Diagnostic Challenges and Pathogenetic Differences in Biomass-Smoke-Induced versus Tobacco-Smoke-Induced COPD: A Comparative Review
Abstract
1. Introduction
Biomass-Smoke-Induced COPD
2. Comparative Analysis of Biomass-Smoke-Induced and Tobacco-Smoke-Induced COPD: Clinical Profiles, Pulmonary Function, and Public Health Implications (Figure 1)
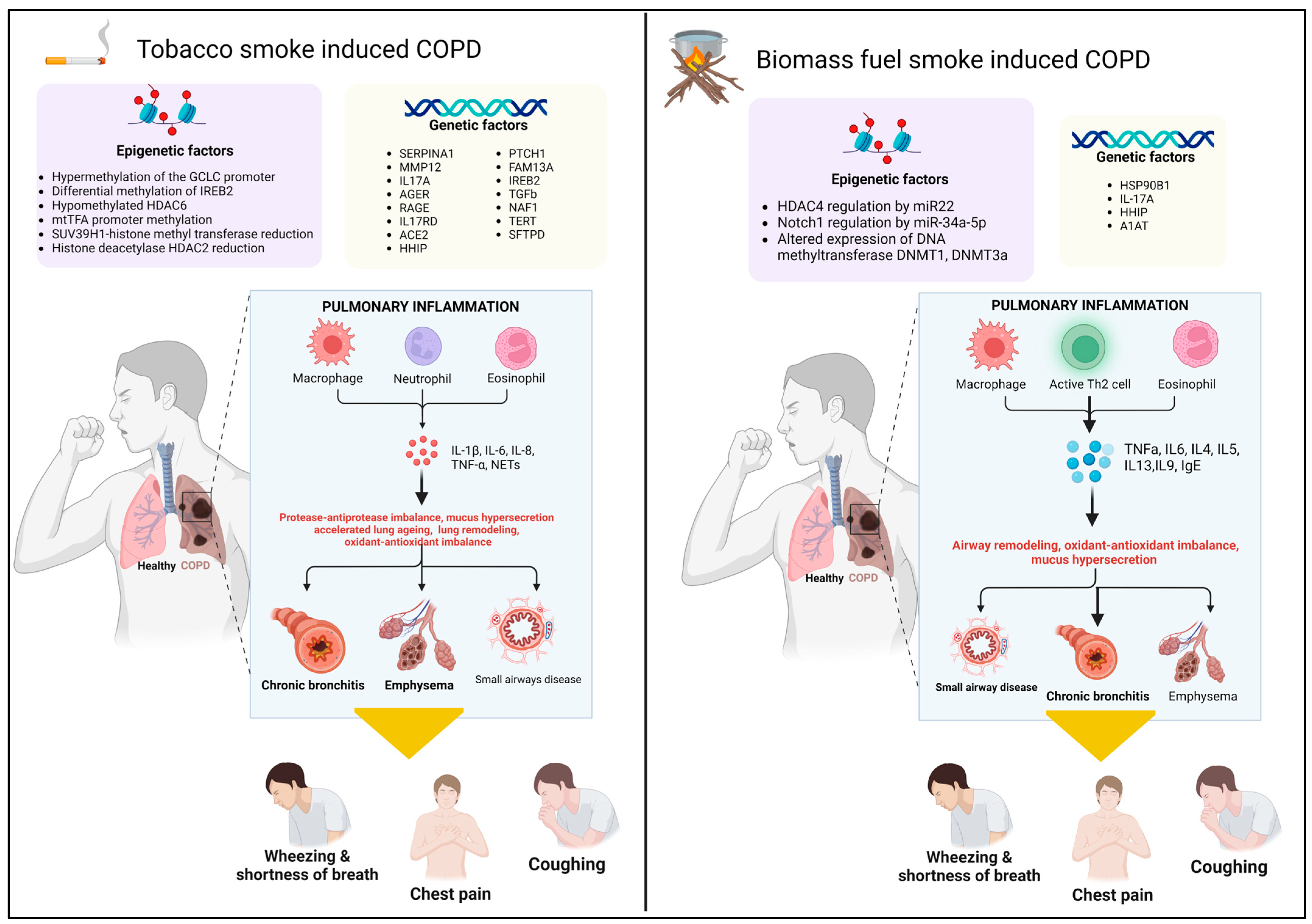
3. Genetic and Epigenetic Factors in COPD Pathogenesis: Cigarette Smoke vs. Biomass Smoke
3.1. Genetic Factors
3.2. Epigenetic Factors
| Gene | Function/Role in COPD | Association with Tobacco-Smoke-Induced COPD | Association with Biomass-Smoke-Induced COPD |
|---|---|---|---|
| SERPINA1 | Encodes α1-antitrypsin, inhibits neutrophil elastase | Deficiency leads to increased risk of emphysema, especially with Z allele [34] | Polymorphisms like PiS (rs17580) noted, severe deficiency less common [45] |
| MMP12 | Degrades elastin, contributing to lung tissue remodeling | Polymorphisms linked to increased susceptibility to emphysema [34] | Not specifically studied in biomass-exposed populations |
| AGER | Encodes receptor for advanced glycation end products (RAGE) | Elevated levels correlate with disease severity and neutrophil recruitment [35] | Not specifically studied in biomass-exposed populations |
| IL-17A | Mediates chronic inflammation and neutrophilia | Associated with inflammation and COPD [36,37] | Polymorphisms (rs2275913, rs8193036) linked to increased risk [43] |
| ACE2 | Receptor for SARS-CoV-2, involved in lung pathology | Upregulated in COPD, suggesting higher COVID-19 susceptibility [38] | The rs3134940-TC genotype of ACE2 had lower sRAGE levels [39] |
| HHIP | Regulates hedgehog signaling, critical for lung development | Protective variants identified, linked to decreased risk [37] | SNPs like rs13118928 and rs1828591 significant in biomass smoke exposure [44] |
| FAM13A | Affects Wnt/β-catenin signaling and fatty acid oxidation | Variants linked to COPD susceptibility [37] | Not specifically studied in biomass-exposed populations |
| IREB2 | Involved in iron metabolism, influencing mitochondrial function | Differential methylation observed, independent of smoking [50] | Not specifically studied in biomass-exposed populations |
| TGFβ2 | Part of the TGFβ pathway, involved in lung tissue repair and inflammation | Less studied in COPD compared to TGFβ1, but implicated [37] | Not specifically studied in biomass-exposed populations |
| NAF1, TERT, TR | Involved in telomere maintenance, linked to early-onset emphysema | Mutations associated with telomere shortening and early COPD onset [40] | Not specifically studied in biomass-exposed populations |
| SFTPD | Surfactant protein D, involved in lung immune response | Associated with COPD risk even in non-smokers [37,41] | Not specifically studied in biomass-exposed populations |
| HSP90B1 | Heat shock protein, involved in protein folding and stress response | Not specifically studied in tobacco smoke COPD | Variant rs2070908 associated with decreased risk of COPD [42] |
| TNF | Encodes TNF-α, a pro-inflammatory cytokine | Polymorphisms associated with smoking-related COPD [46] | No significant association observed with biomass-smoke-induced COPD [46] |
4. A Comparative Analysis of Inflammatory Cells and Mediators in Tobacco Smoke and Biomass Smoke COPD
5. Eosinophilic COPD in Biomass Smoke and Tobacco Smoke: A Comparative Analysis of Inflammatory Profiles and Clinical Implications
6. Oxidative Stress in COPD: Comparing Biomass-Smoke- and Tobacco-Smoke-Induced COPD
6.1. Cellular Responses (Figure 1)
6.2. Oxidative Stress Mechanisms
7. Protease–Antiprotease Imbalance in COPD: A Comparative Analysis of TSCOPD and the Emerging BSCOPD
8. Mucus Hypersecretion in COPD: Tobacco Smoke vs. Biomass Smoke Exposure
9. Airway Remodeling in Biomass Smoke vs. Tobacco Smoke COPD
10. Accelerated Lung Aging in COPD: Tobacco Smoke vs. Biomass Smoke
10.1. Tobacco-Smoke-Induced COPD (TSCOPD) and Accelerated Lung Aging
10.2. Biomass-Smoke-Induced COPD (BSCOPD) and the Need for Research
11. Mechanism of COPD Exacerbation: A Comparative Analysis of TSCOPD and BSCOPD
12. Treatment Recommendations for BSCOPD [16]
- Bronchodilators: these are fundamental for relieving symptoms and improving lung function.
- Pulmonary rehabilitation: a comprehensive program designed to enhance physical fitness and overall quality of life.
- Oxygen therapy: essential for patients experiencing hypoxemia to ensure adequate oxygenation.
- Antibiotics: prescribed during infectious exacerbations to combat respiratory infections.
- Vaccinations: immunization against influenza and pneumococcus is highly recommended to prevent complications.
13. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Chronic Obstructive Pulmonary Disease (COPD). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 20 August 2024).
- The Global Initiative for Chronic Obstructive Lung Diseases. Global Strategy for Diagnosis, Management and Prevention of COPD 2020 Report. 2020. Available online: https://goldcopd.org/gold-reports/ (accessed on 20 August 2024).
- Wang, H.; Meng, R.; Wang, X.; Si, Z.; Zhao, Z.; Lu, H.; Wang, H.; Hu, J.; Zheng, Y.; Chen, J.; et al. A nested case-control study of the effects of dust exposure and smoking on COPD in coal workers. BMC Public Health 2023, 23, 2056. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Janssen, N.; Heederik, D.J.; Smit, L.A.; Vermeulen, R.; Huss, A. Residential proximity to livestock animals and mortality from respiratory diseases in The Netherlands: A prospective census-based cohort study. Environ. Int. 2022, 161, 107140. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Townend, J.; Minelli, C.; Mortimer, K.; Obaseki, D.O.; Al Ghobain, M.; Cherkaski, H.; Denguezli, M.; Gunesekera, K.; Hafizi, H.; Koul, P.A.; et al. The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur. Respir. J. 2017, 49, 1601880. [Google Scholar] [CrossRef]
- Salvi, S.; Barnes, P.J. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest 2010, 138, 3–6. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Capistrano, S.J.; van Reyk, D.; Chen, H.; Oliver, B.G. Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD. Toxics 2017, 5, 36. [Google Scholar] [CrossRef]
- Behera, D.; Jindal, S.K. Respiratory symptoms in Indian women using domestic cooking fuels. Chest 1991, 100, 385–388. [Google Scholar] [CrossRef]
- Ortiz-Quintero, B.; Martínez-Espinosa, I.; Pérez-Padilla, R. Mechanisms of Lung Damage and Development of COPD Due to Household Biomass-Smoke Exposure: Inflammation, Oxidative Stress, MicroRNAs, and Gene Polymorphisms. Cells 2022, 12, 67. [Google Scholar] [CrossRef]
- Perret, J.L.; Abramson, M.J. Biomass smoke COPD: A phenotype or a different disease? Respirology 2018, 23, 124–125. [Google Scholar] [CrossRef]
- Ntritsos, G.; Franek, J.; Belbasis, L.; Christou, M.A.; Markozannes, G.; Altman, P.; Fogel, R.; Sayre, T.; Ntzani, E.E.; Evangelou, E. Gender-specific estimates of COPD prevalence: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Samet, J.M.; Romieu, I.; Bruce, N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax 2000, 55, 518–532. [Google Scholar] [CrossRef]
- Han, M.K.; Martinez, F.J. Host, Gender, and Early-Life Factors as Risks for Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2020, 41, 329–337. [Google Scholar] [CrossRef]
- Pérez-Padilla, R.; Ramirez-Venegas, A.; Sansores-Martinez, R. Clinical Characteristics of Patients with Biomass Smoke-Associated COPD and Chronic Bronchitis, 2004–2014. Chronic Obstr. Pulm. Dis. 2014, 1, 23–32. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.J.; Jung, C.Y.; Shin, H.W.; Lee, B.K. Biomass smoke induced bronchial anthracofibrosis: Presenting features and clinical course. Respir. Med. 2009, 103, 757–765. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, M.; Maldonado Gomez, D.; Torres-Duque, C.A.; Barrero, M.; Jaramillo Villegas, C.; Perez, J.M.; Varon, H. Tomographic and functional findings in severe COPD: Comparison between the wood smoke-related and smokingrelated disease. J. Bras. Pneumol. Publicacao. Soc. Bras. Pneumol. Tisilogia 2013, 39, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Gulati, N.; Fernandes, Y.; Mesquita, A.M.; Sardessai, M.; Lammers, J.W.J.; Hoesein, F.A.M.; Ten Hacken, N.H.T.; van den Berge, M.; Galbán, C.J.; et al. Small airway imaging phenotypes in biomass-and tobacco smoke-exposed patients with COPD. ERJ Open Res. 2017, 3, 00124-2016. [Google Scholar] [CrossRef] [PubMed]
- Sertogullarindan, B.; Gumrukcuoglu, H.A.; Sezgi, C.; Akil, M.A. Frequency of pulmonary hypertension in patients with COPD due to biomass smoke and tobacco smoke. Int. J. Med. Sci. 2012, 9, 406–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brashier, B.; Kajale, S.; Tambe, S.; Kodgule, R.; Londhe, J.; Shetty, D.; Madas, S.; Barnes, P.; Salvi, S. High resolution CT scan (HRCT) thorax differences between biomass-smoke exposure induced COPD (BM COPD) and tobacco-smoking COPD (TS COPD). Eur. Respir. J. 2012, 40 (Suppl. S56), P268. [Google Scholar]
- Moreira, M.A.; Barbosa, M.A.; Queiroz, M.C.; Teixeira, K.I.; Torres, P.P.; Santana Júnior, P.J.; Montadon Júnior, M.E.; Jardim, J.R. Pulmonary changes on HRCT scans in nonsmoking females with COPD due to wood smoke exposure. J. Bras Pneumol. 2013, 39, 155–163. [Google Scholar] [CrossRef]
- Camp, P.G.; Ramirez-Venegas, A.; Sansores, R.H.; Alva, L.F.; McDougall, J.E.; Sin, D.D.; Paré, P.D.; Müller, N.L.; Silva, C.I.; Rojas, C.E.; et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur. Respir. J. 2014, 43, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, O.P.; Semple, S.; Simkhada, P.; Smith, W.C.; Ayres, J.G. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: A systematic review and meta-analysis. Thorax 2010, 65, 221–228. [Google Scholar] [CrossRef]
- Regalado, J.; Pérez-Padilla, R.; Sansores, R.; Páramo Ramirez, J.I.; Brauer, M.; Paré, P.; Vedal, S. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am. J. Respir. Crit. Care Med. 2006, 174, 901–905. [Google Scholar] [CrossRef] [PubMed]
- González-García, M.; Torres-Duque, C.A.; Bustos, A.; Jaramillo, C.; Maldonado, D. Bronchial hyperresponsiveness in women with chronic obstructive pulmonary disease related to wood smoke. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Venegas, A.; Sansores, R.H.; Pérez-Padilla, R.; Regalado, J.; Velázquez, A.; Sánchez, C.; Mayar, M.E. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am. J. Respir. Crit. Care Med. 2006, 173, 393–397. [Google Scholar] [CrossRef]
- Moran-Mendoza, O.; Pérez-Padilla, J.R.; Salazar-Flores, M.; Vazquez-Alfaro, F. Wood smoke-associated lung disease: A clinical, functional, radiological and pathological description. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis. 2008, 12, 1092–1098. [Google Scholar]
- Ramirez-Venegas, A.; Sansores, R.H.; Quintana-Carrillo, R.H.; Velazquez-Uncal, M.; Hernandez-Zenteno, R.J.; Sanchez-Romero, C.; Velazquez-Montero, A.; Flores-Trujillo, F. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am. J. Respir. Crit. Care Med. 2014, 190, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.; Salas, J.; Martinez-Guerra, M.L.; Gómez, A.; Martinez, C.; Portales, A.; Palomar, A.; Villegas, M.; Barrios, R. Pulmonary arterial hypertension and cor pulmonale associated with chronic domestic woodsmoke inhalation. Chest 1993, 103, 12–20. [Google Scholar] [CrossRef]
- Golpe, R.; Sanjuán López, P.; Cano Jiménez, E.; Castro Añón, O.; Pérez de Llano, L.A. Distribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smoke. Arch. Bronconeumol. 2014, 50, 318–324. [Google Scholar] [CrossRef]
- Meneghini, A.C.; Koenigkam-Santos, M.; Pereira, M.C.; Tonidandel, P.R.; Terra-Filho, J.; Cunha, F.Q.; Menezes, M.B.; Vianna, E.O. Biomass smoke COPD has less tomographic abnormalities but worse hypoxemia compared with tobacco COPD. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Medicas Biol. 2019, 52, e8233. [Google Scholar] [CrossRef]
- Sana, A.; Somda, S.M.A.; Meda, N.; Bouland, C. Chronic obstructive pulmonary disease associated with biomass fuel use in women: A systematic review and meta-analysis. BMJ Open Respir. Res. 2018, 5, e000246. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Chang, D.; Lu, G.; Deng, X. Genetic polymorphism and chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cho, M.H.; Zhou, X. What do polymorphisms tell us about the mechanisms of COPD? Clin. Sci. 2017, 131, 2847–2863. [Google Scholar] [CrossRef]
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K.; et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Mellett, M.; Atzei, P.; Horgan, A.; Hams, E.; Floss, T.; Wurst, W.; Fallon, P.G.; Moynagh, P.N. Orphan receptor IL-17RD tunes IL-17A signalling and is required for neutrophilia. Nat. Commun. 2012, 3, 1119. [Google Scholar] [CrossRef]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed]
- Fricke-Galindo, I.; García-Carmona, S.; Alanis-Ponce, J.; Pérez-Rubio, G.; Ramírez-Venegas, A.; Montiel-Lopez, F.; Robles-Hernández, R.; de Jesús Hernández-Zenteno, R.; Rea, D.V.-P.; Bautista-Becerril, B.; et al. sRAGE levels are decreased in plasma and sputum of COPD secondary to biomass-burning smoke and tobacco smoking: Differences according to the rs3134940 AGER variant. Heliyon 2024, 10, e28675. [Google Scholar] [CrossRef]
- Stanley, S.E.; Merck, S.J.; Armanios, M. Telomerase and the Genetics of Emphysema Susceptibility. Implications for Pathogenesis Paradigms and Patient Care. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S447–S451. [Google Scholar] [CrossRef]
- Obeidat, M.; Li, X.; Burgess, S.; Zhou, G.; Fishbane, N.; Hansel, N.N.; Bossé, Y.; Joubert, P.; Hao, K.; Nickle, D.C.; et al. Surfactant protein D is a causal risk factor for COPD: Results of Mendelian randomisation. Eur. Respir. J. 2017, 50, 1700657. [Google Scholar] [CrossRef]
- Ambrocio-Ortiz, E.; Perez-Rubio, G.; Ramirez-Venegas, A.; Hernandez-Zenteno, R.J.; Paredes-Lopez, A.; Sansores, R.H.; Ramirez-Diaz, M.E.; Cruz-Vicente, F.; Martinez-Gomez, M.L.; Falfan-Valencia, R. Protective Role of Genetic Variants in HSP90 Genes-Complex in COPD Secondary to Biomass-Burning Smoke Exposure and Non-Severe COPD Forms in Tobacco Smoking Subjects. Curr. Issues Mol. Biol. 2021, 43, 887–899. [Google Scholar] [CrossRef]
- Ponce-Gallegos, M.A.; Ramírez-Venegas, A.; Falfán-Valencia, R. Th17 profile in COPD exacerbations. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martinez, A.; Perez-Rubio, G.; Ramirez-Venegas, A.; Ramirez-Diaz, M.E.; Cruz-Vicente, F.; Martinez-Gomez, M.L.; Ramos-Martinez, E.; Abarca-Rojano, E.; Falfan-Valencia, R. Participation of HHIP Gene Variants in COPD Susceptibility, Lung Function, and Serum and Sputum Protein Levels in Women Exposed to Biomass-Burning Smoke. Diagnostics 2020, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Gallegos, M.A.; Perez-Rubio, G.; Garcia-Carmona, A.; Garcia-Gomez, J.; Hernandez-Zenteno, R.; Ramirez-Venegas, A.; Falfan-Valencia, R. Haplotype in SERPINA1 (AAT) Is Associated with Reduced Risk for COPD in a Mexican Mestizo Population. Int. J. Mol. Sci. 2019, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Resendiz-Hernandez, J.M.; Ambrocio-Ortiz, E.; Perez-Rubio, G.; Lopez-Flores, L.A.; Abarca-Rojano, E.; Pavon-Romero, G.F.; Flores-Trujillo, F.; de Jesus Hernandez-Zenteno, R.; Camarena, A.; Perez-Rodriguez, M.; et al. TNF promoter polymorphisms areassociated with genetic susceptibility in COPD secondary to tobacco smoking and biomass burning. Int. J. Chron. Obs. Pulmon. Dis. 2018, 13, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Ortega, V.E. Picking the Right Fruit: Intersecting Chronic Obstructive Pulmonary Disease Genome-Wide Association Study Discoveries with Epigenetics. Am. J. Respir. Crit. Care Med. 2018, 197, 1237–1239. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, J.; Li, B.; Liu, S.; Li, X.; Tu, H. Cigarette Smoke-Induced Hypermethylation of the GCLC Gene Is Associated with COPD. Chest 2016, 149, 474–482. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Nedeljkovic, I.; van der Plaat, D.A.; Zhernakova, A.; Lahousse, L.; Brusselle, G.G.; BIOS Consortium; Amin, N.; van Duijn, C.M.; Vonk, J.M.; et al. DNA methylation is associated with lung function in never smokers. Respir. Res. 2019, 20, 268. [Google Scholar]
- Nedeljkovic, I.; Carnero-Montoro, E.; Lahousse, L.; van der Plaat, D.A.; de Jong, K.; Vonk, J.M.; van Diemen, C.C.; Faiz, A.; van den Berge, M.; Obeidat, M.; et al. Understanding the role of the chromosome 15q25.1 in COPD through epigenetics and transcriptomics. Eur. J. Hum. Genet. EJHG 2018, 26, 709–722. [Google Scholar] [CrossRef]
- Peng, H.; Guo, T.; Chen, Z.; Zhang, H.; Cai, S.; Yang, M.; Chen, P.; Guan, C.; Fang, X. Hypermethylation of mitochondrial transcription factor A induced by cigarette smoke is associated with chronic obstructive pulmonary disease. Exp. Lung Res. 2019, 45, 101–111. [Google Scholar] [CrossRef]
- Chen, T.T.; Wu, S.M.; Ho, S.C.; Chuang, H.C.; Liu, C.Y.; Chan, Y.F.; Kuo, L.W.; Feng, P.H.; Liu, W.T.; Chen, K.Y.; et al. SUV39H1 Reduction Is Implicated in Abnormal Inflammation in COPD. Sci. Rep. 2017, 7, 46667. [Google Scholar] [CrossRef]
- Barnes, P.J. Role of HDAC2 in the pathophysiology of COPD. Annu. Rev. Physiol. 2009, 71, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, C.; Schuoler, C.; Bye, H.; Mignan, C.; Rechsteiner, T.; Hillinger, S.; Opitz, I.; Marsland, B.; Faiz, A.; Hiemstra, P.S.; et al. MicroRNA-223 controls the expression of histone deacetylase 2: A novel axis in COPD. J. Mol. Med. 2016, 94, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Gallegos, M.A.; Perez-Rubio, G.; Ambrocio-Ortiz, E.; Partida-Zavala, N.; Hernandez-Zenteno, R.; Flores-Trujillo, F.; Garcia-Gomez, L.; Hernandez-Perez, A.; Ramirez-Venegas, A.; Falfan-Valencia, R. Genetic variants in IL17A and serum levels of IL-17A are associated with COPD related to tobacco smoking and biomass burning. Sci. Rep. 2020, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11 (Suppl. S17), S2129–S2140. [Google Scholar] [CrossRef] [PubMed]
- Golpe, R.; Martín-Robles, I.; Sanjuán-López, P.; Pérez-de-Llano, L.; González-Juanatey, C.; López-Campos, J.L.; Arellano-Orden, E. Differences in systemic inflammation between cigarette and biomass smoke-induced COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2639–2646. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Quintana-Carrillo, R.; Falfán-Valencia, R.; Vargas-Rojas, M.I. Chronic obstructive pulmonary disease induced by exposure to biomass smoke is associated with a Th2 cytokine production profile. Clin. Immunol. 2015, 161, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Eeden, S. Lung Macrophage Phenotypes and Functional Responses: Role in the Pathogenesis of COPD. Int. J. Mol. Sci. 2018, 19, 582. [Google Scholar] [CrossRef]
- Eapen, M.S.; Myers, S.; Walters, E.H.; Sohal, S.S. Airway inflammation in chronic obstructive pulmonary disease (COPD): A true paradox. Expert Rev. Respir. Med. 2017, 11, 827–839. [Google Scholar] [CrossRef]
- Bruzzaniti, S.; Bocchino, M.; Santopaolo, M.; Calì, G.; Stanziola, A.A.; D’Amato, M.; Esposito, A.; Barra, E.; Garziano, F.; Micillo, T.; et al. An immunometabolic pathomechanism for chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2019, 116, 15625–15634. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T. Immune checkpoints in chronic obstructive pulmonary disease. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2017, 26, 170045. [Google Scholar] [CrossRef]
- Wen, L.; Krauss-Etschmann, S.; Petersen, F.; Yu, X. Autoantibodies in Chronic Obstructive Pulmonary Disease. Front. Immunol. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Vishweswaraiah, S.; Thimraj, T.A.; George, L.; Krishnarao, C.S.; Lokesh, K.S.; Siddaiah, J.B.; Ganguly, K. Putative Systemic Biomarkers of Biomass Smoke-Induced Chronic Obstructive Pulmonary Disease among Women in a Rural South Indian Population. Dis. Markers 2018, 2018, 4949175. [Google Scholar] [CrossRef] [PubMed]
- Falfán-Valencia, R.; Ramírez-Venegas, A.; Pérez Lara-Albisua, J.L.; Ramírez-Rodriguez, S.L.; Márquez-García, J.E.; Buendía-Roldan, I.; Gayosso-Gómez, L.V.; Pérez-Padilla, R.; Ortiz-Quintero, B. Smoke exposure from chronic biomass burning induces distinct accumulative systemic inflammatory cytokine alterations compared to tobacco smoking in healthy women. Cytokine 2020, 131, 155089. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.F. Eosinophil Biology in COPD. N. Engl. J. Med. 2017, 377, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kolsum, U.; Brightling, C.E.; Locantore, N.; Agusti, A.; Tal-Singer, R.; ECLIPSE Investigators. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Brightling, C.E. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016, 7, 34–51. [Google Scholar] [CrossRef]
- Tworek, D.; Antczak, A. Eosinophilic COPD—A distinct phenotype of the disease. Adv. Respir. Med. 2017, 85, 271–276. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory endotypes in COPD. Allergy 2019, 74, 1249–1256. [Google Scholar] [CrossRef]
- Doyle, A.D.; Mukherjee, M.; LeSuer, W.E.; Bittner, T.B.; Pasha, S.M.; Frere, J.J.; Ho, T. Eosinophil-derived IL-13 promotes emphysema. Eur. Respir. J. 2019, 53, 1801291. [Google Scholar] [CrossRef]
- Fernandes, L.; Rane, S.; Mandrekar, S.; Mesquita, A.M. Eosinophilic Airway Inflammation in Patients with Stable Biomass Smoke-versus Tobacco Smoke-Associated Chronic Obstructive Pulmonary Disease. J. Health Pollut. 2019, 9, 191209. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, D.K.; Leem, J.H.; Kim, H.C. Associations between Ambient Particulate Matter and Nitrogen Dioxide and Chronic Obstructive Pulmonary Diseases in Adults and Effect Modification by Demographic and Lifestyle Factors. Int. J. Environ. Res. Public Health 2018, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Manullang, A.; Chung, C.L.; Lee, Y.L.; Yuan, T.H.; Tran, H.M.; Makrufardi, F.; Chung, K.F.; Lee, K.Y.; Chang, J.H.; Chuang, H.C. COPD with Eosinophilic Inflammation is Susceptible to Particulate Air Pollution Exposure. Aerosol Air Qual. Res. 2023, 23, 230035. [Google Scholar] [CrossRef]
- García Morales, O.M.; Cañas-Arboleda, A.; Rodríguez Malagón, M.N.; Galindo Pedraza, J.L.; Rodríguez Torres, P.; Avendaño Morales, V.R.; González-Rangel, A.L.; Celis-Preciado, C.A. Blood eosinophils levels in a Colombian cohort of biomass-and tobacco-related COPD patients. Front. Med. 2024, 11, 1321371. [Google Scholar] [CrossRef]
- Yun, J.H.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.M.; Saferali, A.; Vestbo, J.; Tal-Singer, R.; Castaldi, P.J.; Silverman, E.K.; et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047.e10. [Google Scholar] [CrossRef]
- Uribe Echevarría, L.; Leimgruber, C.; García González, J.; Nevado, A.; Álvarez, R.; García, L.N.; Quintar, A.A.; Maldonado, C.A. Evidence of eosinophil extracellular trap cell death in COPD: Does it represent the trigger that switches on the disease? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 885–896. [Google Scholar] [CrossRef]
- Dutta, A.; Ray, M.R.; Banerjee, A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol. Appl. Pharmacol. 2012, 261, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Li, C.; Fang, Z.; Levin-Zaidman, S.; Dezorella, N.; Czech, H.; Martens, P.; Käfer, U.; Gröger, T.; Rüger, C.P.; et al. Toxicity of Water- and Organic-Soluble Wood Tar Fractions from Biomass Burning in Lung Epithelial Cells. Chem. Res. Toxicol. 2021, 34, 1588–1603. [Google Scholar] [CrossRef]
- Cantin, A.M. Cellular response to cigarette smoke and oxidants: Adapting to survive. Proc. Am. Thorac. Soc. 2010, 7, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A.; El Sayed, I.E.T.; Ali, M.M.; Wang, Y.; El Baz, A.; Elarabany, N.; Khalil, A.A. Oxidative stress and inflammation: Elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bull. Natl. Res. Cent. 2024, 48, 16. [Google Scholar] [CrossRef]
- Pérez-Ríos, M.; López-Medina, D.C.; Guerra-Tort, C.; Rey-Brandariz, J.; Varela-Lema, L.; Santiago-Pérez, M.I.; Candal, C.; Montes, A.; López, M.J.; Dalmau, R.; et al. Mortality Attributable to Environmental Tobacco Smoke Exposure in Spain in 2020. Arch. Bronconeumol. 2023, 59, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Cho, C.Y.; Thimmulappa, R.K.; Zhen, L.; Srisuma, S.S.; Kensler, T.W.; Yamamoto, M.; Petrache, I.; Tuder, R.M.; Biswal, S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J. Clin. Investig. 2004, 114, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxidative Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef]
- Fischer, B.M.; Voynow, J.A.; Ghio, A.J. COPD: Balancing oxidants and antioxidants. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T.; et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019, 10, 3145. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Thimmulappa, R.; Thomas, C.M.; Fenwick, P.S.; Chana, K.K.; Donnelly, L.E.; Biswal, S.; Ito, K.; Barnes, P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011, 406, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; Manicone, A.M.; Parks, W.C. Matrix metalloproteinases in emphysema. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 73, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U.; van Overveld, F.J. Role of elastases in the pathogenesis of chronic obstructive pulmonary disease: Implications for treatment. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 27–35. [Google Scholar] [CrossRef]
- Dey, T.; Kalita, J.; Weldon, S.; Taggart, C.C. Proteases and their inhibitors in chronic obstructive pulmonary disease. J. Clin. Med. 2018, 7, 244. [Google Scholar] [CrossRef]
- Greene, C.M.; McElvaney, N.G. Proteases and antiproteases in chronic neutrophilic lung disease—Relevance to drug discovery. Br. J. Pharmacol. 2009, 158, 1048–1058. [Google Scholar] [CrossRef]
- Sun, J.; Bao, J.; Shi, Y.; Zhang, B.; Yuan, L.; Li, J.; Zhang, L.; Sun, M.; Zhang, L.; Sun, W. Effect of simvastatin on MMPs and TIMPs in cigarette smoke-induced rat COPD model. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 717–724. [Google Scholar] [CrossRef]
- Polverino, F.; Rojas-Quintero, J.; Wang, X.; Petersen, H.; Zhang, L.; Gai, X.; Higham, A.; Zhang, D.; Gupta, K.; Rout, A.; et al. A Disintegrin and Metalloproteinase Domain-8: A Novel Protective Proteinase in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Chillappagari, S.; Preuss, J.; Licht, S.; Müller, C.; Mahavadi, P.; Sarode, G.; Vogelmeier, C.; Guenther, A.; Nahrlich, L.; Rubin, B.K.; et al. Altered protease and antiprotease balance during a COPD exacerbation contributes to mucus obstruction. Respir. Res. 2015, 16, 85. [Google Scholar] [CrossRef]
- Martin, C.; Frija-Masson, J.; Burgel, P.R. Targeting mucus hypersecretion: New therapeutic opportunities for COPD? Drugs 2014, 74, 1073–1089. [Google Scholar] [CrossRef]
- Shi, J.; Li, H.; Yuan, C.; Luo, M.; Wei, J.; Liu, X. Cigarette smoke-induced acquired dysfunction of cystic fibrosis transmembrane conductance regulator in the pathogenesis of chronic obstructive pulmonary disease. Oxidative Med. Cell. Longev. 2018, 2018, 6567578. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Chang, E.H.; Chen, Y.; Lu, W.; Kim, M.M.; Niihori, M.; Kim, K.C. MUC1 contributes to goblet cell metaplasia and MUC5AC expression in response to cigarette smoke in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L82–L90. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Rahman, M.; Rinaldi, S.; Koelmel, J.; Lin, E.Z.; Mahesh, P.A.; Ganguly, K. Assessment of wood smoke induced pulmonary toxicity in normal-and chronic bronchitis-like bronchial and alveolar lung mucosa models at air–liquid interface. Respir. Res. 2024, 25, 49. [Google Scholar] [CrossRef]
- Tassew, D.; Fort, S.; Mebratu, Y.; McDonald, J.; Chu, H.W.; Petersen, H.; Tesfaigzi, Y. Effects of Wood Smoke Constituents on Mucin Gene Expression in Mice and Human Airway Epithelial Cells and on Nasal Epithelia of Subjects with a Susceptibility Gene Variant in Tp53. Environ. Health Perspect. 2022, 130, 17010. [Google Scholar] [CrossRef]
- Wang, X.; Polverino, F.; Rojas-Quintero, J.; Zhang, D.; Sánchez, J.; Yambayev, I.; Lindqvist, E.; Virtala, R.; Djukanovic, R.; Davies, D.E.; et al. A Disintegrin and A Metalloproteinase-9 (ADAM9): A Novel Proteinase Culprit with Multifarious Contributions to COPD. Am. J. Respir. Crit. Care Med. 2018, 198, 1500–1518. [Google Scholar] [CrossRef]
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.M.; Herrera, A.M. Fibrotic response of tissue remodeling in COPD. Lung 2011, 189, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ling, M.; Xue, J.; Dai, X.; Sun, Q.; Chen, C.; Liu, Y.; Zhou, L.; Liu, J.; Luo, F.; et al. Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics 2018, 8, 5419–5433. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Venegas, A.; Torres-Duque, C.A.; Guzmán-Bouilloud, N.E.; González-García, M.; Sansores, R.H. Small airway disease in COPD associated to biomass exposure. Rev. Investig. Clínica 2019, 71, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Assad, N.A.; Balmes, J.; Mehta, S.; Cheema, U.; Sood, A. Chronic obstructive pulmonary disease secondary to household air pollution. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2015; Volume 36, pp. 408–421. [Google Scholar]
- Zhao, D.; Zhou, Y.; Jiang, C.; Zhao, Z.; He, F.; Ran, P. Small airway disease: A different phenotype of early stage COPD associated with biomass smoke exposure. Respirology 2018, 23, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Cosio, M.G.; Ghezzo, H.; Salazar, M.; Perez-Padilla, R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int. J. Tuberc. Lung Dis. 2008, 12, 972–977. [Google Scholar]
- Chen, J.; Jiang, C.; Zheng, Y.; Zhao, D.; Wu, F.; Zhao, Z.; Zhao, J.; Li, Q.; Li, B.; Peng, G.; et al. Lung Features in Individuals with Biomass Smoke Exposure Characterized by CT Scan and Changes in Pulmonary Function. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2575–2584. [Google Scholar] [CrossRef]
- Li, S.; Qu, L.; Zhou, L.; Zhan, N.; Liu, L.; Ling, Y.; Li, J. Biomass fuels related-PM2. 5 promotes lung fibroblast-myofibroblast transition through PI3K/AKT/TRPC1 pathway. Ecotoxicol. Environ. Saf. 2024, 276, 116309. [Google Scholar] [CrossRef]
- Vij, N.; Chandramani-Shivalingappa, P.; Van Westphal, C.; Hole, R.; Bodas, M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol. Cell Physiol. 2018, 314, C73–C87. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.; Sundar, I.K.; Gerloff, J.; Li, D.; Rahman, I. Lung cellular senescence is independent of aging in a mouse model of COPD/emphysema. Sci. Rep. 2018, 8, 9023. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, C.A.; de Vries, M.; Costa, R.; Woldhuis, R.R.; Königshoff, M.; Timens, W. Lung ageing and COPD: Is there a role for ageing in abnormal tissue repair? Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2017, 26, 170073. [Google Scholar] [CrossRef]
- Houssaini, A.; Breau, M.; Kebe, K.; Abid, S.; Marcos, E.; Lipskaia, L.; Rideau, D.; Parpaleix, A.; Huang, J.; Amsellem, V.; et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 2018, 3, e93203. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Rashid, K.; Gerloff, J.; Rangel-Moreno, J.; Li, D.; Rahman, I. Genetic ablation of histone deacetylase 2 leads to lung cellular senescence and lymphoid follicle formation in COPD/emphysema. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4955–4971. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Lee, C.G.; Kim, L.K. COPD as a Disease of Immunosenescence. Yonsei Med. J. 2019, 60, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, Q.; Javed, R.; Zhong, J.; Gao, H.; Liang, H. Effect of tobacco smoking on the epigenetic age of human respiratory organs. Clin. Epigenetics 2019, 11, 183. [Google Scholar] [CrossRef]
- Anzueto, A.; Miravitlles, M. Chronic Obstructive Pulmonary Disease Exacerbations: A Need for Action. Am. J. Med. 2018, 131, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dy, R.; Sethi, S. The lung microbiome and exacerbations of COPD. Curr. Opin. Pulm. Med. 2016, 22, 196–202. [Google Scholar] [CrossRef]
- Footitt, J.; Mallia, P.; Durham, A.L.; Ho, W.E.; Trujillo-Torralbo, M.B.; Telcian, A.G.; Del Rosario, A.; Chang, C.; Peh, H.Y.; Kebadze, T.; et al. Oxidative and Nitrosative Stress and Histone Deacetylase-2 Activity in Exacerbations of COPD. Chest 2016, 149, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Nachmias, N.; Langier, S.; Brzezinski, R.Y.; Siterman, M.; Stark, M.; Etkin, S.; Avriel, A.; Schwarz, Y.; Shenhar-Tsarfaty, S.; Bar-Shai, A. NLRP3 inflammasome activity is upregulated in an in-vitro model of COPD exacerbation. PLoS ONE 2019, 14, e0214622. [Google Scholar] [CrossRef]
- Cho, J.; Lee, C.H.; Hwang, S.S.; Kim, K.U.; Lee, S.H.; Park, H.Y.; Park, S.J.; Min, K.H.; Oh, Y.M.; Yoo, K.H.; et al. Risk of acute exacerbations in chronic obstructive pulmonary disease associated with biomass smoke compared with tobacco smoke. BMC Pulm. Med. 2019, 19, 68. [Google Scholar] [CrossRef]
| Radiological Feature | TSCOPD | BSCOPD | References |
|---|---|---|---|
| Emphysema | Predominantly emphysema-predominant phenotype, with a higher percentage of emphysema and larger emphysematous spaces. | Less emphysema overall; when present, centrilobular or panlobular patterns observed. Emphysema is not as predominant. | [21,22] |
| Air Trapping | Less prominent, though present. | More air trapping, indicating an airway-predominant phenotype. | [22] |
| Bronchial Wall Thickening | Present, with a trend towards thicker walls. | Significant bronchial wall thickening, especially in wood-smoke-induced COPD. | [21,23] |
| Bronchiectasis | Less common | Common in wood-smoke-induced COPD | [22] |
| Mosaic Perfusion Pattern, Parenchymal Bands, Tree-in-Bud Pattern, Laminar Atelectasis | Not typically reported | observed in wood-smoke-induced COPD | [22] |
| Lung Volumes | Generally larger lung volumes | Smaller lung volumes | [21] |
| Oxygen Saturation | Less impact on oxygen saturation during rest and exercise | Lower oxygen saturation at rest and during exercise, indicating worse hypoxemia. | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, J.; Singh, S.; Greeshma, M.V.; Mahesh, P.A.; Mabalirajan, U. Diagnostic Challenges and Pathogenetic Differences in Biomass-Smoke-Induced versus Tobacco-Smoke-Induced COPD: A Comparative Review. Diagnostics 2024, 14, 2154. https://doi.org/10.3390/diagnostics14192154
Dutta J, Singh S, Greeshma MV, Mahesh PA, Mabalirajan U. Diagnostic Challenges and Pathogenetic Differences in Biomass-Smoke-Induced versus Tobacco-Smoke-Induced COPD: A Comparative Review. Diagnostics. 2024; 14(19):2154. https://doi.org/10.3390/diagnostics14192154
Chicago/Turabian StyleDutta, Joytri, Sabita Singh, Mandya V. Greeshma, Padukudru Anand Mahesh, and Ulaganathan Mabalirajan. 2024. "Diagnostic Challenges and Pathogenetic Differences in Biomass-Smoke-Induced versus Tobacco-Smoke-Induced COPD: A Comparative Review" Diagnostics 14, no. 19: 2154. https://doi.org/10.3390/diagnostics14192154
APA StyleDutta, J., Singh, S., Greeshma, M. V., Mahesh, P. A., & Mabalirajan, U. (2024). Diagnostic Challenges and Pathogenetic Differences in Biomass-Smoke-Induced versus Tobacco-Smoke-Induced COPD: A Comparative Review. Diagnostics, 14(19), 2154. https://doi.org/10.3390/diagnostics14192154







