Quantitative Assessment of Polarization and Elastic Properties of Endometrial Tissue for Precancer/Cancer Diagnostics Using Multimodal Optical Coherence Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Experiment Design
2.2. Multimodal Optical Coherence Tomography (MM OCT)
2.3. Histological Examination
2.4. Statistical Analysis
3. Results and Discussion
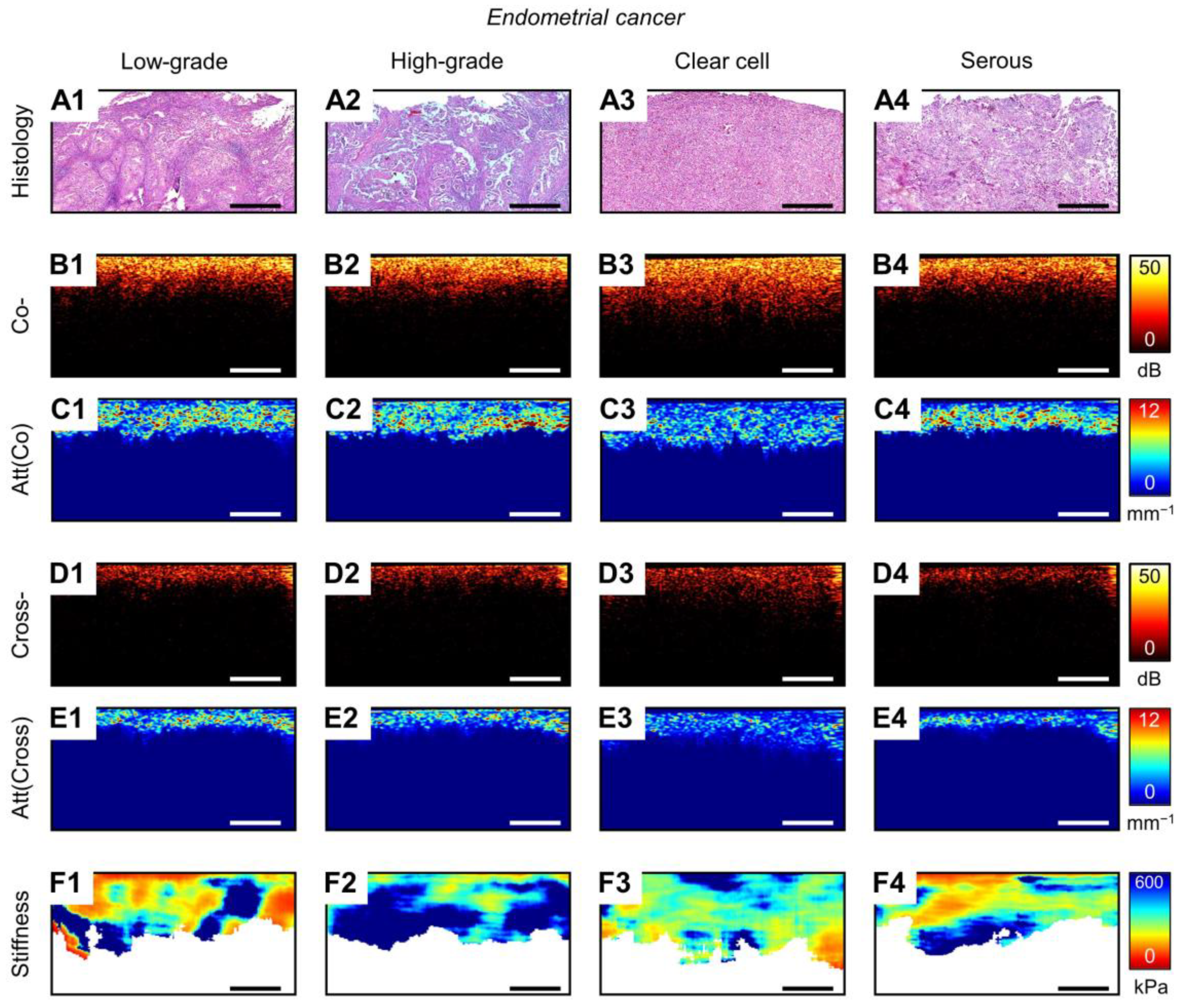
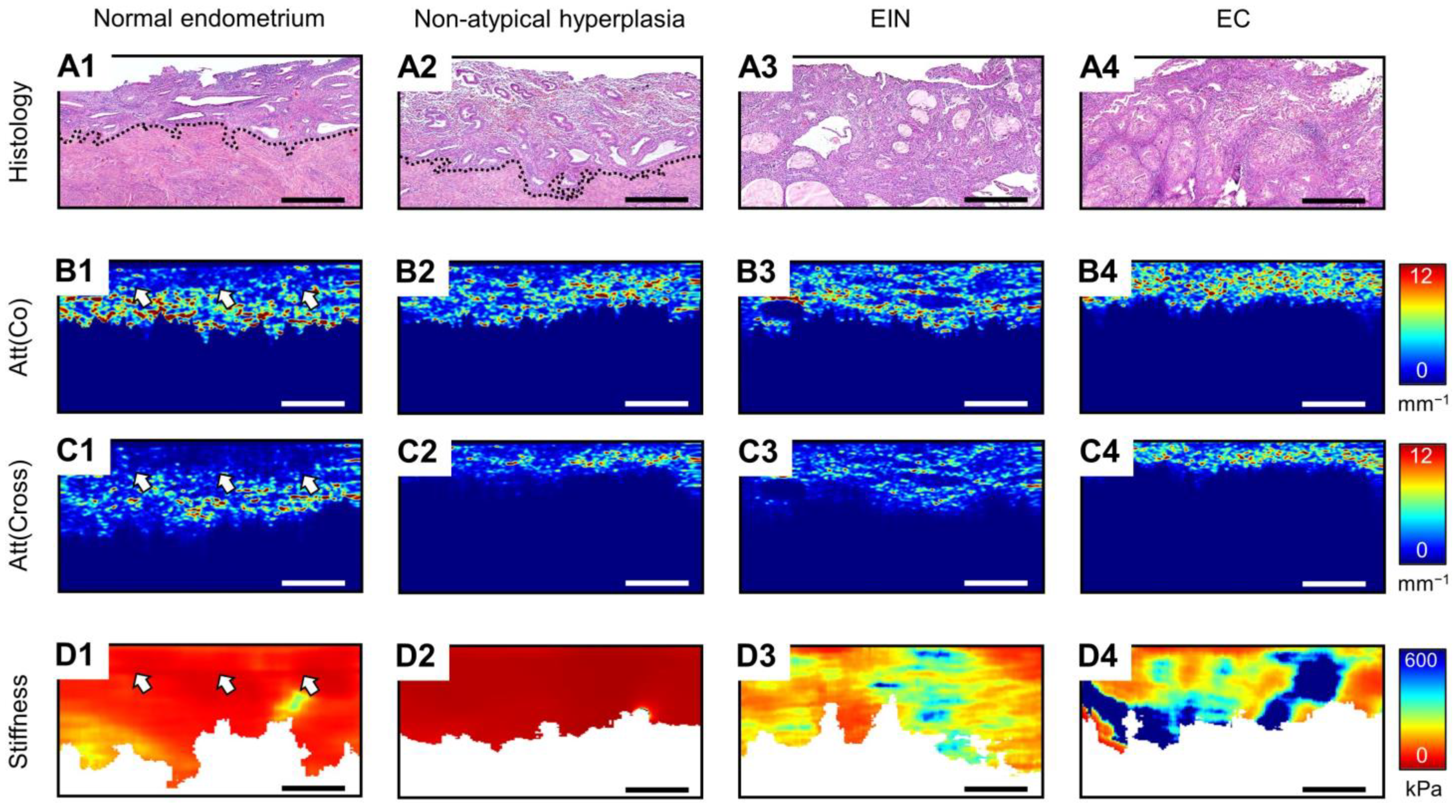
3.1. MM OCT Analysis of Polarization and Elastic Properties of Endometrial Tissue
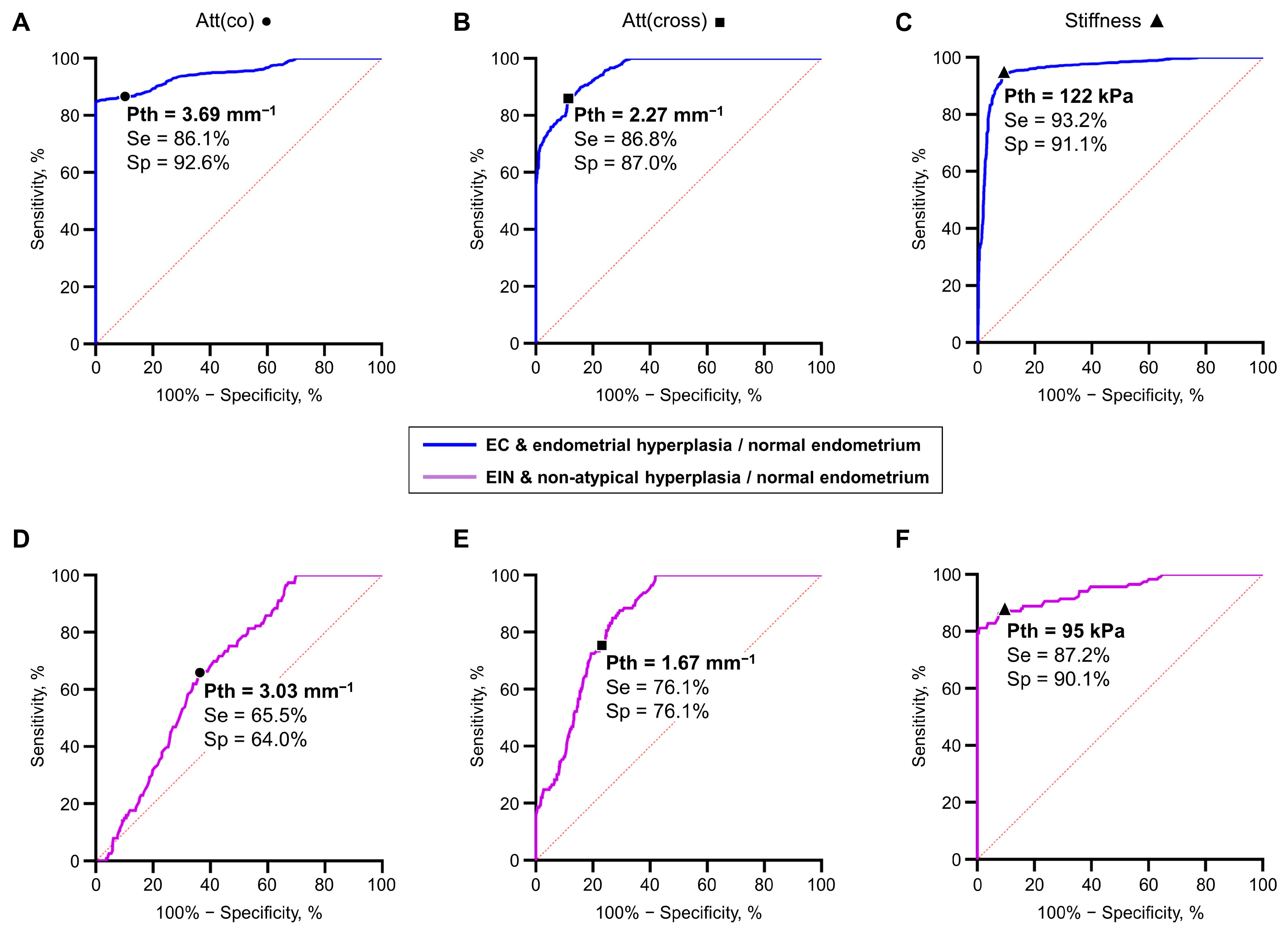
3.2. Diagnostics Parameters of MM OCT Criteria in EIN/EC Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Hutt, S.; Tailor, A.; Ellis, P.; Michael, A.; Butler-Manuel, S.; Chatterjee, J. The role of biomarkers in endometrial cancer and hyperplasia: A literature review. Acta Oncol. 2019, 58, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Nees, L.K.; Heublein, S.; Steinmacher, S.; Juhasz-Böss, I.; Brucker, S.; Tempfer, C.B.; Wallwiener, M. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch. Gynecol. Obstet. 2022, 306, 407–421. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Critchley, H.O.; Williams, A.R.; Arends, M.J.; Saunders, P.T. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod. Update 2017, 23, 232–254. [Google Scholar] [CrossRef]
- Reed, S.D.; Newton, K.M.; Clinton, W.L.; Epplein, M.; Garcia, R.; Allison, K.; Voigt, L.F.; Weiss, N.S. Incidence of endometrial hyperplasia. Am. J. Obstet. Gynecol. 2009, 200, 678.e1–678.e6. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Chen, H.; Strickland, A.L.; Castrillon, D.H. Histopathologic diagnosis of endometrial precancers: Updates and future directions. Semin. Diagn. Pathol. 2022, 39, 137–147. [Google Scholar] [CrossRef]
- Hedrick Ellenson, L.; Ronnett, B.M.; Kurman, R.J. Precursors of Endometrial Carcinoma. In Blaustein’s Pathology of the Female Genital Tract; Kurman, R.J., Hedrick Ellenson, L., Ronnett, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 439–472. [Google Scholar]
- Brinton, L.A.; Felix, A.S.; McMeekin, D.S.; Creasman, W.T.; Sherman, M.E.; Mutch, D.; Cohn, D.E.; Walker, J.L.; Moore, R.G.; Downs, L.S.; et al. Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol. Oncol. 2013, 129, 277–284. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Vitale, S.G.; Buzzaccarini, G.; Riemma, G.; Pacheco, L.A.; Di Spiezio Sardo, A.; Carugno, J.; Chiantera, V.; Török, P.; Noventa, M.; Haimovich, S.; et al. Endometrial biopsy: Indications, techniques and recommendations. An evidence-based guideline for clinical practice. J. Gynecol. Obstet. Hum. Reprod. 2023, 52, 102588. [Google Scholar] [CrossRef] [PubMed]
- Adambekov, S.; Goughnour, S.L.; Mansuria, S.; Donnellan, N.; Elishaev, E.; Villanueva, H.J.; Edwards, R.P.; Bovbjerg, D.H.; Linkov, F. Patient and provider factors associated with endometrial Pipelle sampling failure. Gynecol. Oncol. 2017, 144, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Sanam, M.; Majid, M.M. Comparison the Diagnostic Value of Dilatation and Curettage Versus Endometrial Biopsy by Pipelle—A Clinical Trial. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 4971–4975. [Google Scholar] [CrossRef] [PubMed]
- Gil González, Y.; Pérez Morales, M.E.; Emergi Zhrigen, Y.; Santana Suárez, M.A.; Pérez Matos, C.; Nieto Naya, M.; Ocón Padrón, L.; Laseca Modrego, M.; Arencibia Sánchez, O.; Sánchez Sánchez, V.; et al. Role of hysteroscopy during conservative management of atypical endometrial hyperplasia and early-stage endometrial cancer in patients who desire pregnancy. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2022, 42, 3435–3440. [Google Scholar] [CrossRef]
- Svirsky, R.; Smorgick, N.; Rozowski, U.; Sagiv, R.; Feingold, M.; Halperin, R.; Pansky, M. Can we rely on blind endometrial biopsy for detection of focal intrauterine pathology? Am. J. Obstet. Gynecol. 2008, 199, 115.e1–115.e3. [Google Scholar] [CrossRef]
- Loffer, F.D. The Time Has Come to Quit Relying on a Blind Endometrial Biopsy or Dilation and Curettage to Rule Out Malignant Endometrial Changes. J. Minim. Invasive Gynecol. 2019, 26, 1207–1208. [Google Scholar] [CrossRef]
- Carugno, J.; Marbin, S.J.; LaganÀ, A.S.; Vitale, S.G.; Alonso, L.; Sardo, A.D.S.; Haimovich, S. New development on hysteroscopy for endometrial cancer diagnosis: State of the art. Minerva Medica 2021, 112, 12–19. [Google Scholar] [CrossRef]
- Bilir, E.; Kahramanoğlu, İ. The role of hysteroscopy in fertility preservation in endometrial cancer and atypical endometrial hyperplasia: A semi-systematic literature review. Arch. Gynecol. Obstet. 2023, 308, 1113–1126. [Google Scholar] [CrossRef]
- Bedner, R.; Rzepka-Górska, I. Hysteroscopy with directed biopsy versus dilatation and curettage for the diagnosis of endometrial hyperplasia and cancer in perimenopausal women. Eur. J. Gynaecol. Oncol. 2007, 28, 400–402. [Google Scholar]
- Garuti, G.; Cellani, F.; Garzia, D.; Colonnelli, M.; Luerti, M. Accuracy of hysteroscopic diagnosis of endometrial hyperplasia: A retrospective study of 323 patients. J. Minim. Invasive Gynecol. 2005, 12, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Koutlaki, N.; Dimitraki, M.; Zervoudis, S.; Skafida, P.; Nikas, I.; Mandratzi, J.; Liberis, A.; Liberis, V. Hysteroscopy and endometrial cancer. Diagnosis and influence on prognosis. Gynecol. Surg. 2010, 7, 335–341. [Google Scholar] [CrossRef][Green Version]
- Garuti, G.; Mirra, M.; Luerti, M. Hysteroscopic view in atypical endometrial hyperplasias: A correlation with pathologic findings on hysterectomy specimens. J. Minim. Invasive Gynecol. 2006, 13, 325–330. [Google Scholar] [CrossRef]
- De Franciscis, P.; Riemma, G.; Schiattarella, A.; Cobellis, L.; Guadagno, M.; Vitale, S.G.; Mosca, L.; Cianci, A.; Colacurci, N. Concordance between the Hysteroscopic Diagnosis of Endometrial Hyperplasia and Histopathological Examination. Diagnostics 2019, 9, 142. [Google Scholar] [CrossRef]
- Vitale, S.G.; Riemma, G.; Carugno, J.; Chiofalo, B.; Vilos, G.A.; Cianci, S.; Budak, M.S.; Lasmar, B.P.; Raffone, A.; Kahramanoglu, I. Hysteroscopy in the management of endometrial hyperplasia and cancer in reproductive aged women: New developments and current perspectives. Transl. Cancer Res. 2020, 9, 7767–7777. [Google Scholar] [CrossRef]
- Garuti, G.; Sagrada, P.F.; Frigoli, A.; Fornaciari, O.; Finco, A.; Mirra, M.; Soligo, M. Hysteroscopic biopsy compared with endometrial curettage to assess the preoperative rate of atypical hyperplasia underestimating endometrial carcinoma. Arch. Gynecol. Obstet. 2023, 308, 971–979. [Google Scholar] [CrossRef]
- Leitgeb, R.; Placzek, F.; Rank, E.; Krainz, L.; Haindl, R.; Li, Q.; Liu, M.; Andreana, M.; Unterhuber, A.; Schmoll, T.; et al. Enhanced medical diagnosis for dOCTors: A perspective of optical coherence tomography. J. Biomed. Opt. 2021, 26, 100601. [Google Scholar] [CrossRef] [PubMed]
- Kirillin, M.; Motovilova, T.; Shakhova, N. Optical coherence tomography in gynecology: A narrative review. J. Biomed. Opt. 2017, 22, 121709. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, A.; Gelikonov, V.; Gelikonov, G.; Feldchtein, F.; Kuranov, R.; Gladkova, N.; Shakhova, N.; Snopova, L.; Shakhov, A.; Kuznetzova, I.; et al. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt. Express 1997, 1, 432–440. [Google Scholar] [CrossRef]
- Law, T.S.M.; Wu, F.; Xu, H.; Wang, C.C.; Li, T.C. Endometrium imaging using real-time rotational optical coherence tomography imaging system: A pilot, prospective and ex-vivo study. Medicine 2019, 98, e17738. [Google Scholar] [CrossRef]
- Ding, B.; Jinyuan, T.; Tao, K.; Ding, Z.; Yang, S. A pilot and ex-vivo study of examination of endometrium tissue by catheter based optical coherence tomography. BMC Med. Imaging 2022, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, A.; Sherstnev, E.; Kiseleva, E.; Achkasova, K.; Potapov, A.; Yashin, K.; Sirotkina, M.; Gelikonov, G.; Matkivsky, V.; Shilyagin, P.; et al. Depth-resolved method for attenuation coefficient calculation from optical coherence tomography data for improved biological structure visualization. J. Biophotonics 2023, 16, e202100392. [Google Scholar] [CrossRef] [PubMed]
- Gelikonov, V.M.; Romashov, V.N.; Shabanov, D.V.; Ksenofontov, S.Y.; Terpelov, D.A.; Shilyagin, P.A.; Gelikonov, G.V.; Vitkin, I.A. Cross-Polarization Optical Coherence Tomography with Active Maintenance of the Circular Polarization of a Sounding Wave in a Common Path System. Radiophys. Quantum Electron. 2018, 60, 897–911. [Google Scholar] [CrossRef]
- Kiseleva, E.; Kirillin, M.; Feldchtein, F.; Vitkin, A.; Sergeeva, E.; Zagaynova, E.; Streltzova, O.; Shakhov, B.; Gubarkova, E.; Gladkova, N. Differential diagnosis of human bladder mucosa pathologies in vivo with cross-polarization optical coherence tomography. Biomed. Opt. Express 2015, 6, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Q.; Tan, J.; Liu, Y.M.; Li, Y.Z.; You, F.F.; Zhang, M.Y.; Chen, Q.; Zou, K.; Sun, X. Diagnostic accuracy of optical coherence tomography for bladder cancer: A systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2019, 27, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Gubarkova, E.V.; Kiseleva, E.B.; Sirotkina, M.A.; Vorontsov, D.A.; Achkasova, K.A.; Kuznetsov, S.S.; Yashin, K.S.; Matveyev, A.L.; Sovetsky, A.A.; Matveev, L.A.; et al. Diagnostic Accuracy of Cross-Polarization OCT and OCT-Elastography for Differentiation of Breast Cancer Subtypes: Comparative Study. Diagnostics 2020, 10, 994. [Google Scholar] [CrossRef]
- Gubarkova, E.; Kiseleva, E.; Moiseev, A.; Vorontsov, D.; Kuznetsov, S.; Plekhanov, A.; Karabut, M.; Sirotkina, M.; Gelikonov, G.; Gamayunov, S.; et al. Intraoperative Assessment of Breast Cancer Tissues after Breast-Conserving Surgery Based on Mapping the Attenuation Coefficients in 3D Cross-Polarization Optical Coherence Tomography. Cancers 2023, 15, 2663. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Hepburn, M.S.; Mowla, A.; Kennedy, B.F. Strain and elasticity imaging in compression optical coherence elastography: The two-decade perspective and recent advances. J. Biophotonics 2021, 14, e202000257. [Google Scholar] [CrossRef]
- Kennedy, B.F.; McLaughlin, R.A.; Kennedy, K.M.; Chin, L.; Wijesinghe, P.; Curatolo, A.; Tien, A.; Ronald, M.; Latham, B.; Saunders, C.M.; et al. Investigation of Optical Coherence Microelastography as a Method to Visualize Cancers in Human Breast Tissue. Cancer Res. 2015, 75, 3236–3245. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Gubarkova, E.V.; Sirotkina, M.A.; Sovetsky, A.A.; Vorontsov, D.A.; Matveev, L.A.; Kuznetsov, S.S.; Bogomolova, A.Y.; Vorontsov, A.Y.; Matveyev, A.L.; et al. Compression OCT-elastography combined with speckle-contrast analysis as an approach to the morphological assessment of breast cancer tissue. Biomed. Opt. Express 2023, 14, 3037–3056. [Google Scholar] [CrossRef]
- Li, C.; Guan, G.; Ling, Y.; Hsu, Y.T.; Song, S.; Huang, J.T.; Lang, S.; Wang, R.K.; Huang, Z.; Nabi, G. Detection and characterisation of biopsy tissue using quantitative optical coherence elastography (OCE) in men with suspected prostate cancer. Cancer Lett. 2015, 357, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yuting, L.; Li, C.; Zhou, K.; Guan, G.; Appleton, P.L.; Lang, S.; McGloin, D.; Huang, Z.; Nabi, G. Microscale characterization of prostate biopsies tissues using optical coherence elastography and second harmonic generation imaging. Lab. Investig. 2018, 98, 380–390. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Sirotkina, M.A.; Gubarkova, E.V.; Kiseleva, E.B.; Sovetsky, A.A.; Karabut, M.M.; Zagainov, V.E.; Kuznetsov, S.S.; Maslennikova, A.V.; Zagaynova, E.V.; et al. Towards targeted colorectal cancer biopsy based on tissue morphology assessment by compression optical coherence elastography. Front. Oncol. 2023, 13, 1121838. [Google Scholar] [CrossRef] [PubMed]
- Plekhanov, A.A.; Kozlov, D.S.; Shepeleva, A.A.; Kiseleva, E.B.; Shimolina, L.E.; Druzhkova, I.N.; Plekhanova, M.A.; Karabut, M.M.; Gubarkova, E.V.; Gavrina, A.I.; et al. Tissue Elasticity as a Diagnostic Marker of Molecular Mutations in Morphologically Heterogeneous Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 5337. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Ethical principles for medical research involving human subjects. Eur. J. Emerg. Med. 2001, 8, 221–223. [Google Scholar] [CrossRef]
- Vermij, L.; Smit, V.; Nout, R.; Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gubarkova, E.V.; Sovetsky, A.A.; Sirotkina, M.A.; Gelikonov, G.V.; Zagaynova, E.V.; Gladkova, N.D.; Vitkin, A. Practical obstacles and their mitigation strategies in compressional optical coherence elastography of biological tissues. J. Innov. Opt. Health Sci. 2017, 10, 1742006. [Google Scholar] [CrossRef]
- Shilyagin, P.A.; Gelikonov, G.V.; Gelikonov, V.M.; Moiseev, A.A.; Terpelov, D.A. Achromatic registration of quadrature components of the optical spectrum in spectral domain optical coherence tomography. Quantum Electron. 2014, 44, 664. [Google Scholar] [CrossRef]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; de Boer, J.F. Depth-resolved model-based reconstruction of attenuation coefficients in optical coherence tomography. Biomed. Opt. Express 2014, 5, 322–337. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Chin, L.; McLaughlin, R.A.; Latham, B.; Saunders, C.M.; Sampson, D.D.; Kennedy, B.F. Quantitative micro-elastography: Imaging of tissue elasticity using compression optical coherence elastography. Sci. Rep. 2015, 5, 15538. [Google Scholar] [CrossRef]
- Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Gelikonov, G.V.; Moiseev, A.A.; Zaitsev, V.Y. Vector method for strain estimation in phase-sensitive optical coherence elastography. Laser Phys. Lett. 2018, 15, 065603. [Google Scholar] [CrossRef]
- Zykov, A.A.; Matveyev, A.L.; Sovetsky, A.A.; Matveev, L.A.; Zaitsev, V.Y. Vector method of strain estimation in OCT-elastography with adaptive choice of scale for estimating interframe phase-variation gradients. Laser Phys. Lett. 2023, 20, 095601. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Gubarkova, E.V.; Sovietsky, A.A.; Zaitsev, V.Y.; Matveev, L.A.; Matveyev, A.L.; Timofeeva, L.B.; Kuznetsov, S.S.; Zagaynova, E.V.; Gladkova, N.D.; et al. Optical Coherence Elastography for Non-Invasive Monitoring of Tumor Elasticity under Chemotherapy: Pilot Study. Sovrem. Tehnol. V Med. 2018, 10, 43–51. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Sirotkina, M.A.; Sovetsky, A.A.; Gubarkova, E.V.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Zagaynova, E.V.; Gladkova, N.D.; Zaitsev, V.Y. Histological validation of in vivo assessment of cancer tissue inhomogeneity and automated morphological segmentation enabled by Optical Coherence Elastography. Sci. Rep. 2020, 10, 11781. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Feuerman, M.; Miller, A.R. Relationships between statistical measures of agreement: Sensitivity, specificity and kappa. J. Eval. Clin. Pract. 2008, 14, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Baak, J.P.; Mutter, G.L.; Robboy, S.; van Diest, P.J.; Uyterlinde, A.M.; Orbo, A.; Palazzo, J.; Fiane, B.; Løvslett, K.; Burger, C.; et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer 2005, 103, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Owings, R.A.; Quick, C.M. Endometrial intraepithelial neoplasia. Arch. Pathol. Lab. Med. 2014, 138, 484–491. [Google Scholar] [CrossRef]
- Van Den Bosch, T.; Verbakel, J.Y.; Valentin, L.; Wynants, L.; De Cock, B.; Pascual, M.A.; Leone, F.P.G.; Sladkevicius, P.; Alcazar, J.L.; Votino, A.; et al. Typical ultrasound features of various endometrial pathologies described using International Endometrial Tumor Analysis (IETA) terminology in women with abnormal uterine bleeding. Ultrasound Obstet. Gynecol. 2021, 57, 164–172. [Google Scholar] [CrossRef]
- Sbarra, M.; Lupinelli, M.; Brook, O.R.; Venkatesan, A.M.; Nougaret, S. Imaging of Endometrial Cancer. Radiol. Clin. N. Am. 2023, 61, 609–625. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Valous, N.A.; Hadjidemetriou, S.; Teichgräber, U.; Malich, A. Quantitative Assessment of Breast-Tumor Stiffness Using Shear-Wave Elastography Histograms. Diagnostics 2022, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, K.; He, X.; Chen, T.; Jiang, W.; Zhou, J.; Liu, Y.; Guo, D. Diagnostic Accuracy of Liver and Spleen Stiffness in Magnetic Resonance Elastography for the Detection of Gastroesophageal Varices: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 3527. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Esnal-Zufiaurre, A.; Arends, M.J.; Herrington, C.S.; Collins, F.; Williams, A.R.W.; Saunders, P.T.K. Improving the Diagnosis of Endometrial Hyperplasia Using Computerized Analysis and Immunohistochemical Biomarkers. Front. Reprod. Health 2022, 4, 896170. [Google Scholar] [CrossRef] [PubMed]
- Heremans, R.; Van Den Bosch, T.; Valentin, L.; Wynants, L.; Pascual, M.A.; Fruscio, R.; Testa, A.C.; Buonomo, F.; Guerriero, S.; Epstein, E.; et al. Ultrasound features of endometrial pathology in women without abnormal uterine bleeding: Results from the International Endometrial Tumor Analysis study (IETA3). Ultrasound Obstet. Gynecol. 2022, 60, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Long, B.J.; Sherman, M.E.; Lemens, M.A.; Podratz, K.C.; Hopkins, M.R.; Ahlberg, L.J.; Mc Guire, L.J.; Laughlin-Tommaso, S.K.; Bakkum-Gamez, J.N.; et al. Risk assessment of endometrial cancer and endometrial intraepithelial neoplasia in women with abnormal bleeding and implications for clinical management algorithms. Am. J. Obstet. Gynecol. 2020, 223, 549.e1–549.e13. [Google Scholar] [CrossRef]
- Levine, D.; Gupta, S.C.; Kwan, C.; Brook, A.; Jorgensen, E.M.; Kappler, A.; Hecht, J.L. The Sonographic Appearance of Endometrial Intraepithelial Neoplasia. J. Ultrasound Med. 2022, 41, 1723–1737. [Google Scholar] [CrossRef]
- Matias-Guiu, X.; Prat, J. Molecular pathology of endometrial carcinoma. Histopathology 2013, 62, 111–123. [Google Scholar] [CrossRef]
- Lax, S.F. Pathology of Endometrial Carcinoma. Adv. Exp. Med. Biol. 2017, 943, 75–96. [Google Scholar] [CrossRef]
- Stenbäck, F. Collagen type III formation and distribution in the uterus: Effects of hormones and neoplasm development. Oncology 1989, 46, 326–334. [Google Scholar] [CrossRef]
- Shi, J.W.; Lai, Z.Z.; Yang, H.L.; Yang, S.L.; Wang, C.J.; Ao, D.; Ruan, L.Y.; Shen, H.H.; Zhou, W.J.; Mei, J.; et al. Collagen at the maternal-fetal interface in human pregnancy. Int. J. Biol. Sci. 2020, 16, 2220–2234. [Google Scholar] [CrossRef]
- Stachowicz, N.; Smoleń, A.; Ciebiera, M.; Łoziński, T.; Poziemski, P.; Borowski, D.; Czekierdowski, A. Risk Assessment of Endometrial Hyperplasia or Endometrial Cancer with Simplified Ultrasound-Based Scoring Systems. Diagnostics 2021, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Mhawech-Fauceglia, P. Pathology of the Uterine Corpus. In Handbook of Gynecology; Shoupe, D., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1215–1232. [Google Scholar]
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the corpus uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155, 45–60. [Google Scholar] [CrossRef]
- Faria, S.C.; Devine, C.E.; Rao, B.; Sagebiel, T.; Bhosale, P. Imaging and Staging of Endometrial Cancer. Semin. Ultrasound CT MRI 2019, 40, 287–294. [Google Scholar] [CrossRef]
- Bourdel, N.; Chauvet, P.; Tognazza, E.; Pereira, B.; Botchorishvili, R.; Canis, M. Sampling in Atypical Endometrial Hyperplasia: Which Method Results in the Lowest Underestimation of Endometrial Cancer? A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2016, 23, 692–701. [Google Scholar] [CrossRef]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Zorzato, P.C.; Dababou, S.; Bosco, M.; Torella, M.; Braga, A.; Frigerio, M.; Gardella, B.; Cianci, S.; Laganà, A.S.; et al. Conservative Management of Atypical Endometrial Hyperplasia and Early Endometrial Cancer in Childbearing Age Women. Medicina 2022, 58, 1256. [Google Scholar] [CrossRef]
- De Rocco, S.; Buca, D.; Oronzii, L.; Petrillo, M.; Fanfani, F.; Nappi, L.; Liberati, M.; D’Antonio, F.; Scambia, G.; Leombroni, M.; et al. Reproductive and pregnancy outcomes of fertility-sparing treatments for early-stage endometrial cancer or atypical hyperplasia: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 273, 90–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhang, J.; Wang, C.; Wang, Y.; Chen, H.; Shan, L.; Huo, J.; Gu, J.; Ma, X. Deep learning model for classifying endometrial lesions. J. Transl. Med. 2021, 19, 10. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sone, K.; Noda, K.; Yoshida, K.; Toyohara, Y.; Kato, K.; Inoue, F.; Kukita, A.; Taguchi, A.; Nishida, H.; et al. Automated system for diagnosing endometrial cancer by adopting deep-learning technology in hysteroscopy. PLoS ONE 2021, 16, e0248526. [Google Scholar] [CrossRef]
- Raimondo, D.; Raffone, A.; Salucci, P.; Raimondo, I.; Capobianco, G.; Galatolo, F.A.; Cimino, M.; Travaglino, A.; Maletta, M.; Ferla, S.; et al. Detection and Classification of Hysteroscopic Images Using Deep Learning. Cancers 2024, 16, 1315. [Google Scholar] [CrossRef]
- Harika, B.; Subbaiah, M.; Maurya, D.K. Diagnostic Accuracy of Hysteroscopic Scoring System in Predicting Endometrial Malignancy and Atypical Endometrial Hyperplasia. J. Mid-Life Health 2021, 12, 206–210. [Google Scholar] [CrossRef]
- Capasso, I.; Cucinella, G.; Wright, D.E.; Takahashi, H.; De Vitis, L.A.; Gregory, A.V.; Kim, B.; Reynolds, E.; Fumagalli, D.; Occhiali, T.; et al. Artificial intelligence model for enhancing the accuracy of transvaginal ultrasound in detecting endometrial cancer and endometrial atypical hyperplasia. Int. J. Gynecol. Cancer 2024, 005652, ijgc-2024. [Google Scholar] [CrossRef] [PubMed]
- Tanos, V.; Neofytou, M.; Tanos, P.; Pattichis, C.S.; Pattichis, M.S. Computer-Aided Diagnosis by Tissue Image Analysis as an Optical Biopsy in Hysteroscopy. Int. J. Mol. Sci. 2022, 23, 12782. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.D.; Drake, W.K.; Rice, P.F.; Long, D.J.; Shir, H.; Walton, R.H.M.; Reed, M.N.; Galvez, D.; Gorman, T.; Heusinkveld, J.M.; et al. Iterative prototyping based on lessons learned from the falloposcope in vivo pilot study experience. J. Biomed. Opt. 2023, 28, 121206. [Google Scholar] [CrossRef]
- Law, T.S.M.; Cheung, W.C.; Wu, F.; Zhang, R.; Chung, J.P.W.; Wang, C.C.; Chen, X.; Li, T.C. Endometrial Vascularization Characterized by Optical Coherence Tomography and Immunohistochemistry in Women Undergoing In Vitro Fertilization-Embryo Transfer Treatment. Medicina 2019, 55, 81. [Google Scholar] [CrossRef]
- Miranda, C.; Barkley, J.; Smith, B. Intrauterine photoacoustic and ultrasound imaging probe. J. Biomed. Opt. 2018, 23, 046008. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, J.; Wang, T.; Fang, J.; Su, H.; Xiao, Z.; Peng, Y.; Liang, X.; Gong, X.; Chen, Z. Imaging biomarker for quantitative analysis of endometrial injury based on optical coherence tomography/ultrasound integrated imaging mode. J. Biophotonics 2023, 16, e202300113. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Ksenofontov, S.Y.; Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Zykov, A.A.; Gelikonov, G.V. Real-Time Strain and Elasticity Imaging in Phase-Sensitive Optical Coherence Elastography Using a Computationally Efficient Realization of the Vector Method. Photonics 2021, 8, 527. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Es’haghian, S.; Chin, L.; McLaughlin, R.A.; Sampson, D.D.; Kennedy, B.F. Optical palpation: Optical coherence tomography-based tactile imaging using a compliant sensor. Opt. Lett. 2014, 39, 3014–3017. [Google Scholar] [CrossRef]
- Foo, K.Y.; Kennedy, K.M.; Zilkens, R.; Allen, W.M.; Fang, Q.; Sanderson, R.W.; Anstie, J.; Dessauvagie, B.F.; Latham, B.; Saunders, C.M.; et al. Optical palpation for tumor margin assessment in breast-conserving surgery. Biomed. Opt. Express 2021, 12, 1666–1682. [Google Scholar] [CrossRef]
- Grechkanev, G.O.; Plekhanov, A.A.; Loginova, M.M.; Avetisyan, E.A.; Shepeleva, A.A.; Zaitseva, A.M.; Ushanova, A.A.; Gamayunov, S.V.; Sirotkina, M.A.; Zaitsev, V.Y.; et al. First experience of using multimodal optical coherence tomography for diagnostics of hyperplastic processes in the endometrium. Russ. Bull. Obstet.-Gynecol. 2023, 23, 66–72. [Google Scholar] [CrossRef]

| Endometrial Morphology | MM OCT Parameters | ||
|---|---|---|---|
| Att(co), mm−1 | Att(cross), mm−1 | Stiffness, kPa | |
| Normal endometrium | |||
| Proliferative | 1.73 [1.50; 1.99] | 0.61 [0.47; 0.76] | 60 [47; 73] |
| Secretory | 2.43 [2.16; 2.72] | 1.37 [1.01; 1.65] | 51 [37; 66] |
| Atrophic | 2.96 [2.66; 3.26] | 0.75 [0.56; 1.01] | 69 [31; 85] |
| Endometrial hyperplasia | |||
| Non-atypical | 3.16 [2.69; 3.30] | 2.15 [1.62; 2.53] | 25 [6; 34] |
| EIN | 3.14 [2.90; 3.32] | 2.02 [1.69; 2.72] | 172 [124; 305] |
| EC | |||
| Low-grade | 5.37 [5.14; 6.07] | 4.56 [3.45; 5.19] | 311 [192; 497] |
| High-grade | 5.20 [4.88; 5.93] | 3.88 [3.06; 4.70] | 414 [318; 635] |
| Clear cell | 3.25 [2.83; 3.46] | 2.32 [1.98; 2.68] | 327 [273; 468] |
| Serous | 5.33 [5.19; 5.61] | 2.37 [1.95; 3.04] | 343 [239; 524] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plekhanov, A.A.; Grechkanev, G.O.; Avetisyan, E.A.; Loginova, M.M.; Kiseleva, E.B.; Shepeleva, A.A.; Moiseev, A.A.; Sovetsky, A.A.; Gubarkova, E.V.; Anina, A.A.; et al. Quantitative Assessment of Polarization and Elastic Properties of Endometrial Tissue for Precancer/Cancer Diagnostics Using Multimodal Optical Coherence Tomography. Diagnostics 2024, 14, 2131. https://doi.org/10.3390/diagnostics14192131
Plekhanov AA, Grechkanev GO, Avetisyan EA, Loginova MM, Kiseleva EB, Shepeleva AA, Moiseev AA, Sovetsky AA, Gubarkova EV, Anina AA, et al. Quantitative Assessment of Polarization and Elastic Properties of Endometrial Tissue for Precancer/Cancer Diagnostics Using Multimodal Optical Coherence Tomography. Diagnostics. 2024; 14(19):2131. https://doi.org/10.3390/diagnostics14192131
Chicago/Turabian StylePlekhanov, Anton A., Gennady O. Grechkanev, Elena A. Avetisyan, Maria M. Loginova, Elena B. Kiseleva, Anastasia A. Shepeleva, Alexander A. Moiseev, Alexander A. Sovetsky, Ekaterina V. Gubarkova, Anastasia A. Anina, and et al. 2024. "Quantitative Assessment of Polarization and Elastic Properties of Endometrial Tissue for Precancer/Cancer Diagnostics Using Multimodal Optical Coherence Tomography" Diagnostics 14, no. 19: 2131. https://doi.org/10.3390/diagnostics14192131
APA StylePlekhanov, A. A., Grechkanev, G. O., Avetisyan, E. A., Loginova, M. M., Kiseleva, E. B., Shepeleva, A. A., Moiseev, A. A., Sovetsky, A. A., Gubarkova, E. V., Anina, A. A., Shutova, A. M., Gamayunov, S. V., Gelikonov, G. V., Zaitsev, V. Y., Sirotkina, M. A., & Gladkova, N. D. (2024). Quantitative Assessment of Polarization and Elastic Properties of Endometrial Tissue for Precancer/Cancer Diagnostics Using Multimodal Optical Coherence Tomography. Diagnostics, 14(19), 2131. https://doi.org/10.3390/diagnostics14192131










