1.000 | 0.00% |
| ESRD, n (%) | 5 | (1.7) | 5 | (1.7) | 1.00 | (0.29–3.49) | 1.000 | 0.00% |
| GCS, median (IQR) | 13 | (8–15) | 13 | (7–15) | - | 0.878 | 1.26% | |
| ISS, median (IQR) | 16 | (16–25) | 16 | (16–24) | - | 0.819 | 1.87% | |
| Outcomes | ||||||||
| Mortality, n (%) | 47 | (15.8) | 35 | (11.7) | 1.41 | (0.88–2.25) | 0.154 | - |
| Hospital stay (days) | 19.5 | ±16.7 | 17.4 | ±16.5 | - | 0.121 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Wu, S.-C.; Yen, Y.-H.; Yang, J.C.-S.; Hsu, S.-Y.; Hsieh, C.-H. Assessing the Predictive Utility of the C-Reactive Protein-to-Lymphocyte Ratio for Mortality in Isolated Traumatic Brain Injury: A Single-Center Retrospective Analysis. Diagnostics 2024, 14, 2065. https://doi.org/10.3390/diagnostics14182065
Huang C-Y, Wu S-C, Yen Y-H, Yang JC-S, Hsu S-Y, Hsieh C-H. Assessing the Predictive Utility of the C-Reactive Protein-to-Lymphocyte Ratio for Mortality in Isolated Traumatic Brain Injury: A Single-Center Retrospective Analysis. Diagnostics. 2024; 14(18):2065. https://doi.org/10.3390/diagnostics14182065
Chicago/Turabian StyleHuang, Ching-Ya, Shao-Chun Wu, Yuan-Hao Yen, Johnson Chia-Shen Yang, Shiun-Yuan Hsu, and Ching-Hua Hsieh. 2024. "Assessing the Predictive Utility of the C-Reactive Protein-to-Lymphocyte Ratio for Mortality in Isolated Traumatic Brain Injury: A Single-Center Retrospective Analysis" Diagnostics 14, no. 18: 2065. https://doi.org/10.3390/diagnostics14182065







