Neuroimaging Correlates of Post-COVID-19 Symptoms: A Functional MRI Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Data
2.2. MRI Data Acquisition and Processing
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 16 August 2024).
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236,379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Wang, Y.-R.; Wang, Q.-H.; Chen, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; Hu, T.; Liu, X.-D.; et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol. Neurodegener. 2021, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Sabbatino, F.; De Pascale, D.; Sellitto, C.; Stefanelli, B.; Bertini, N.; De Simone, M.; Liguori, L.; Di Paola, I.; et al. Long COVID: Clinical Framing, Biomarkers, and Therapeutic Approaches. J. Pers. Med. 2023, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, A.; Norouzi, S.; Dehdarirad, H.; Aghlmand, S.; Yusefzadeh, H.; Javan-Noughabi, J. The global economic burden of COVID-19 disease: A comprehensive systematic review and meta-analysis. Syst. Rev. 2024, 13, 68. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ghaderi, S. Advanced magnetic resonance neuroimaging techniques: Feasibility and applications in long or post-COVID-19 syndrome—A review. Ann. Med. Surg. 2024, 86, 1584–1589. [Google Scholar] [CrossRef]
- Drew, P.J. Vascular and neural basis of the BOLD signal. Curr. Opin. Neurobiol. 2019, 58, 61–69. [Google Scholar] [CrossRef]
- Edison, P. Brain connectivity and COVID-19. Brain Connect. 2021, 11, 251–252. [Google Scholar] [CrossRef]
- Fu, Z.; Tu, Y.; Calhoun, V.D.; Zhang, Y.; Zhao, Q.; Chen, J.; Meng, Q.; Lu, Z.; Hu, L. Dynamic functional network connectivity associated with post-traumatic stress symptoms in COVID-19 survivors. Neurobiol. Stress 2021, 15, 100377. [Google Scholar] [CrossRef]
- Andrei Appelt, P.; Taciana Sisconetto, A.; Baldo Sucupira, K.S.M.; Neto, E.M.; Chagas, T.J.; Bazan, R.; Moura Cabral, A.; Andrade, A.O.; de Souza, L.A.P.S.; José Luvizutto, G. Changes in Electrical Brain Activity and Cognitive Functions Following Mild to Moderate COVID-19: A one-Year Prospective Study After Acute Infection. Clin. EEG Neurosci. 2022, 53, 543–557. [Google Scholar] [CrossRef]
- Niroumand Sarvandani, M.; Sheikhi Koohsar, J.; Rafaiee, R.; Saeedi, M.; Seyedhosseini Tamijani, S.M.; Ghazvini, H.; Sheibani, H. COVID-19 and the Brain: A Psychological and Resting-state Functional Magnetic Resonance Imagin (fMRI) Study of the Whole-brain Functional Connectivity. Basic Clin. Neurosci. 2023, 14, 753–771. [Google Scholar] [CrossRef]
- Tanashyan, M.; Morozova, S.; Raskurazhev, A.; Kuznetsova, P. A prospective randomized, double-blind placebo-controlled study to evaluate the effectiveness of neuroprotective therapy using functional brain MRI in patients with post-covid chronic fatigue syndrome. Biomed. Pharmacother. 2023, 168, 115723. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.S.; Evans, A.C.; McKinstry, R.C.; Almli, C.R.; Collins, D.L. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 2009, 47 (Suppl. S1). [Google Scholar] [CrossRef]
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 2023, 146, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ryan, M.C.; Liang, H.; Zhang, X.; Cunningham, E.; Wang, J.; Wilson, E.; Herskovits, E.H.; Kottilil, S.; Ernst, T.M. Changes in Brain Activation Patterns During Working Memory Tasks in People With Post-COVID Condition and Persistent Neuropsychiatric Symptoms. Neurology 2023, 100, e2409–e2423. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Long COVID syndrome-associated brain fog. J. Med. Virol. 2022, 94, 979–984. [Google Scholar] [CrossRef]
- Azcue, N.; Gómez-Esteban, J.C.; Acera, M.; Tijero, B.; Fernandez, T.; Ayo-Mentxakatorre, N.; Pérez-Concha, T.; Murueta-Goyena, A.; Lafuente, J.V.; Prada, Á.; et al. Brain fog of post-COVID-19 condition and Chronic Fatigue Syndrome, same medical disorder? J. Transl. Med. 2022, 20, 569. [Google Scholar] [CrossRef]
- Ishii, A.; Tanaka, M.; Yamano, E.; Watanabe, Y. The neural substrates of physical fatigue sensation to evaluate ourselves: A magnetoencephalography study. Neuroscience 2014, 261, 60–67. [Google Scholar] [CrossRef]
- Raizen, D.; Bhavsar, R.; Keenan, B.T.; Liu, P.Z.; Kegelman, T.P.; Chao, H.H.; Vapiwala, N.; Rao, H. Increased posterior cingulate cortex blood flow in cancer-related fatigue. Front. Neurol. 2023, 14, 1135462. [Google Scholar] [CrossRef]
- Sliwinska, M.W.; Khadilkar, M.; Campbell-Ratcliffe, J.; Quevenco, F.; Devlin, J.T. Early and sustained supramarginal gyrus contributions to phonological processing. Front. Psychol. 2012, 3, 161. [Google Scholar] [CrossRef]
- Wiener, M.; Hamilton, R.; Turkeltaub, P.; Matell, M.S.; Coslett, H.B. Fast forward: Supramarginal gyrus stimulation alters time measurement. J. Cogn. Neurosci. 2010, 22, 23–31. [Google Scholar] [CrossRef]
- Herath, P.; Carmichael, M.; Murphy, A.; Bonilha, L.; Newman-Norlund, R.; Rorden, C.; Davis, M. Cortical Substrate of Supraspinal Fatigue following Exhaustive Aerobic Exercise Localizes to a Large Cluster in the Anterior Premotor Cortex. Front. Neurol. 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, K.; Giakoumettis, D. Chapter 1—Basic knowledge on neuroanatomy and neurophysiology of the central nervous system. In Neuroimaging in Neurogenic Communication Disorders; Academic Press: Cambridge, MA, USA, 2023; pp. 1–30. ISBN 9780128238752. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Z.; Wang, T.; Yang, X.; Feng, F.; Fan, L.; Jiang, J. Connectivity pattern differences bilaterally in the cerebellum posterior lobe in healthy subjects after normal sleep and sleep deprivation: A resting-state functional MRI study. Neuropsychiatr. Dis. Treat. 2015, 11, 1279–1289. [Google Scholar] [CrossRef]
- De Simone, M.; De Feo, R.; Choucha, A.; Ciaglia, E.; Fezeu, F. Enhancing Sleep Quality: Assessing the Efficacy of a Fixed Combination of Linden, Hawthorn, Vitamin B1, and Melatonin. Med. Sci. 2024, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Tedjasukmana, R.; Budikayanti, A.; Islamiyah, W.R.; Witjaksono, A.; Hakim, M. Sleep disturbance in post COVID-19 conditions: Prevalence and quality of life. Front. Neurol. 2023, 13, 1095606. [Google Scholar] [CrossRef]
- Casamento-Moran, A.; Mooney, R.A.; Chib, V.S.; Celnik, P.A. Cerebellar Excitability Regulates Physical Fatigue Perception. J. Neurosci. 2023, 43, 3094–3106. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.M.; Garssen, B.; Cull, A.; de Haes, J.C. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br. J. Cancer 1996, 73, 241–245. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef]
- Logothetis, N. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]

| Characteristic | Control N = 20 1 | Post-COVID N = 30 1 | p-Value 2 |
|---|---|---|---|
| Age | 42 (37, 47) | 34 (27, 50) | 0.4 |
| Gender | 0.092 | ||
| F | 10 (50%) | 22 (73%) | |
| M | 10 (50%) | 8 (27%) | |
| MoCA | 28.00 (26.50, 29.00) | 28.00 (27.00, 29.00) | 0.7 |
| MFI-20 | 23 (18, 28) | 63 (54, 74) | <0.001 |
| T | PFWEcorr | Number of Voxels | Peak MNI Coordinate | |
|---|---|---|---|---|
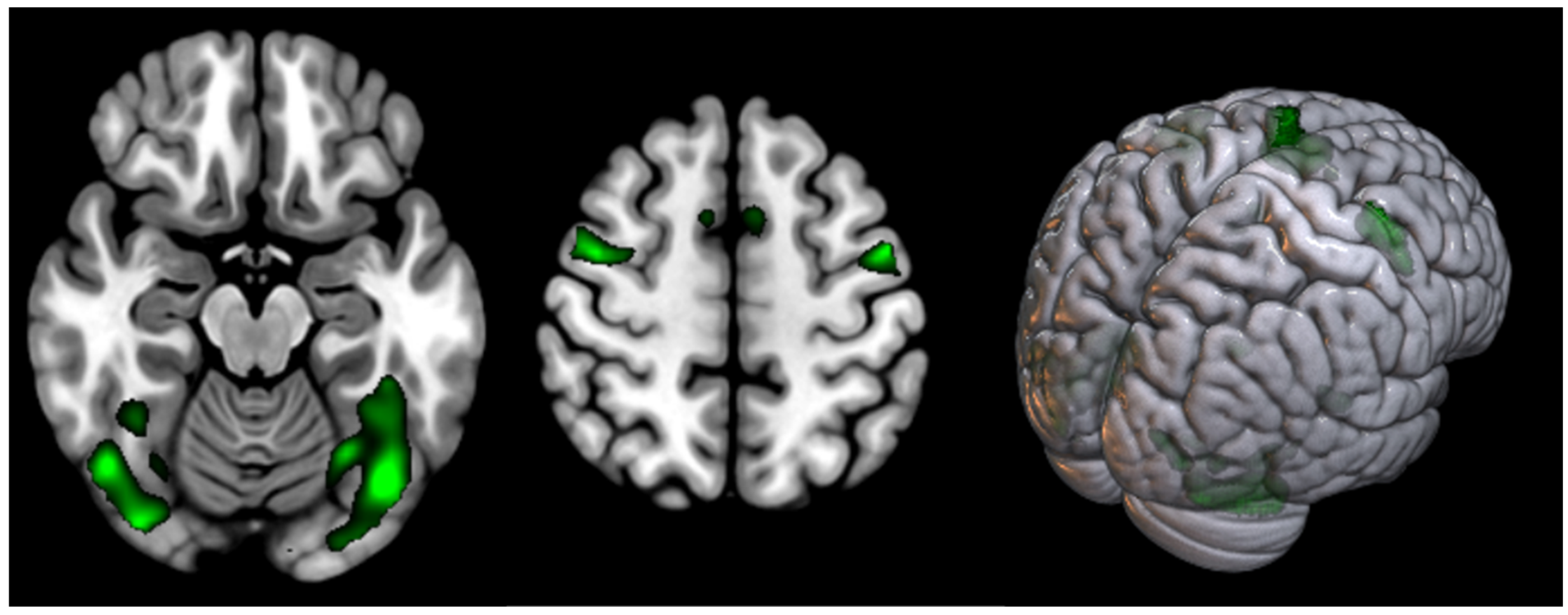
| Supplementary motor cortex | 11.98 | <0.000 | 799 | 0 0 68 |
| Occipital cortex (Fusiform gyrus) (L) | 10.34 | <0.001 | 1507 | 60 8 30 |
| Occipital cortex (Fusiform gyrus) (R) | 9.24 | <0.005 | 706 | 44 −66 −20 |
| Precentral gyrus (L) (BA6) | <0.007 | 608 | −54 −2 48 | |
| Precentral gyrus (R) (BA6) | 8.67 | <0.009 | 221 | 42 −2 56 |
| T | PFWEcorr | Number of Voxels | Peak MNI Coordinate | |
|---|---|---|---|---|
| Supplementary motor cortex | 10.98 | <0.000 | 1002 | 2 8 58 |
| Occipital cortex (Fusiform gyrus) and Cerebellum Posterior Lobe (L + R) | 16.96 | <0.000 | 13,405 | −26 −86 −16 |
| Precentral gyrus (L) (BA6) | 14.38 | <0.000 | 1739 | −48 −2 46 |
| Precentral gyrus (R) (BA6) | 10.73 | <0.000 | 2186 | 52 14 44 |
| Parietal Superior Lobule (L) (BA7) | 9.21 | <0.000 | 1066 | −24 −60 46 |
| Parietal Superior Lobule (R) (BA7) | 8.43 | <0.000 | 315 | 28 −58 46 |
| T | Puncorr | Number of Voxels | Peak MNI Coordinate | |
|---|---|---|---|---|
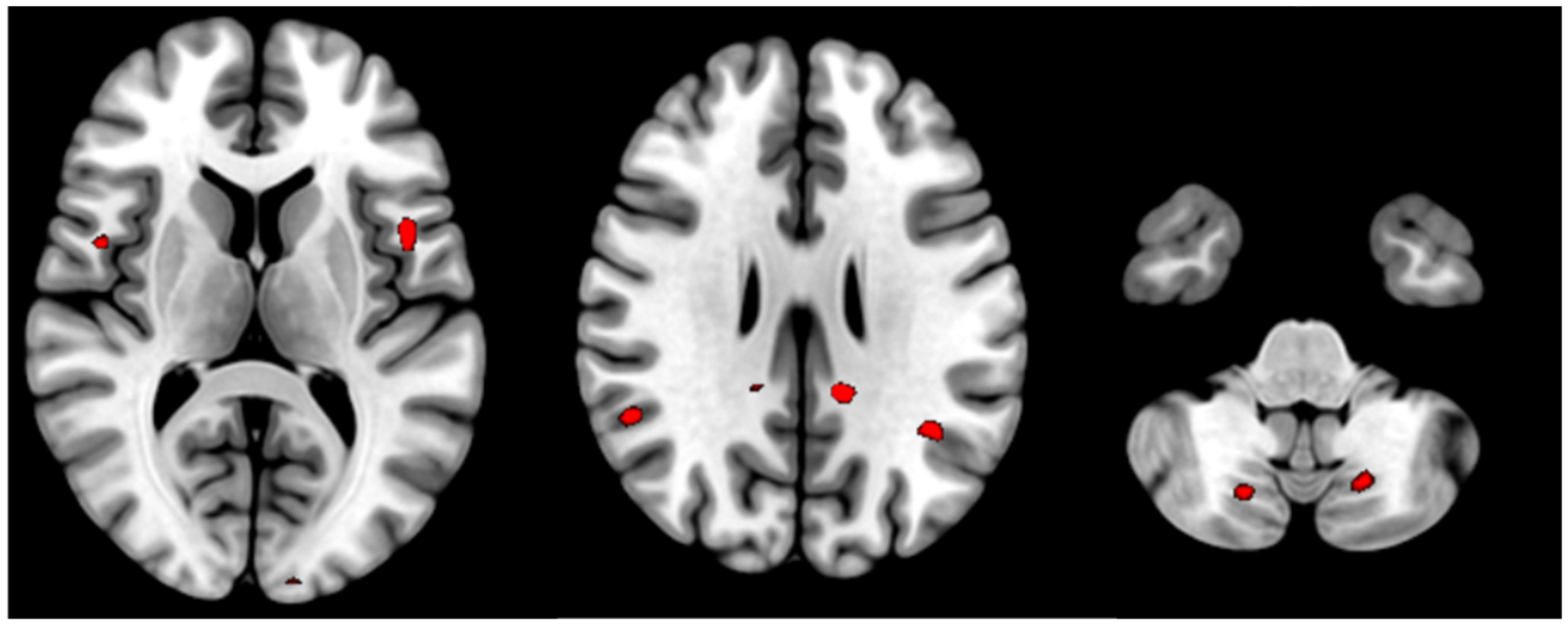
| Posterior cingulate cortex (L) | 4.06 | <0.000 | 14 | −12 −42 28 |
| Posterior cingulate cortex (R) | 3.32 | 0.001 | 2 | 6 −44 50 |
| Supramarginal gyrus (L) | 3.37 | <0.000 | 23 | −38 −54 30 |
| Supramarginal gyrus (R) | 3.49 | <0.000 | 22 | 52 −50 22 |
| Opercular part of Precentral gyrus (L) | 3.77 | <0.000 | 12 | −46 8 8 |
| Opercular part of Precentral gyrus (R) | 3.47 | 0.001 | 11 | 48 4 10 |
| Cerebellum Posterior Lobe (8) (L) | 3.33 | 0.001 | 67 | −18 −68 −42 |
| Cerebellum Posterior Lobe (8) (R) | 3.25 | 0.001 | 34 | 18 −70 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanashyan, M.M.; Kuznetsova, P.I.; Morozova, S.N.; Annushkin, V.A.; Raskurazhev, A.A. Neuroimaging Correlates of Post-COVID-19 Symptoms: A Functional MRI Approach. Diagnostics 2024, 14, 2180. https://doi.org/10.3390/diagnostics14192180
Tanashyan MM, Kuznetsova PI, Morozova SN, Annushkin VA, Raskurazhev AA. Neuroimaging Correlates of Post-COVID-19 Symptoms: A Functional MRI Approach. Diagnostics. 2024; 14(19):2180. https://doi.org/10.3390/diagnostics14192180
Chicago/Turabian StyleTanashyan, Marine M., Polina I. Kuznetsova, Sofya N. Morozova, Vladislav A. Annushkin, and Anton A. Raskurazhev. 2024. "Neuroimaging Correlates of Post-COVID-19 Symptoms: A Functional MRI Approach" Diagnostics 14, no. 19: 2180. https://doi.org/10.3390/diagnostics14192180
APA StyleTanashyan, M. M., Kuznetsova, P. I., Morozova, S. N., Annushkin, V. A., & Raskurazhev, A. A. (2024). Neuroimaging Correlates of Post-COVID-19 Symptoms: A Functional MRI Approach. Diagnostics, 14(19), 2180. https://doi.org/10.3390/diagnostics14192180






