Establishing Neuron-Specific Enolase Reference Intervals: A Comparative Analysis of Partitioned Approach- and Gender-Based Continuous Age- and Season-Related Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Detection of NSE, ProGRP, and Hemolysis Index
2.3. RI Partitioning
2.4. Statistical Analysis
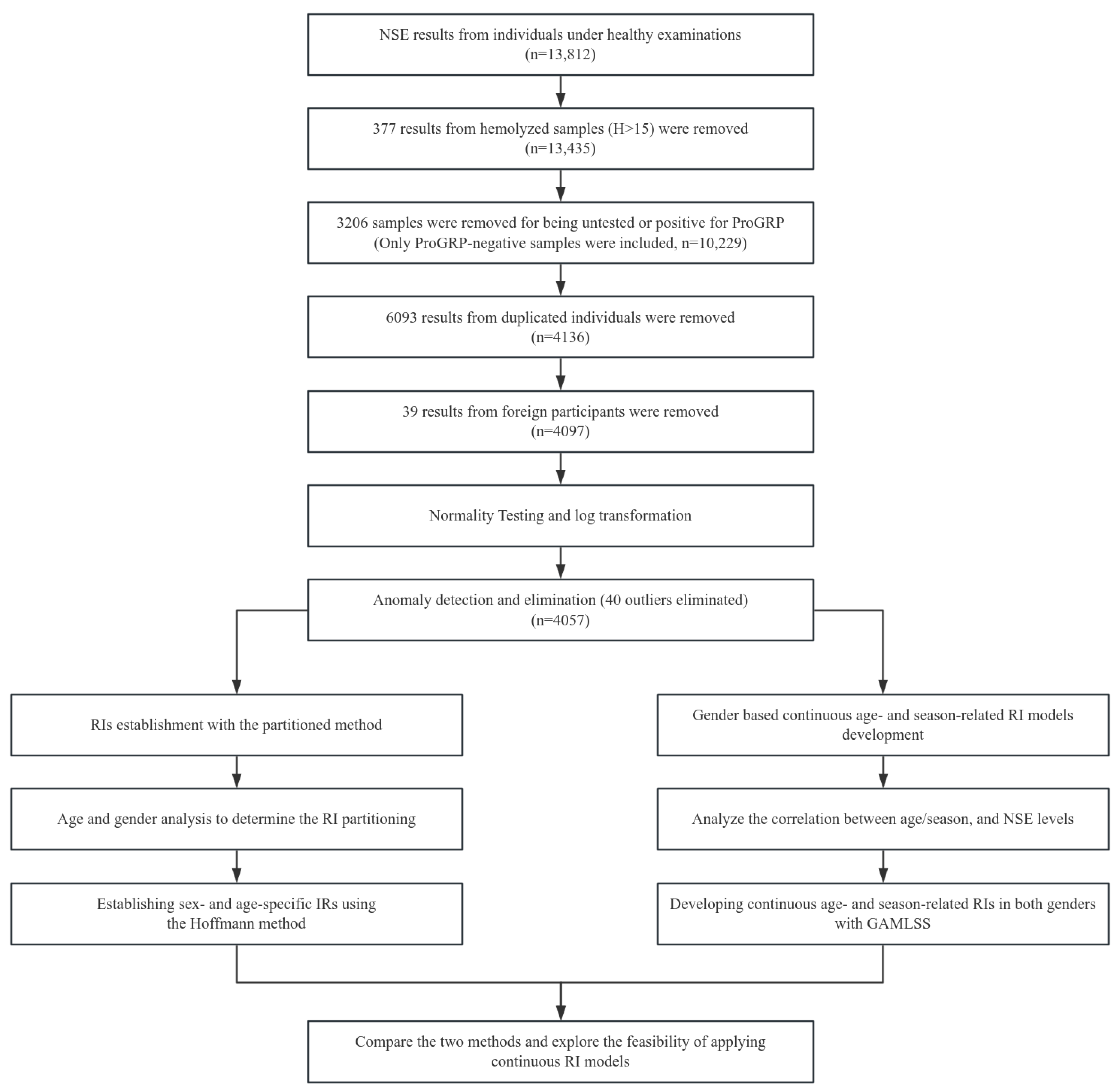
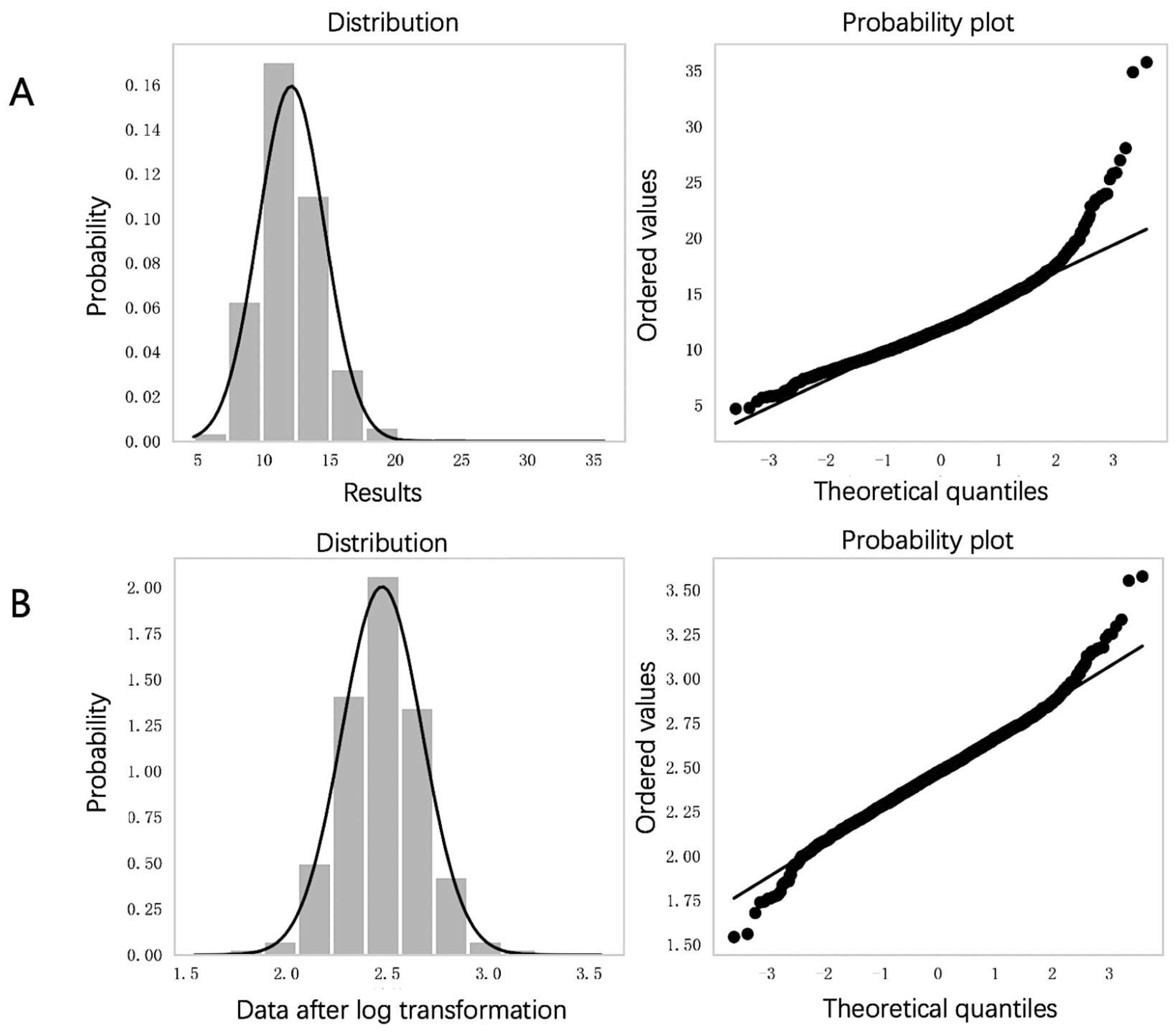
2.4.1. Data Preprocessing
2.4.2. Establishment of Partitioned RIs for NSE
2.4.3. Development of Gender-Based Continuous Age- or Season-Dependent RI Models
2.4.4. Development of Gender-Based Continuous Age- and Season-Integrated RI Models
2.4.5. Software
3. Results
3.1. Data Distribution and Anomaly Elimination
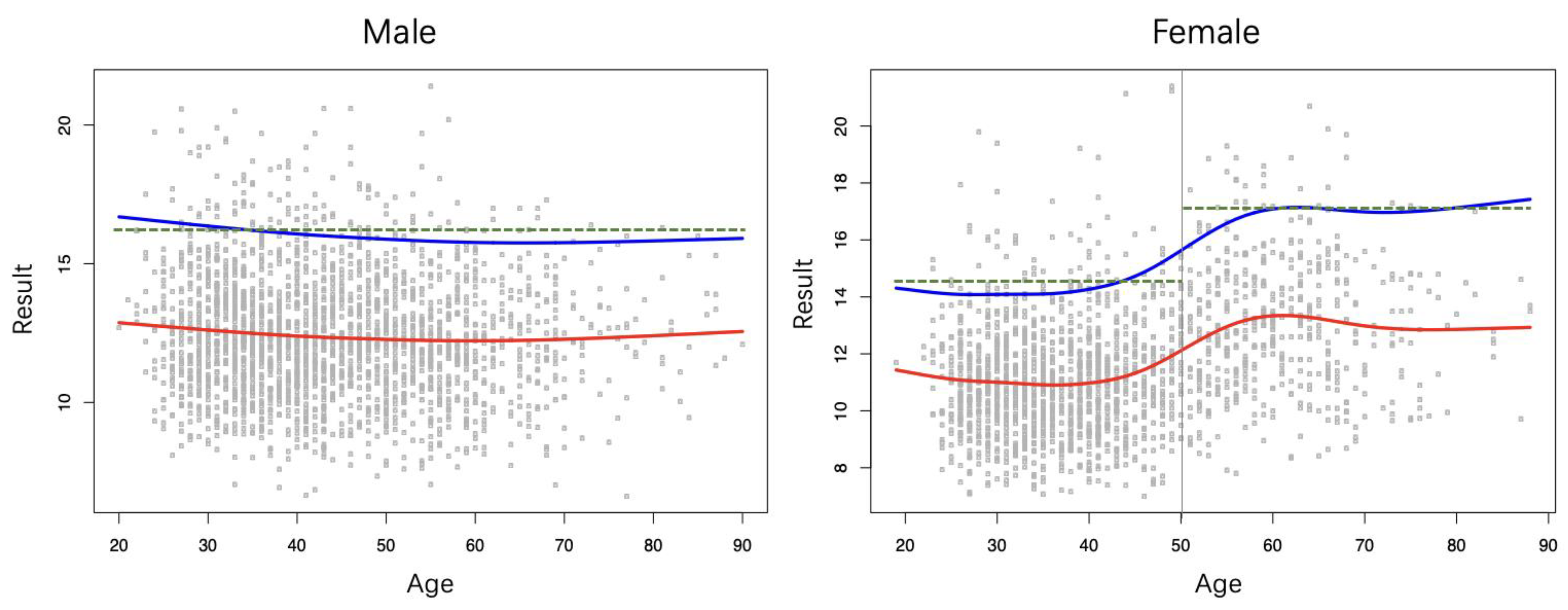
3.2. Gender and Age Grouping Analysis
3.3. Sex- and Age-Specific RIs with the Partitioned Approach
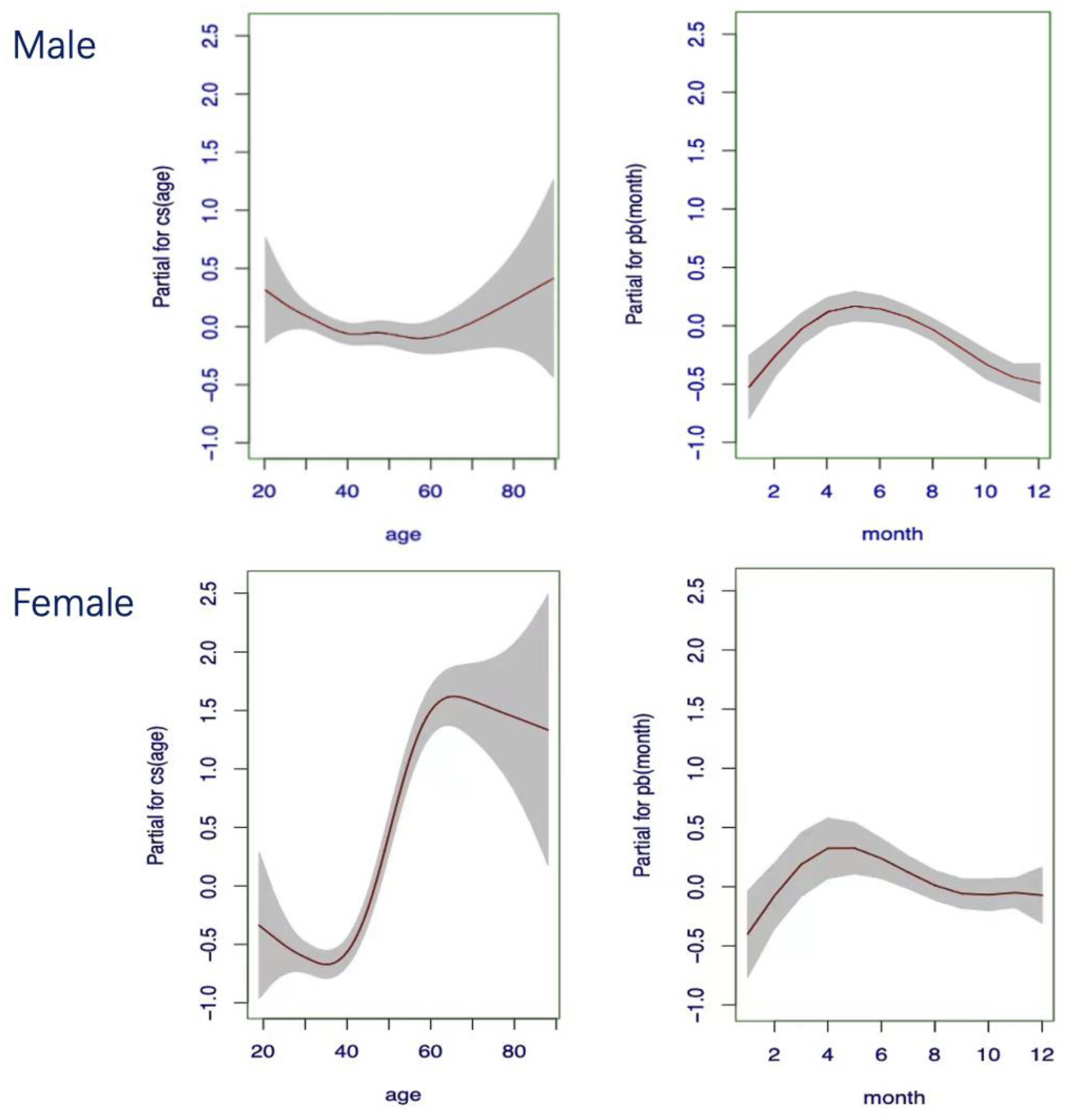
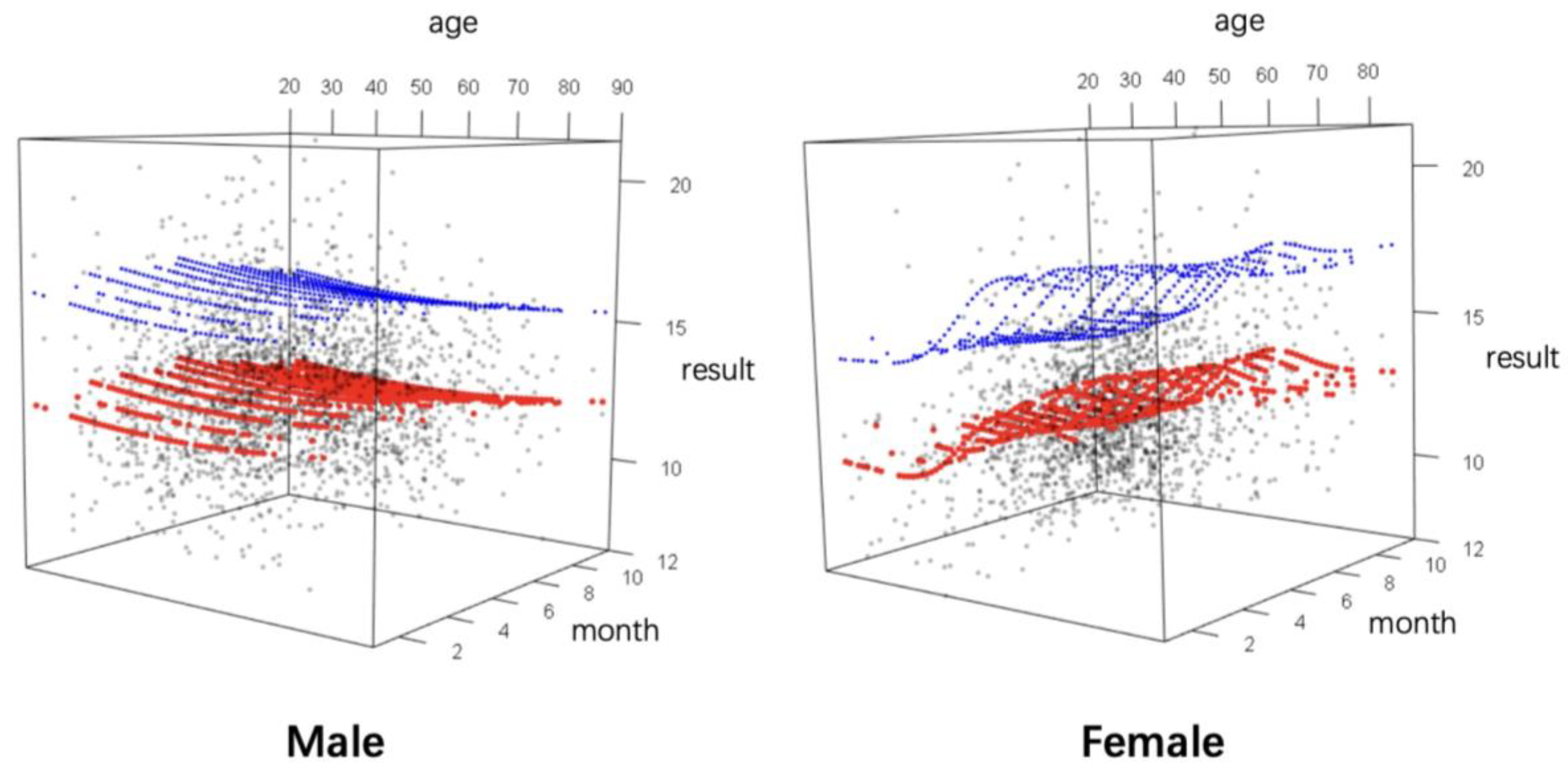
3.4. Gender-Based Continuous Age-Dependent RIs
3.5. Gender-Based Continuous Season-Dependent RIs
3.6. Gender-Based Continuous Age- and Season-Integrated RIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, J.; Qiu, X.; Li, Y.; Wang, Z.; Luan, Y. miR-27a regulates the self renewal of the H446 small cell lung cancer cell line in vitro. Oncol. Rep. 2013, 29, 161–168. [Google Scholar] [CrossRef]
- Fizazi, K.; Cojean, I.; Pignon, J.P.; Rixe, O.; Gatineau, M.; Hadef, S.; Arriagada, R.; Baldeyrou, P.; Comoy, E.; Le Chevalier, T. Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: An early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer 1998, 82, 1049–1055. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Jassem, E.; Bonfrer, J.M.; Mooi, W.J.; van Tinteren, H. Serum neuron-specific enolase and lactate dehydrogenase as predictors of response to chemotherapy and survival in non-small cell lung cancer. Semin. Oncol. 1992, 19, 37–43. [Google Scholar]
- Wick, M.R.; Scheithauer, B.W.; Kovacs, K. Neuron-specific enolase in neuroendocrine tumors of the thymus, bronchus, and skin. Am. J. Clin. Pathol. 1983, 79, 703–707. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Mariani, P.; Lomanto, D.; Carlei, F.; Lezoche, E.; Speranza, V. Serum neuron-specific enolase in diagnosis and follow-up of gastrointestinal neuroendocrine tumors. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 1992, 13, 352. [Google Scholar]
- Tapia, F.J.; Polak, J.M.; Barbosa, A.J.; Bloom, S.R.; Marangos, P.J.; Dermody, C.; Pearse, A.G. Neuron-specific enolase is produced by neuroendocrine tumours. Lancet 1981, 1, 808–811. [Google Scholar] [CrossRef]
- Böhmer, A.E.; Oses, J.P.; Schmidt, A.P.; Perón, C.S.; Krebs, C.L.; Oppitz, P.P.; D’Avila, T.T.; Souza, D.O.; Portela, L.V.; Stefani, M.A. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 2011, 68, 1624–1630; discussion 1630–1631. [Google Scholar] [CrossRef] [PubMed]
- Amoo, M.; Henry, J.; O’Halloran, P.J.; Brennan, P.; Husien, M.B.; Campbell, M.; Caird, J.; Javadpour, M.; Curley, G.F. S100B, GFAP, UCH-L1 and NSE as predictors of abnormalities on CT imaging following mild traumatic brain injury: A systematic review and meta-analysis of diagnostic test accuracy. Neurosurg. Rev. 2022, 45, 1171–1193. [Google Scholar] [CrossRef]
- Horn, P.S.; Pesce, A.J. Reference intervals: An update. Clin. Chim. Acta 2003, 334, 5–23. [Google Scholar] [CrossRef]
- CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory. In Approved Guideline, 3rd ed.; EP28A3C; CLSI EP28A3CE; CLSI: Wayne, PA, USA, 2010. [Google Scholar]
- Miao, Q.; Cai, B.; Gao, X.; Su, Z.; Zhang, J. The establishment of neuron-specific enolase reference interval for the healthy population in southwest China. Sci. Rep. 2020, 10, 6332. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.D.; Haeckel, R.; Loh, T.P.; Sikaris, K.; Streichert, T.; Katayev, A.; Barth, J.H.; Ozarda, Y. Indirect methods for reference interval determination—Review and recommendations. Clin. Chem. Lab. Med. 2018, 57, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Obstfeld, A.E.; Patel, K.; Boyd, J.C.; Drees, J.; Holmes, D.T.; Ioannidis, J.P.A.; Manrai, A.K. Data Mining Approaches to Reference Interval Studies. Clin. Chem. 2021, 67, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Zierk, J.; Arzideh, F.; Rechenauer, T.; Haeckel, R.; Rascher, W.; Metzler, M.; Rauh, M. Age- and Sex-Specific Dynamics in 22 Hematologic and Biochemical Analytes from Birth to Adolescence. Clin. Chem. 2015, 61, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Higgins, V.; McCudden, C.; Adeli, K. Continuous reference intervals for 38 biochemical markers in healthy children and adolescents: Comparisons to traditionally partitioned reference intervals. Clin. Biochem. 2019, 73, 82–89. [Google Scholar] [CrossRef]
- Cao, B.; Peng, Y.; Song, W.; Peng, X.; Hu, L.; Liu, Z.; Liu, Z.; Gong, C.; Ni, X. Pediatric Continuous Reference Intervals of Serum Insulin-like Growth Factor 1 Levels in a Healthy Chinese Children Population—Based on PRINCE Study. Endocr. Pr. 2022, 28, 696–702. [Google Scholar] [CrossRef]
- Chen, D.; Ni, J.; Lin, X.; Wei, X.; Zhou, Y.; Wang, Y.; Bai, B. Quantitative Analyses of Hemolysis Interference and Preparation of Reference Chart for Five Hemolysis-Interfered Clinical Tests. Labeled Immunoasays 2022, 29, 847–851. [Google Scholar]
- Ferraro, S.; Braga, F.; Luksch, R.; Terenziani, M.; Caruso, S.; Panteghini, M. Measurement of Serum Neuron-Specific Enolase in Neuroblastoma: Is There a Clinical Role? Clin. Chem. 2020, 66, 667–675. [Google Scholar] [CrossRef]
- Motoyoshi, N.; Tsutsui, M.; Soman, K.; Shirasaka, T.; Narita, T.; Kunioka, S.; Naya, K.; Yamazaki, D.; Narita, M.; Kamiya, H. Neuron-specific enolase levels immediately following cardiovascular surgery is modulated by hemolysis due to cardiopulmonary bypass, making it unsuitable as a brain damage biomarker. J. Artif. Organs 2024, 27, 100–107. [Google Scholar] [CrossRef]
- Ramont, L.; Thoannes, H.; Volondat, A.; Chastang, F.; Millet, M.C.; Maquart, F.X. Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: Implications in clinical practice. Clin. Chem. Lab. Med. 2005, 43, 1215–1217. [Google Scholar] [CrossRef]
- George, D. SPSS for Windows Step by Step: A Simple Study Guide and Reference, 17.0 Update, 10/e; Pearson Education: Chennai, India, 2011. [Google Scholar]
- Demir, S. Comparison of normality tests in terms of sample sizes under different skewness and Kurtosis coefficients. Int. J. Assess. Tools Educ. 2022, 9, 397–409. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Płaczkowska, S.; Terpińska, M.; Piwowar, A. Establishing laboratory-specific reference intervals for TSH and fT4 by use of the indirect Hoffman method. PLoS ONE 2022, 17, e0261715. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Lichtinghagen, R.; Wosniok, W. Simple estimation of reference intervals from routine laboratory data. LaboratoriumsMedizin 2016, 39, 000010151520150104. [Google Scholar] [CrossRef]
- Schneider, J.; Philipp, M.; Salewski, L.; Velcovsky, H.G. Pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in therapy control of patients with small-cell lung cancer. Clin. Lab. 2003, 49, 35–42. [Google Scholar]
- Nisman, B.; Heching, N.; Biran, H.; Barak, V.; Peretz, T. The prognostic significance of circulating neuroendocrine markers chromogranin a, pro-gastrin-releasing peptide and neuron-specific enolase in patients with advanced non-small-cell lung cancer. Tumour Biol. 2006, 27, 8–16. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Wang, L.; Zhou, Y.; Shen, Y.; Xu, F.; Chen, Y. Selective application of neuroendocrine markers in the diagnosis and treatment of small cell lung cancer. Clin. Chim. Acta 2020, 509, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Hu, L.; Peng, Y.; Yan, R.; Ni, X. Comparison of four algorithms on establishing continuous reference intervals for pediatric analytes with age-dependent trend. BMC Med. Res. Methodol. 2020, 20, 136. [Google Scholar] [CrossRef]
- Jørgensen, L.G.M.; Løber, J.; Carlsen, N.L.T.; Momsen, G.; Hirsch, F.R. Serum neuron specific enolase (S-NSE) reference interval evaluation by time-resolved immunofluorometry compared with a radioimmunoassay. Clin. Chim. Acta 1996, 249, 77–91. [Google Scholar] [CrossRef]
- Lu, M.H.; Ju, S.Q.; Cong, H.; Yang, S.M.; Zhu, W.C. Investigate of Serum NSE Reference Range of Healthy People in Nantong with Electrochemiluminescence. J. Mod. Lab. Med. 2018, 33, 59–62. [Google Scholar]
- Liu, Q.; Fan, J.; Xu, A.; Yao, L.; Li, Y.; Wang, W.; Liang, W.; Yang, F. Distribution of serum neuron-specific enolase and the establishment of a population reference interval in healthy adults. J. Clin. Lab. Anal. 2019, 33, e22863. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Alves, S.E.; Bulloch, K.; Weiland, N.G. Ovarian steroids and the brain: Implications for cognition and aging. Neurology 1997, 48, S8–S15. [Google Scholar] [CrossRef]
- McEwen, B.; Akama, K.; Alves, S.; Brake, W.G.; Bulloch, K.; Lee, S.; Li, C.; Yuen, G.; Milner, T.A. Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. USA 2001, 98, 7093–7100. [Google Scholar] [CrossRef] [PubMed]
- Monk, D.; Brodaty, H. Use of estrogens for the prevention and treatment of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2000, 11, 1–10. [Google Scholar] [CrossRef]
- Pasquali, R.; Baraldi, G.; Casimirri, F.; Mattioli, L.; Capelli, M.; Melchionda, N.; Capani, F.; Labo, G. Seasonal variations of total and free thyroid hormones in healthy men: A chronobiological study. Acta Endocrinol. 1984, 107, 42–48. [Google Scholar] [CrossRef]
- Berry, D.J.; Hyppönen, E.; Cortina-Borja, M. Investigating the association of vitamin D seasonality on inflammatory and hemostatic markers. Chronobiol. Int. 2013, 30, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, A.E.; Levitan, R.D.; Houle, S.; Wilson, A.A.; Nobrega, J.N.; Meyer, J.H. Increased Seasonal Variation in Serotonin Transporter Binding in Seasonal Affective Disorder. Neuropsychopharmacology 2016, 41, 2447–2454. [Google Scholar] [CrossRef]
- Matlabs Almousawi, N.; Jasim, R. Relationship of Serotonin to Neuron Specific Enolase In Serum Samples of Patients with Advanced Stages of Cancer Tumors. Eur. Chem. Bull. 2017, 6, 350. [Google Scholar] [CrossRef]
- Szturmowicz, M.; Burakowski, J.; Tomkowski, W.; Sakowicz, A.; Filipecki, S. Neuron-Specific Enolase in Non-Neoplastic Lung Diseases, a Marker of Hypoxemia? Int. J. Biol. Markers 1998, 13, 150–153. [Google Scholar] [CrossRef]
- Anju, M.; Maiya, A.G.; Hande, M.; Binu, V.S. Effect of photobiomodulation on serum neuron specific enolase (NSE) among patients with diabetic peripheral neuropathy—A pilot study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1061–1063. [Google Scholar] [CrossRef]
- Eworo, R.E.; Fabian, U.A.; Thomas, C.; Luke, U.O.; Usoro, C.A.; Johnson, D.D.; Egom, O.U.; Ntamu, A.N.; Nsonwu Anyanwu, C.A. Evaluation of serum cotinine, total protein, thiocyanate, neuron specific enolase and uric acid levels of cigarette smokers in calabar metropolis, nigeria: A cross sectional study. Glob. J. Pure Appl. Sci. 2023, 29, 55–62. [Google Scholar] [CrossRef]
- Glei, D.A.; Goldman, N.; Lin, Y.H.; Weinstein, M. Age-Related Changes in Biomarkers: Longitudinal Data from a Population-Based Sample. Res. Aging 2011, 33, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xia, L.; Cheng, X.; Wu, J.; Yin, Y.; Hou, L.; Li, X.; Guo, X.; Lin, S.; Qiu, L. Establishment of variation source and age-related reference interval models for 22 common biochemical analytes in older people using real-world big data mining. Age Ageing 2020, 49, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Stasinopoulos, D.; Rigby, R.; Heller, G.Z.; Voudouris, V.; Bastiani, F.D. Flexible Regression and Smoothing: Using GAMLSS in R; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]

| Age | Gender | |||
|---|---|---|---|---|
| Male | Female | |||
| Group | n | Group | n | |
| 19–29 | 1 | 236 | 7 | 269 |
| 30–39 | 2 | 690 | 8 | 583 |
| 40–49 | 3 | 602 | 9 | 436 |
| 50–59 | 4 | 445 | 10 | 276 |
| 60–69 | 5 | 198 | 11 | 187 |
| ≥70 | 6 | 72 | 12 | 63 |
| Total | — | 2243 | — | 1814 |
| Age Group | Male | Female | ||
|---|---|---|---|---|
| Meandiff | p | Meandiff | p | |
| (19–29) vs. (30–39) | −0.009 | 0.983 | −0.002 | 1 |
| (19–29) vs. (40–49) | −0.023 | 0.562 | 0.018 | 0.787 |
| (19–29) vs. (50–59) | −0.04 | 0.065 | 0.163 | 0 |
| (19–29) vs. (60–69) | −0.036 | 0.306 | 0.176 | 0 |
| (19–29) vs. (>70) | −0.016 | 0.987 | 0.158 | 0 |
| (30–39) vs. (40–49) | −0.013 | 0.759 | 0.02 | 0.477 |
| (30–39) vs. (50–59) | −0.03 | 0.058 | 0.165 | 0 |
| (30–39) vs. (60–69) | −0.026 | 0.451 | 0.178 | 0 |
| (30–39) vs. (>70) | −0.006 | 1 | 0.16 | 0 |
| (40–49) vs. (50–59) | −0.017 | 0.652 | 0.145 | 0 |
| (40–49) vs. (60–69) | −0.013 | 0.952 | 0.158 | 0 |
| (40–49) vs. (>70) | 0.007 | 1 | 0.14 | 0 |
| (50–59) vs. (60–69) | 0.004 | 1 | 0.013 | 0.968 |
| (50–59) vs. (>70) | 0.024 | 0.899 | −0.005 | 1 |
| (60–69) vs. (>70) | 0.02 | 0.967 | −0.018 | 0.978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhu, D.; Zhang, M.; Wang, T.; Han, N.; Ge, T.; Ma, X.; Wu, A.; Li, R.; Zhao, X. Establishing Neuron-Specific Enolase Reference Intervals: A Comparative Analysis of Partitioned Approach- and Gender-Based Continuous Age- and Season-Related Models. Diagnostics 2024, 14, 2226. https://doi.org/10.3390/diagnostics14192226
Zhao H, Zhu D, Zhang M, Wang T, Han N, Ge T, Ma X, Wu A, Li R, Zhao X. Establishing Neuron-Specific Enolase Reference Intervals: A Comparative Analysis of Partitioned Approach- and Gender-Based Continuous Age- and Season-Related Models. Diagnostics. 2024; 14(19):2226. https://doi.org/10.3390/diagnostics14192226
Chicago/Turabian StyleZhao, Haibin, Dong Zhu, Miaomiao Zhang, Tengjiao Wang, Ning Han, Tinglei Ge, Xiaoming Ma, Anxin Wu, Runqing Li, and Xiuying Zhao. 2024. "Establishing Neuron-Specific Enolase Reference Intervals: A Comparative Analysis of Partitioned Approach- and Gender-Based Continuous Age- and Season-Related Models" Diagnostics 14, no. 19: 2226. https://doi.org/10.3390/diagnostics14192226
APA StyleZhao, H., Zhu, D., Zhang, M., Wang, T., Han, N., Ge, T., Ma, X., Wu, A., Li, R., & Zhao, X. (2024). Establishing Neuron-Specific Enolase Reference Intervals: A Comparative Analysis of Partitioned Approach- and Gender-Based Continuous Age- and Season-Related Models. Diagnostics, 14(19), 2226. https://doi.org/10.3390/diagnostics14192226





