Application of Digital Analysis for Assessment of Coronary Sub-Occlusions in Autopsy Pathology: It Is Time to Move beyond Histology Alone
Abstract
1. Background
2. Materials and Methods
2.1. Cases Selection
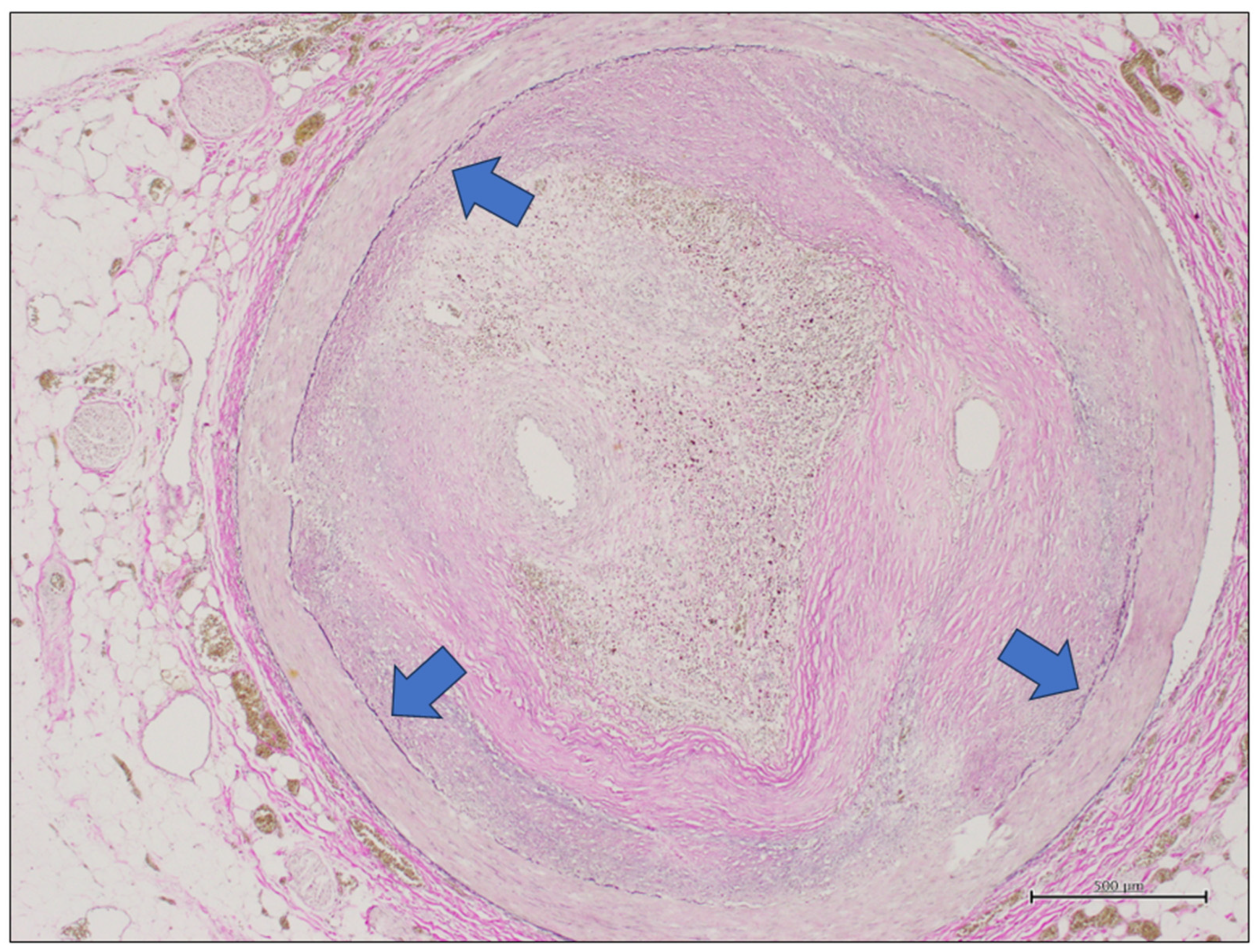
2.2. Coronary Artery Gross Evaluation and Sampling, Histology, and Ancillary Technique
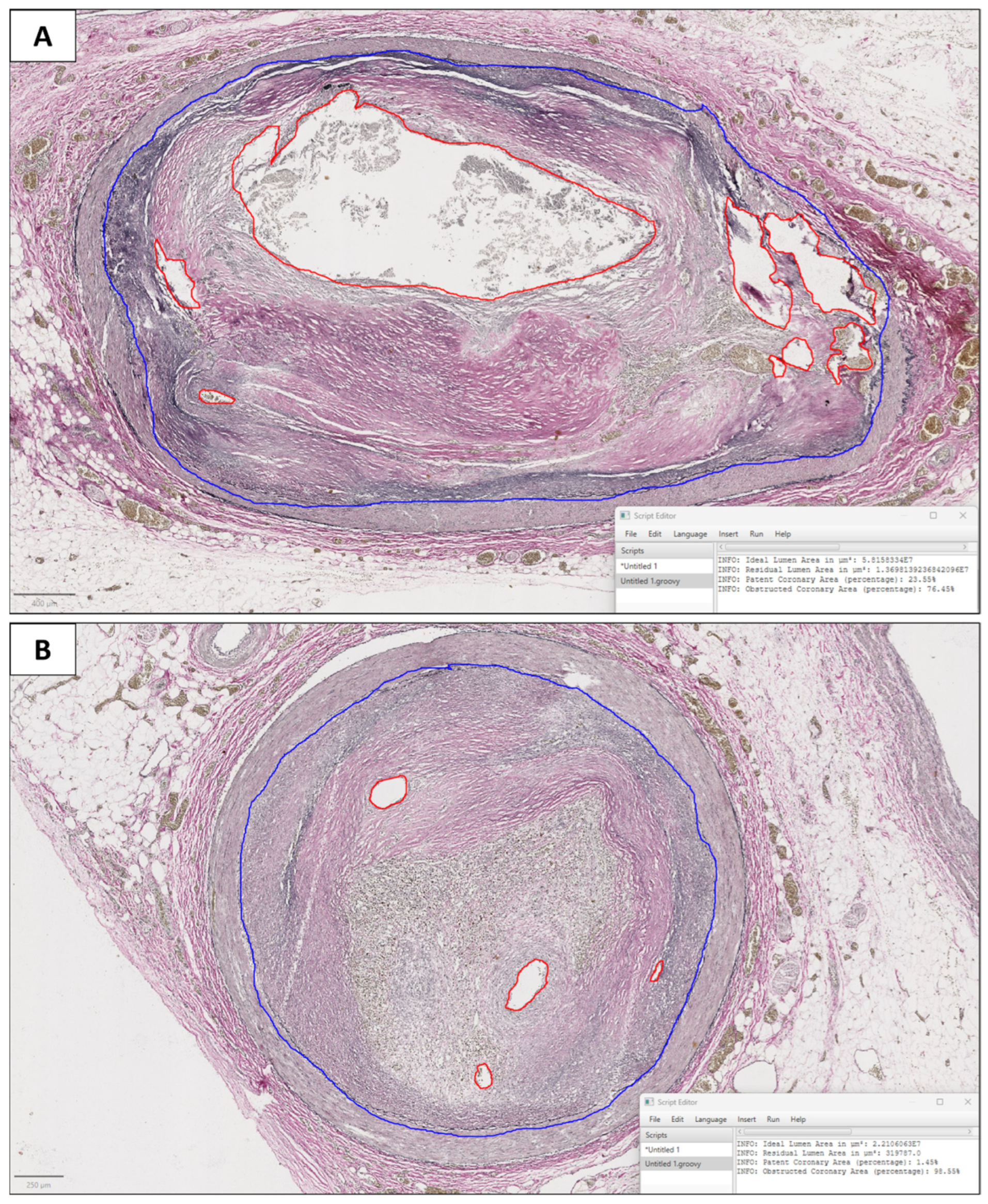
2.3. Digital Pathology
2.4. Pathologist Evaluation
2.5. Statistical Analysis
2.6. Hardware and Software
3. Results
3.1. General Features of the Series
3.2. Digital Pathology Analysis
3.3. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Michaud, K.; Basso, C.; d’Amati, G.; Giordano, C.; Kholová, I.; Preston, S.D.; Rizzo, S.; Sabatasso, S.; Sheppard, M.N.; Vink, A.; et al. Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification. Virchows Arch. 2020, 476, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.; Diletti, R.; Hoogendijk, M.G.; van der Graaf, M.; Zijlstra, F.; Szili-Torok, T.; Yap, S.C. Vulnerability for ventricular arrhythmias in patients with chronic coronary total occlusion. Expert. Rev. Cardiovasc. Ther. 2020, 18, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001, 345, 1473–1482. [Google Scholar] [CrossRef]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, D.; Dai, N.; Shin, D.; Choi, K.H.; Kim, S.M.; Kim, H.K.; Jeon, K.H.; Ha, S.J.; Lee, K.Y.; et al. Anatomic and Hemodynamic Plaque Characteristics for Subsequent Coronary Events. Front. Cardiovasc. Med. 2022, 9, 871450. [Google Scholar] [CrossRef]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef]
- Fraggetta, F.; L’Imperio, V.; Ameisen, D.; Carvalho, R.; Leh, S.; Kiehl, T.R.; Serbanescu, M.; Racoceanu, D.; Della Mea, V.; Polonia, A.; et al. Best Practice Recommendations for the Implementation of a Digital Pathology Workflow in the Anatomic Pathology Laboratory by the European Society of Digital and Integrative Pathology (ESDIP). Diagnostics 2021, 11, 2167. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Ghuran, A.V.; Camm, A.J. Ischaemic heart disease presenting as arrhythmias. Br. Med. Bull. 2001, 59, 193–210. [Google Scholar] [CrossRef]
- Friberg, N.; Ljungberg, O.; Berglund, E.; Berglund, D.; Ljungberg, R.; Alafuzoff, I.; Englund, E. Cause of death and significant disease found at autopsy. Virchows Arch. 2019, 475, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Z.H.; Olgin, J.E.; Vittinghoff, E.; Ursell, P.C.; Kim, A.S.; Sporer, K.; Yeh, C.; Colburn, B.; Clark, N.M.; Khan, R.; et al. Prospective Countywide Surveillance and Autopsy Characterization of Sudden Cardiac Death: POST SCD Study. Circulation 2018, 137, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Sabatasso, S.; Mangin, P.; Fracasso, T.; Moretti, M.; Docquier, M.; Djonov, V. Early markers for myocardial ischemia and sudden cardiac death. Int. J. Leg. Med. 2016, 130, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Campobasso, C.P.; Dell’Erba, A.S.; Addante, A.; Zotti, F.; Marzullo, A.; Colonna, M.F. Sudden cardiac death and myocardial ischemia indicators: A comparative study of four immunohistochemical markers. Am. J. Forensic Med. Pathol. 2008, 29, 154–161. [Google Scholar] [CrossRef]
- D’Abbronzo, G.; D’Antonio, A.; De Chiara, A.; Panico, L.; Sparano, L.; Diluvio, A.; Sica, A.; Svanera, G.; Franco, R.; Ronchi, A. Development of an Artificial-Intelligence-Based Tool for Automated Assessment of Cellularity in Bone Marrow Biopsies in Ph-Negative Myeloproliferative Neoplasms. Cancers 2024, 16, 1687. [Google Scholar] [CrossRef]
- Cazzato, G.; Massaro, A.; Colagrande, A.; Trilli, I.; Ingravallo, G.; Casatta, N.; Lupo, C.; Ronchi, A.; Franco, R.; Maiorano, E.; et al. Artificial Intelligence Applied to a First Screening of Naevoid Melanoma: A New Use of Fast Random Forest Algorithm in Dermatopathology. Curr. Oncol. 2023, 30, 6066–6078. [Google Scholar] [CrossRef]
- Mohammadi, M.; Fell, C.; Morrison, D.; Syed, S.; Konanahalli, P.; Bell, S.; Bryson, G.; Arandjelović, O.; Harrison, D.J.; Harris-Birtill, D. Automated reporting of cervical biopsies using artificial intelligence. PLOS Digit. Health 2024, 3, e0000381. [Google Scholar] [CrossRef]
- Ford, J.C.; O’Rourke, K.; Veinot, J.P.; Walley, V.M. The histologic estimation of coronary artery stenoses: Accuracy and the effect of lumen shape. Cardiovasc. Pathol. 2001, 10, 91–96. [Google Scholar] [CrossRef]
- Barth, R.F.; Kellough, D.A.; Allenby, P.; Blower, L.E.; Hammond, S.H.; Allenby, G.M.; Buja, L.M. Assessment of atherosclerotic luminal narrowing of coronary arteries based on morphometrically generated visual guides. Cardiovasc. Pathol. 2017, 29, 53–60. [Google Scholar] [CrossRef]
- Aimo, A.; Di Paolo, M.; Castiglione, V.; Modena, M.; Barison, A.; Benvenuti, M.; Bugelli, V.; Campobasso, C.P.; Guidi, B.; Camici, P.G.; et al. Scared to death: Emotional stress causing fatal myocardial infarction with nonobstructed coronary arteries in women. JACC. Case Rep. 2020, 2, 2400–2403. [Google Scholar] [CrossRef]
- Baronti, A.; Gentile, F.; Manetti, A.C.; Scatena, A.; Pellegrini, S.; Pucci, A.; Franzini, M.; Castiglione, V.; Maiese, A.; Giannoni, A.; et al. Myocardial infarction following COVID-19 vaccine administration: Post hoc, ergo propter hoc? Viruses 2022, 14, 1644. [Google Scholar] [CrossRef] [PubMed]
- Gitto, L.; Serinelli, S.; Busardò, F.P.; Panebianco, V.; Bolino, G.; Maiese, A. Can post-mortem computed tomography be considered an alternative for autopsy in deaths due to hemopericardium? J. Geriatr. Cardiol. 2014, 11, 363–367. [Google Scholar] [CrossRef] [PubMed]
| Autoptic Cases Included in the Series | ||||
|---|---|---|---|---|
| Subject | Sex | Age | Cause of Death | Coronary Atherosclerosis |
| A | m | 51 | Burns, sepsis, DIC | Fibrosis plaque (uncomplicated atherosclerosis) |
| B | f | 83 | Hypothermia | Initial atherosclerosis |
| C | f | 64 | Subarachnoid haemorrhage | Fibrosis plaque (uncomplicated atherosclerosis) |
| D | m | 62 | SCD due to arrhythmia | Fibrosis plaque (uncomplicated atherosclerosis) |
| E | m | 59 | SCD due to arrhythmia | Complicated atherosclerosis |
| F | f | 76 | SCD due to arrhythmia | Fibrosis plaque (uncomplicated atherosclerosis) |
| G | m | 77 | Polytrauma, DIC | Complicated atherosclerosis |
| H | m | 29 | Myocardial infarction | Complicated atherosclerosis |
| I | m | 76 | Polytrauma, DIC | Fibrosis plaque (uncomplicated atherosclerosis) |
| J | m | 53 | SCD due to arrhythmia | Fibrosis plaque (uncomplicated atherosclerosis) |
| K | m | 56 | Myocardial infarction | Complicated atherosclerosis |
| Percentage of Lumen Stenosis Evaluated by Eye Versus Digital Image Analysis | |||
|---|---|---|---|
| Subject | Section | Eye (%) | Digital (%) |
| A | 1 | 55 | 65.6 |
| 2 | 50 | 60 | |
| 3 | 55 | 65 | |
| 4 | 85 | 83 | |
| B | 5 | 35 | 41.6 |
| 6 | 35 | 35.2 | |
| C | 7 | 50 | 54 |
| 8 | 30 | 37.6 | |
| 9 | 40 | 42.1 | |
| D | 10 | 75 | 77.8 |
| 11 | 80 | 84.1 | |
| 12 | 65 | 57.8 | |
| 13 | 85 | 82.2 | |
| 14 | 80 | 83.6 | |
| 15 | 70 | 76.7 | |
| 16 | 50 | 54 | |
| E | 17 | 90 | 91 |
| 18 | 85 | 91.6 | |
| 19 | 80 | 79.6 | |
| 20 | 95 | 91.6 | |
| 21 | 40 | 53.4 | |
| F | 22 | 65 | 71.4 |
| G | 23 | 45 | 53 |
| 24 | 47 | 55.5 | |
| H | 25 | 92 | 85.7 |
| 26 | 80 | 81.8 | |
| 27 | 82 | 76.1 | |
| 28 | 83 | 66.4 | |
| 29 | 77 | 75.9 | |
| 30 | 55 | 73.3 | |
| 31 | 75 | 71.7 | |
| I | 32 | 65 | 64.2 |
| 33 | 92 | 82 | |
| 34 | 62 | 62.6 | |
| 35 | 62 | 62.7 | |
| 36 | 90 | 87.1 | |
| 37 | 82 | 71 | |
| 38 | 88 | 82.8 | |
| 39 | 78 | 68.3 | |
| J | 40 | 70 | 66 |
| 41 | 70 | 73.1 | |
| 42 | 80 | 78.6 | |
| 43 | 80 | 76.5 | |
| 44 | 75 | 71.8 | |
| K | 45 | 99 | 99 |
| 46 | 98 | 96.9 | |
| 47 | 98 | 98.6 | |
| 48 | 90 | 94.5 | |
| 49 | 65 | 73.5 | |
| 50 | 65 | 67.7 | |
| Lin Agreement Index between Eye Evaluation and Digital Evaluation | |
|---|---|
| Lumen Stenosis Range | Lin Agreement Index |
| Overall | 0.923 |
| 60–80% | 0.798 |
| 65–75% | 0.516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Abbronzo, G.; Franco, R.; Salzillo, C.; Campobasso, C.P.; Municinò, M.; Feola, A.; Ronchi, A. Application of Digital Analysis for Assessment of Coronary Sub-Occlusions in Autopsy Pathology: It Is Time to Move beyond Histology Alone. Diagnostics 2024, 14, 2115. https://doi.org/10.3390/diagnostics14192115
D’Abbronzo G, Franco R, Salzillo C, Campobasso CP, Municinò M, Feola A, Ronchi A. Application of Digital Analysis for Assessment of Coronary Sub-Occlusions in Autopsy Pathology: It Is Time to Move beyond Histology Alone. Diagnostics. 2024; 14(19):2115. https://doi.org/10.3390/diagnostics14192115
Chicago/Turabian StyleD’Abbronzo, Giuseppe, Renato Franco, Cecilia Salzillo, Carlo Pietro Campobasso, Maurizio Municinò, Alessandro Feola, and Andrea Ronchi. 2024. "Application of Digital Analysis for Assessment of Coronary Sub-Occlusions in Autopsy Pathology: It Is Time to Move beyond Histology Alone" Diagnostics 14, no. 19: 2115. https://doi.org/10.3390/diagnostics14192115
APA StyleD’Abbronzo, G., Franco, R., Salzillo, C., Campobasso, C. P., Municinò, M., Feola, A., & Ronchi, A. (2024). Application of Digital Analysis for Assessment of Coronary Sub-Occlusions in Autopsy Pathology: It Is Time to Move beyond Histology Alone. Diagnostics, 14(19), 2115. https://doi.org/10.3390/diagnostics14192115








