The Endoscopic Management of Zenker’s Diverticulum: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
3. Clinical Assessment
| Dysphagia | Regurgitation | Weight Loss | Retrosternal Pain | Halitosis | Cough | Hoarseness | Pneumonia | Max Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [18] | No | 0 | 4 | ||||||||||||||
| Solids | 1 | ||||||||||||||||
| Semisolids | 2 | ||||||||||||||||
| Solids | 3 | ||||||||||||||||
| Aphagia | 4 | ||||||||||||||||
| [17] | No | 0 | No | 0 | None | 0 | No | 0 | 12 | ||||||||
| Occasional | 1 | Occasional | 1 | <5 kg | 1 | Occasional | 1 | ||||||||||
| Daily | 2 | Daily | 2 | 5–10 kg | 2 | Daily | 2 | ||||||||||
| Each meal | 3 | Each meal | 3 | >10 kg | 3 | Each meal | 3 | ||||||||||
| [20] | No | 0 | No | 0 | None | 0 | No | 0 | No | 0 | No | 0 | No | 0 | 16 | ||
| Solids | 1 | Occasional | 1 | 0.45–4.54 kg | 1 | Occasional | 1 | Occasional | 1 | Occasional | 1 | Yes | 2 | ||||
| Semisolids | 2 | Daily | 2 | 4.99–9.07 kg | 2 | Daily | 2 | Daily | 2 | Daily | 2 | ||||||
| Solids | 3 | >9.07 kg | 3 | ||||||||||||||
| [22] | No | 0 | No | 0 | None | 0 | 10 | ||||||||||
| Solids | 1 | >1/week and <1/day | 1 | Recurrent chest infections OR unintentional weight loss >5 kg over last 3 months | 1 | ||||||||||||
| Semisolids | 2 | ≥1/day | 2 | Recurrent chest infections and unintentional weight loss | 2 | ||||||||||||
| Solids | 3 | Each meal | 3 | ||||||||||||||
| Difficulty swallowing saliva | 4 | Choking or coughing | 4 | ||||||||||||||
4. Zenker’s Diverticulum Surgical Treatment
5. Endoscopic Management
5.1. Flexible Endoscopic Septum Division (FESD)
5.2. Zenker-Peroral Endoscopic Myotomy (Z-POEM)
5.3. Peroral Endoscopic Septotomy (POES)
5.4. Hybrid Techniques
5.4.1. Submucosal Injection Techniques
5.4.2. Submucosal Tunneling Techniques
6. Comparative Data
6.1. Surgery vs. Endoscopy
6.2. Endoscopy vs. Endoscopy
7. Future Perspectives
7.1. Terminology and Abbreviations
7.2. High-Quality Evidence
7.3. Endoscopic Treatment Algorithm
7.4. Standardization of Pre- and Post-Procedural Management
7.5. Validated Symptom Scores
7.6. Endoscopic Training
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, I.J.; Gabb, M.; Panagopoulos, V.; Jamieson, G.G.; Dodds, W.J.; Dent, J.; Shearman, D.J. Pharyngeal (Zenker’s) diverticulum is a disorder of upper esophageal sphincter opening. Gastroenterology 1992, 103, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Watemberg, S.; Landau, O.; Avrahami, R. Zenker’s diverticulum: Reappraisal. Am. J. Gastroenterol. 1996, 91, 1494. [Google Scholar] [PubMed]
- Maselli, R. Flexible endoscopic treatment for Zenker’s diverticulum: From the lumen to the third space. Ann. Gastroenterol. 2021, 34, 149–154. [Google Scholar] [CrossRef]
- Fair, L.; Ward, M.A. Modern approaches to treating Zenker’s diverticulum. Curr. Opin. Gastroenterol. 2023, 39, 333–339. [Google Scholar] [CrossRef]
- Weusten, B.L.A.M.; Barret, M.; Bredenoord, A.J.; Familiari, P.; Gonzalez, J.-M.; van Hooft, J.E.; Lorenzo-Zúñiga, V.; Louis, H.; Martinek, J.; van Meer, S.; et al. Endoscopic management of gastrointestinal motility disorders—Part 2: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 600–614. [Google Scholar] [CrossRef]
- Jain, D.; Sharma, A.; Shah, M.; Patel, U.; Thosani, N.; Singhal, S. Efficacy and Safety of Flexible Endoscopic Management of Zenker’s Diverticulum. J. Clin. Gastroenterol. 2018, 52, 369–385. [Google Scholar] [CrossRef]
- Familiari, P. Endoscopic treatment of Zenker’s diverticulum: A never ending (r)evolution. Endoscopy 2022, 54, 352–353. [Google Scholar] [CrossRef]
- Mondragón, O.V.H.; Pineda, M.O.S.; Valencia, J.M.B. Zenker’s diverticulum: Submucosal tunneling endoscopic septum division (Z-POEM). Dig. Endosc. 2017, 30, 124. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Spadaccini, M.; Belletrutti, P.J.; Galtieri, P.A.; Fugazza, A.; Anderloni, A.; Carrara, S.; Di Leo, M.; Pellegatta, G.; Cappello, A.; et al. Peroral endoscopic septotomy for short-septum Zenker’s diverticulum. Endoscopy 2020, 52, 563–568. [Google Scholar] [CrossRef]
- Pugliese, F.; Dioscoridi, L.; Italia, A.; Forgione, A.; Cintolo, M.; Forti, E.; Bonato, G.; Massad, M.; Mutignani, M. Peroral Endoscopic Diverticulotomy (POED) for Zenker Diverticulum Using Submucosal Injection to Perform a Complete Myotomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2020, 30, e30–e32. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Pinho, R.; Rodrigues, J.; Correia, J.; Freitas, T. Tunneling-free peroral endoscopic septotomy for Zenker diverticulum. VideoGIE 2023, 8, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, G.; Maurommatis, E.; Koumentakis, C.; Tsevgas, I.; Zachariadis, D.; Bazerbachi, F. Single-tunnel Zenker’s diverticulum peroral endoscopic myotomy. Endoscopy 2023, 55, E604–E605. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, F.; Mandarino, F.V.; Verga, M.C.; Dell’ANna, G.; Fasulo, E.; Barchi, A.; Fanti, L.; Viale, E.; Esposito, D.; Napolitano, M.; et al. Modified Per-Oral Endoscopic Septo-Miotomy (m-POESM) for Zenker Diverticulum management: Results from a prospective cohort at a tertiary referral center. Endoscopy 2024, 56, S396. [Google Scholar] [CrossRef]
- Hu, H.; Wang, M.; Zhu, L.; Zhou, P. Endoscopic Transversal Incision and Longitudinal Septostomy (TILS): An Updated Technique for Treating Esophageal Diverticulum. Dig. Dis. 2020, 38, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, G. Mucosotomy at the top of the septum facilitates tunneling and clipping during peroral endoscopic myotomy for Zenker’s diverticulum (Z-POEM). Ann. Gastroenterol. 2020, 33, 101. [Google Scholar] [CrossRef]
- Azzolini, F.; Mandarino, F.; Verga, C.; Dell’ANna, G.; Fasulo, E.; Barchi, A.; Fanti, L.; Viale, E.; Esposito, D.; Napolitano, M.; et al. OC.12.4: Modified Per-Oral Endoscopic Septo-Miotomy (m-POESM) for Zenker Diverticulum management: Results from a prospective cohort at a tertiary referral center. Dig. Liver Dis. 2024, 56, S190. [Google Scholar] [CrossRef]
- Eckardt, V.F.; Aignherr, C.; Bernhard, G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992, 103, 1732–1738. [Google Scholar] [CrossRef]
- Dakkak, M.; Bennett, J.R. A New Dysphagia Score With Objective Validation. J. Clin. Gastroenterol. 1992, 14, 99–100. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Vespa, E.; Barchi, A.; Fasulo, E.; Sinagra, E.; Azzolini, F.; Danese, S. Precision Endoscopy in Peroral Myotomies for Motility Disorders of the Upper Gastrointestinal Tract: Current Insights and Prospective Avenues—A Comprehensive Review. Life 2023, 13, 2143. [Google Scholar] [CrossRef]
- Kothari, T.H.; Kothari, S.; Bittner, K.; Ullah, A.; Kaul, V. Su1137 The “Kothari-Haber” Scoring System: A Prospective Evaluation of Pre- and Post-Procedure Outcomes for Patients Undergoing Endoscopic Myotomy for Zenker’s Diverticulum. Gastrointest. Endosc. 2018, 87, AB289–AB290. [Google Scholar] [CrossRef]
- Che, S.Y.W.; Joseph, S.; Kuchta, K.; Amundson, J.R.; VanDruff, V.N.; Ishii, S.; Zimmermann, C.J.; Hedberg, H.M.; Ujiki, M.B. Outcomes after per-oral endoscopic myotomy for Zenker’s diverticula (Z-POEM) and correlation with impedance planimetry (FLIP). Surg. Endosc. 2024, 38, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, S.; Siau, K.; Lee, M.; Shalmani, H.M.; Kuwai, T.; Priestnall, L.; Muhammad, H.; Hall, A.; Mulder, C.J.; Neumann, H.; et al. Zenker’s Diverticulum: Can Protocolised Measurements with Barium SWALLOW Predict Severity and Treatment Outcomes? The “Zen-Rad” Study. Dysphagia 2021, 36, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.K.; Mendoza, J.; Kallogjeri, D.; Hardi, A.C.; Bradley, J.P. Comparison of Surgical Treatments for Zenker Diverticulum A Systematic Review and Network Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Postma, G.N. Zenker Diverticulum—Which Surgical Approach Is Superior? JAMA Otolaryngol. Head Neck Surg. 2016, 142, 401–403. [Google Scholar] [CrossRef]
- Bonavina, L.; Bona, D.; Abraham, M.; Saino, G.; Abate, E. Long-term results of endosurgical and open surgical approach for Zenker diverticulum. World J. Gastroenterol. 2007, 13, 2586–2589. [Google Scholar] [CrossRef]
- Beard, K.; Swanström, L.L. Zenker’s diverticulum: Flexible versus rigid repair. J. Thorac. Dis. 2017, 9, S154–S162. [Google Scholar] [CrossRef]
- Albers, D.V.; Kondo, A.; Bernardo, W.M.; Sakai, P.; Moura, R.N.; Silva, G.L.R.; Ide, E.; Tomishige, T.; de Moura, E.G.H. Endoscopic versus surgical approach in the treatment of Zenker’s diverticulum: Systematic review and meta-analysis. Endosc. Int. Open 2016, 4, E678–E686. [Google Scholar] [CrossRef]
- da Silva, B.A.M.; Germade, A.; Citores, L.P.; Antolin, S.M.; Santos, F.; Barranco, F.S.; Millán, A.P.; Arisqueta, F.I. Diverticulotomía endoscópica utilizando Ligasure™. Gastroenterol. Y Hepatol. 2017, 40, 80–84. [Google Scholar] [CrossRef]
- Rudler, F.; de Chambrun, G.P.; Lallemant, B.; Garrel, R.; Pouderoux, P.; Ramdani, M.; Caillo, L.; Reynaud, C.; Valats, J.-C.; Blanc, P. Management of the Zenker diverticulum: Multicenter retrospective comparative study of open surgery and rigid endoscopy versus flexible endoscopy. Surg. Endosc. 2023, 37, 7064–7072. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y.-F.; Hu, Y.; Chen, L.-Q. Surgical Treatment of Zenker’s Diverticulum. Dig. Surg. 2013, 30, 207–218. [Google Scholar] [CrossRef]
- Ishaq, S.; Sultan, H.; Siau, K.; Kuwai, T.; Mulder, C.J.; Neumann, H. New and emerging techniques for endoscopic treatment of Zenker’s diverticulum: State-of-the-art review. Dig. Endosc. 2018, 30, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Pagano, N.; Fumagalli, U.; Peracchia, A.; Narne, S.; Malesci, A.; Rosati, R. Transoral treatment of Zenker diverticulum: Flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis. Esophagus 2011, 24, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Bizzotto, A.; Iacopini, F.; Landi, R.; Costamagna, G. Zenker’s diverticulum: Exploring treatment options. Acta Otorhinolaryngol. Ital. 2013, 33, 219–229. [Google Scholar]
- Case, D.J.; Baron, T.H. Flexible endoscopic management of Zenker diverticulum: The Mayo Clinic experience. Mayo Clin. Proc. 2010, 85, 719–722. [Google Scholar] [CrossRef]
- Mulder, C.; Costamagna, G.; Sakai, P. Zenker’s diverticulum: Treatment using a flexible endoscope. Endoscopy 2001, 33, 991–997. [Google Scholar] [CrossRef]
- Mulder, C.J. Zapping Zenker’s diverticulum: Gastroscopic treatment. Can. J. Gastroenterol. Hepatol. 1999, 13, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Perbtani, Y.; Suarez, A.; Wagh, M.S. Techniques and efficacy of flexible endoscopic therapy of Zenker’s diverticulum. World J. Gastrointest. Endosc. 2015, 7, 206–212. [Google Scholar] [CrossRef]
- Christiaens, P.; De Roock, W.; Van Olmen, A.; Moons, V.; D’HAens, G. Treatment of Zenker’s diverticulum through a flexible endoscope with a transparent oblique-end hood attached to the tip and a monopolar forceps. Endoscopy 2007, 39, 137–140. [Google Scholar] [CrossRef]
- Rabenstein, T.; May, A.; Michel, J.; Manner, H.; Pech, O.; Gossner, L.; Ell, C. Argon plasma coagulation for flexible endoscopic Zenker’s diverticulotomy. Endoscopy 2007, 39, 141–145. [Google Scholar] [CrossRef]
- Vogelsang, A.; Preiss, C.; Neuhaus, H.; Schumacher, B. Endotherapy of Zenker’s diverticulum using the needle-knife technique: Long-term follow-up. Endoscopy 2007, 39, 131–136. [Google Scholar] [CrossRef]
- Ferreira, L.E.V.V.C.; Simmons, D.T.; Baron, T.H. Zenker’s diverticula: Pathophysiology, clinical presentation, and flexible endoscopic management. Dis. Esophagus 2008, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huberty, V.; El Bacha, S.; Blero, D.; Le Moine, O.; Hassid, S.; Devière, J. Endoscopic treatment for Zenker’s diverticulum: Long-term results (with video). Gastrointest. Endosc. 2013, 77, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Antonello, A.; Ishaq, S.; Zanatta, L.; Cesarotto, M.; Costantini, M.; Battaglia, G. The role of flexible endotherapy for the treatment of recurrent Zenker’s diverticula after surgery and endoscopic stapling. Surg. Endosc. 2016, 30, 2351–2357. [Google Scholar] [CrossRef]
- Ishaq, S.; Siau, K.; Lee, M.; Sultan, H.; Mohaghegh, S.H.; Kuwai, T.; Mulder, C.J.; Neumann, H. Long-term success of flexible endoscopic septal division with the stag beetle knife for Zenker’s diverticulum: A tertiary center study. Dis. Esophagus 2020, 33, doaa019. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Deliwala, S.; Chandan, S.; Ramai, D.; Ali, H.; Kassab, L.L.; Facciorusso, A.; Kochhar, G.S. Effectiveness and safety of stag beetle knife (SB knife) in management of Zenker’s diverticulum: A systematic review and meta-analysis. Dis. Esophagus 2024, 37, doad069. [Google Scholar] [CrossRef]
- Juin, C.; Barret, M.; Belle, A.; Abouali, E.; Leblanc, S.; Oudjit, A.; Dohan, A.; Coriat, R.; Chaussade, S. Endoscopic treatment of Zenker’s diverticulum by complete septotomy: Initial experience in 19 patients. Endosc. Int. Open 2020, 08, E885–E890. [Google Scholar] [CrossRef]
- Ishaq, S.; Hassan, C.; Antonello, A.; Tanner, K.; Bellisario, C.; Battaglia, G.; Anderloni, A.; Correale, L.; Sharma, P.; Baron, T.H.; et al. Flexible endoscopic treatment for Zenker’s diverticulum: A systematic review and meta-analysis. Gastrointest. Endosc. 2016, 83, 1076–1089.e5. [Google Scholar] [CrossRef]
- Brueckner, J.; Schneider, A.; Messmann, H.; Gölder, S.K. Long-term symptomatic control of Zenker diverticulum by flexible endoscopic mucomyotomy with the hook knife and predisposing factors for clinical recurrence. Scand. J. Gastroenterol. 2016, 51, 666–671. [Google Scholar] [CrossRef]
- Repici, A.; Cappello, A.; Spadaccini, M.; Nicoletti, R.; Carrara, S.; Fugazza, A.; Galtieri, P.A.; Lamonaca, L.; Romana, C.; Badalamenti, M.; et al. Cap-Assisted Endoscopic Septotomy of Zenker’s Diverticulum: Early and Long-Term Outcomes. Am. J. Gastroenterol. 2021, 116, 1853–1858. [Google Scholar] [CrossRef]
- Yang, J.; Novak, S.; Ujiki, M.; Hernández, Ó.; Desai, P.; Benias, P.; Lee, D.; Chang, K.; Brieau, B.; Barret, M.; et al. An international study on the use of peroral endoscopic myotomy in the management of Zenker’s diverticulum. Gastrointest. Endosc. 2020, 91, 163–168. [Google Scholar] [CrossRef]
- Elkholy, S.; El-Sherbiny, M.; Delano-Alonso, R.; Herrera-Esquivel, J.d.J.; Valenzuela-Salazar, C.; Rodriguez-Parra, A.; Del Rio-Suarez, I.; Vargas-Madrigal, J.; Akar, T.; Günay, S.; et al. Peroral endoscopic myotomy as treatment for Zenker’s diverticulum (Z-POEM): A multi-center international study. Esophagus 2021, 18, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Mondragón, O.H.; Pioche, M.; Steinway, S.N.; Nieto, J.; Ujiki, M.B.; VanDruff, V.N.; Kim, R.E.; Canakis, A.; Tantau, M.; et al. Zenker’s peroral endoscopic myotomy for management of large Zenker’s diverticulum. Endoscopy 2023, 55, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Steinway, S.; Zhang, L.; Amundson, J.; Nieto, J.; Desai, P.; Jacques, J.; Bejjani, M.; Pioche, M.; Kumta, N.; Hernandez-Mondragon, O.; et al. Long-term outcomes of Zenker’s peroral endoscopic myotomy (Z-POEM) for treatment of Zenker’s diverticulum. Endosc. Int. Open 2023, 11, E607–E612. [Google Scholar] [CrossRef] [PubMed]
- Budnicka, A.; Januszewicz, W.; Białek, A.B.; Spychalski, M.; Reguła, J.; Kaminski, M.F. Peroral Endoscopic Myotomy in the Management of Zenker’s Diverticulum: A Retrospective Multicenter Study. J. Clin. Med. 2021, 10, 187. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Xia, H.; Shi, L.; Zeng, X.; Jiang, J.; Ren, W.; Peng, Y.; Lü, M.; Tang, X. The role of peroral endoscopic myotomy for Zenker’s diverticulum: A systematic review and meta-analysis. Surg. Endosc. 2022, 36, 2749–2759. [Google Scholar] [CrossRef]
- Mittal, C.; Diehl, D.L.; Draganov, P.V.; Jamil, L.H.; Khalid, A.; Khara, H.S.; Khullar, V.; Law, R.; Lo, S.K.; Mathew, A.; et al. Practice patterns, techniques, and outcomes of flexible endoscopic myotomy for Zenker’s diverticulum: A retrospective multicenter study. Endoscopy 2021, 53, 346–353. [Google Scholar] [CrossRef]
- Klingler, M.J.; Landreneau, J.P.; Strong, A.T.; Barajas-Gamboa, J.S.; Tat, C.; Tu, C.; Fathalizadeh, A.; Kroh, M.; Rodriguez, J.; Sanaka, M.R.; et al. Endoscopic mucosal incision and muscle interruption (MIMI) for the treatment of Zenker’s diverticulum. Surg. Endosc. 2021, 35, 3896–3904. [Google Scholar] [CrossRef]
- Spadaccini, M.; Maselli, R.; Chandrasekar, V.T.; Patel, H.K.; Fugazza, A.; Galtieri, P.A.; Pellegatta, G.; Attardo, S.; Carrara, S.; Anderloni, A.; et al. Submucosal tunnelling techniques for Zenker’s diverticulum: A systematic review of early outcomes with pooled analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, e78–e83. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Nieto, J.; Ngamruengphong, S.; Repici, A.; Khashab, M.A. Zenker’s diverticulum: Advancing beyond the tunnel. VideoGIE 2021, 6, 562–567. [Google Scholar] [CrossRef]
- Pugliese, F.; Dioscoridi, L.; Forgione, A.; Forti, E.; Cintolo, M.; Mutignani, M. Cricopharyngeal myotomy with flexible endoscope for Zenker’s diverticulum using hook knife and endoclips (with video describing an objective measurement of the cutting length). Esophagus 2018, 15, 122–126. [Google Scholar] [CrossRef]
- Al Ghamdi, S.S.; Farha, J.; Moran, R.A.; Pioche, M.; Moll, F.; Yang, D.J.; Mondragón, O.V.H.; Ujiki, M.; Wong, H.; Tantau, A.; et al. Zenker’s peroral endoscopic myotomy, or flexible or rigid septotomy for Zenker’s diverticulum: A multicenter retrospective comparison. Endoscopy 2022, 54, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Swei, E.; Pokala, S.K.; Menard-Katcher, P.; Wagh, M.S. Comparison of Zenker’s per-oral endoscopic myotomy (Z-POEM) with standard flexible endoscopic septotomy for Zenker’s diverticulum: A prospective study with 2-year follow-up. Surg. Endosc. 2023, 37, 6818–6823. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, D.P.C.; Hirsch, B.; de Oliveira, G.H.P.; Kum, A.S.T.; Mahmood, S.; Bernardo, W.M.; Sharma, N.R.; De Moura, E.G.; De Moura, D.T.H. Flexible Endoscopic Versus Rigid Endoscopy or Surgery for the Management of Zenker’s Diverticulum: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e43021. [Google Scholar] [CrossRef]
- Benites-Goñi, H.; Bardalez-Cruz, P.; Medina-Morales, B.; Asencios-Cusihuallpa, J.; Marin-Calderón, L. Peroral endoscopic septotomy for Zenker’s diverticulum with additional cut of mucosal flap: Step by step. VideoGIE 2024, 9, 226–228. [Google Scholar] [CrossRef]
- Holland, A.M.; Lorenz, W.R.; Ricker, A.B.; Mead, B.S.; Scarola, G.T.; Colavita, P.D. Cricopharyngomyotomy: Outcomes of flexible endoscopic management of small and medium sized Zenker’s diverticulum. Am. J. Surg. 2024, 2, 115823. [Google Scholar] [CrossRef]
- Schoeman, S.; Kobayashi, R.; Marcon, N.; May, G.; Mosko, J.; Teshima, C. Outpatient flexible endoscopic diverticulotomy for the management of Zenker’s diverticulum: A retrospective analysis of a large single-center cohort. Gastrointest. Endosc. 2023, 97, 226–231.e2. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Iacopini, F.; Bizzotto, A.; Familiari, P.; Tringali, A.; Perri, V.; Bella, A. Prognostic variables for the clinical success of flexible endoscopic septotomy of Zenker’s diverticulum. Gastrointest. Endosc. 2016, 83, 765–773. [Google Scholar] [CrossRef]
- Lohr, K.N.; Zebrack, B.J. Using patient-reported outcomes in clinical practice: Challenges and opportunities. Qual. Life Res. 2009, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Neilson, L.J.; Sharp, L.; Patterson, J.M.; von Wagner, C.; Hewitson, P.; McGregor, L.M.; Rees, C.J. The Newcastle ENDOPREM™: A validated patient reported experience measure for gastrointestinal endoscopy. BMJ Open Gastroenterol. 2021, 8, e000653. [Google Scholar] [CrossRef]
- Seaman, D.L.; Levy, J.d.l.M.; Gostout, C.J.; Rajan, E.; Herman, L.; Knipschield, M. An animal training model for endoscopic treatment of Zenker’s diverticulum. Gastrointest. Endosc. 2007, 65, 1050–1053. [Google Scholar] [CrossRef]

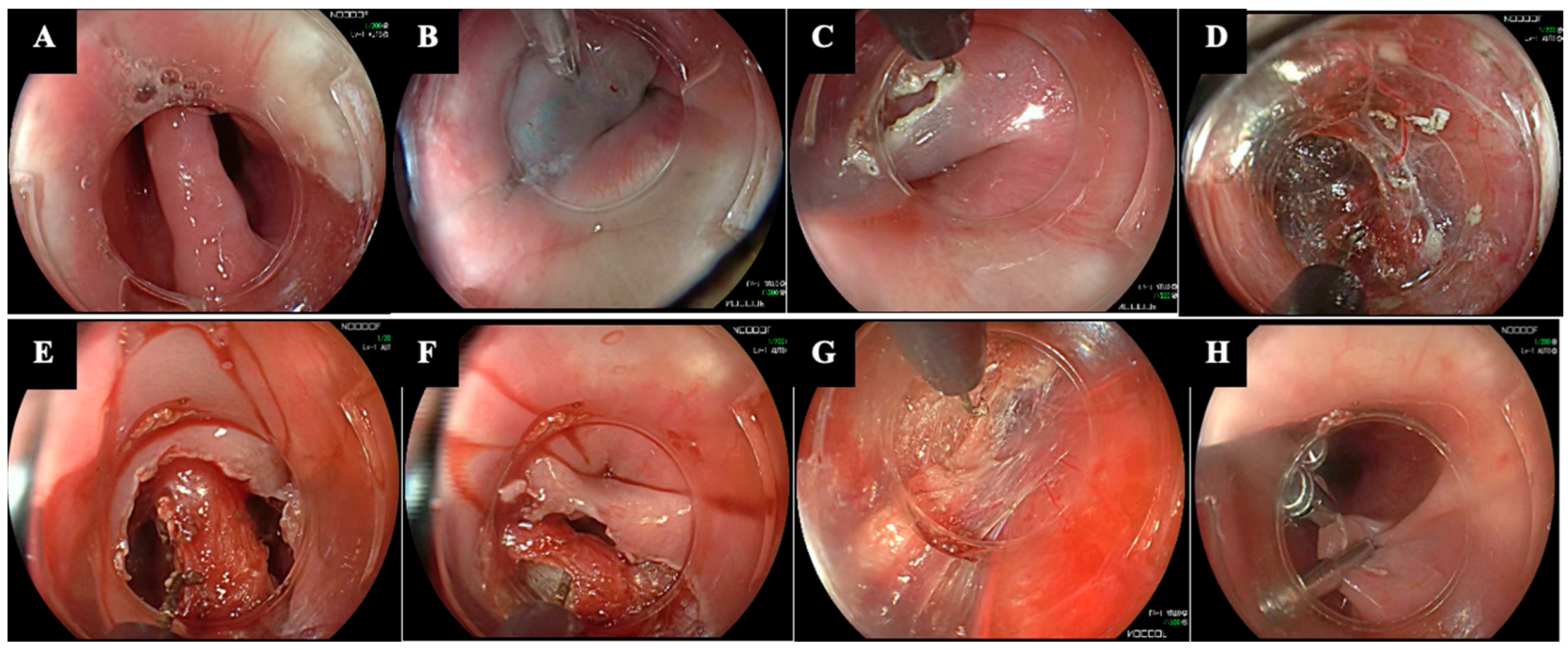
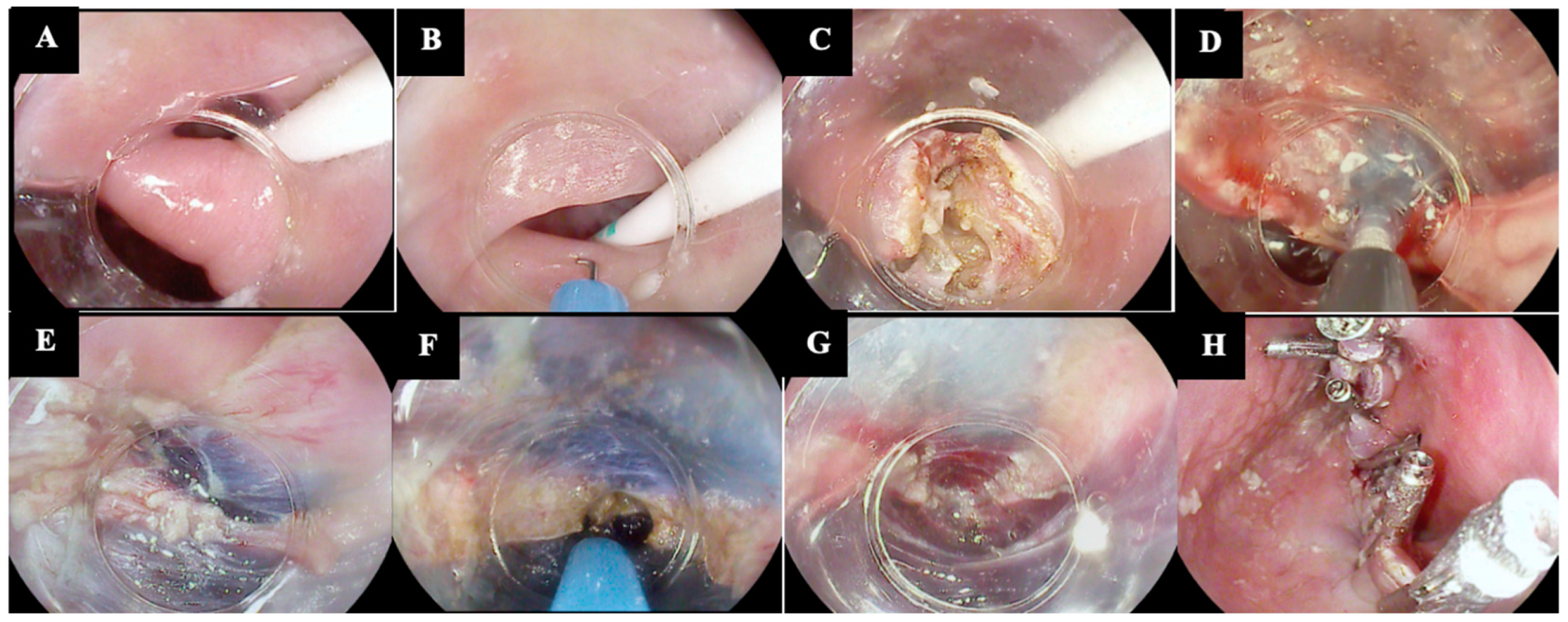
| Technique | Authors, Year of Publication | Study Design | Technical Peculiarities | Pros |
|---|---|---|---|---|
| POED | Pugliese F. et al., 2020 [10] | Case series (5 patients) |
|
|
| TILS | Hu H. et al., 2020 [14] | Case report |
|
|
| SING | Azzolini F. et al., 2024 [13] | Prospective series (9 patients) |
|
|
| Z-POEM with mucosotomy | Zhang L. Y. et al., 2021 [59] | Case series (4 patients) |
|
|
| Single-tunnel Z-POEM | Mavrogenis G. et al., 2023 [12] | Case report |
|
|
| Tunneling-free Z-POEM or R-POES | Estevinho M. M. et al., 2023 [11] | Case series (4 patients) |
|
|
| Author (Year) | Study Design | Technique (n Patients) | Technical Success (%) | Clinical Success (%) | Procedural Time (min) | Adverse Events (%) | Recurrence Rate (%) |
|---|---|---|---|---|---|---|---|
| Mittal et al. (2020) [56] | Retrospective | POES (24) | 95.8 | 90.2 | NA | 16.7 | NA |
| FESD (137) | 97.1 | 75.2 | NA | 6.6 | NA | ||
| Spadaccini et al. (2021) [58] | Meta-analysis | Z-POEM (133) | 97.1 | 94.1 | 46.4 | NA | NA |
| POES (63) | 96.5 | 91.1 | 20.4 | NA | NA | ||
| Klinger et al. (2021) [57] | Retrospective | POES (19) | 94.7 | 89.5 | 27.0 | 15.8 | 11.7 |
| FESD (7) | 100 | 100 | 22.0 | 28.6 | 42.9 | ||
| Al Ghamdi et al. (2022) [61] | Retrospective | Z-POEM (119) | 95.0 | 92.7 | 46.13 (p < 0.001) | 16.8 (p < 0.05) | 14.7 |
| FESD (86) | 95.3 | 86.7 | 33.7 (p < 0.001) | 2.3 (p < 0.05) | 9.2 | ||
| RES (40) | 87.5 | 89.2 | 54.0 | 30.0 (p < 0.05) | 9.1 | ||
| Swei et al. (2023) [62] | Prospective | Z-POEM (13) | 100 | 100 | 43.9 | 0 | 0 |
| FESD (15) | 100 | 86.7 | 60.2 | 6.7 | 0.8 | ||
| Rudler et al. (2023) [29] | Retrospective | OPEN SURGERY (48) | 100 | 97 | 85 (p < 0.001) | 10 | 4 (p = 0.003) |
| RES (52) | 88 (p = 0.04) | 79 (p = 0.016) | 45 (p < 0.001) | 19 | 26 | ||
| FESD (44) | 98 | 90 | 47 (p < 0.001) | 13 | 29 (p = 0.001) | ||
| Albers et al. (2016) [27] | Meta-analysis | ENDOSCOPY 300 | NA | NA | 42 (p < 0.00001) | 9.3 (p = 0.02) | 13 |
| SURGERY 296 | NA | NA | 47 | 14.8 | 6.4 (p = 0.02) | ||
| Cadena Aguirre et al. (2023) [63] | Meta-analysis | OPEN SURGERY (336) | NA | 93.9 | NA | 9.8 | NA |
| RES (453) | 79 | 69.7 | NA | 4.7 | NA | ||
| FESD (492) | 92.9 | 88 | NA | 7.4 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Anna, G.; Fasulo, E.; Fanizza, J.; Barà, R.; Vespa, E.; Barchi, A.; Cecinato, P.; Fuccio, L.; Annese, V.; Malesci, A.; et al. The Endoscopic Management of Zenker’s Diverticulum: A Comprehensive Review. Diagnostics 2024, 14, 2155. https://doi.org/10.3390/diagnostics14192155
Dell’Anna G, Fasulo E, Fanizza J, Barà R, Vespa E, Barchi A, Cecinato P, Fuccio L, Annese V, Malesci A, et al. The Endoscopic Management of Zenker’s Diverticulum: A Comprehensive Review. Diagnostics. 2024; 14(19):2155. https://doi.org/10.3390/diagnostics14192155
Chicago/Turabian StyleDell’Anna, Giuseppe, Ernesto Fasulo, Jacopo Fanizza, Rukaia Barà, Edoardo Vespa, Alberto Barchi, Paolo Cecinato, Lorenzo Fuccio, Vito Annese, Alberto Malesci, and et al. 2024. "The Endoscopic Management of Zenker’s Diverticulum: A Comprehensive Review" Diagnostics 14, no. 19: 2155. https://doi.org/10.3390/diagnostics14192155
APA StyleDell’Anna, G., Fasulo, E., Fanizza, J., Barà, R., Vespa, E., Barchi, A., Cecinato, P., Fuccio, L., Annese, V., Malesci, A., Azzolini, F., Danese, S., & Mandarino, F. V. (2024). The Endoscopic Management of Zenker’s Diverticulum: A Comprehensive Review. Diagnostics, 14(19), 2155. https://doi.org/10.3390/diagnostics14192155






