Return-to-Play Post-Myocarditis for Athletes: To Play or Not to Play?
Abstract
:1. Introduction
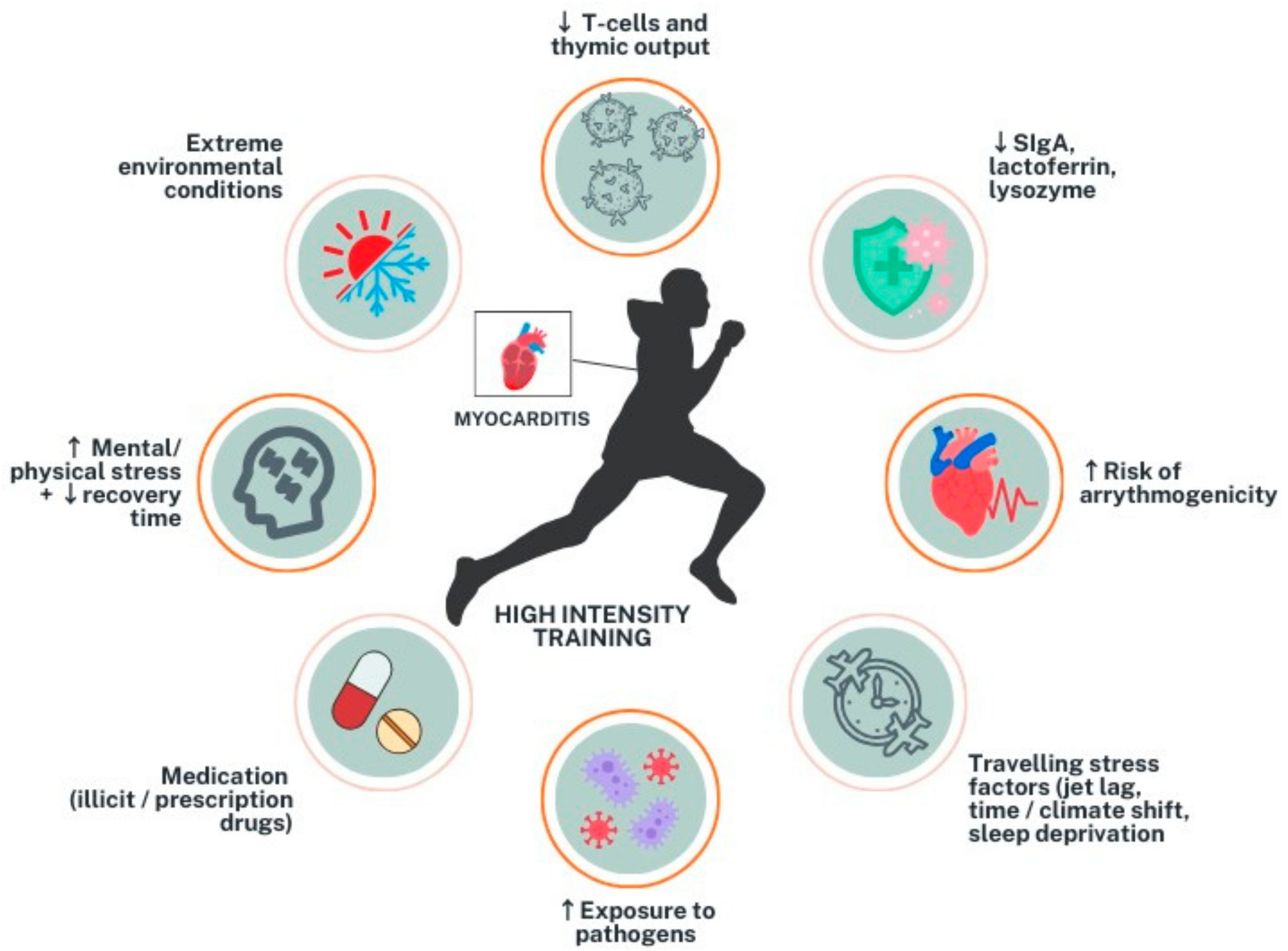
2. Myocarditis in Athletes
3. Clinical Presentation and Diagnosis
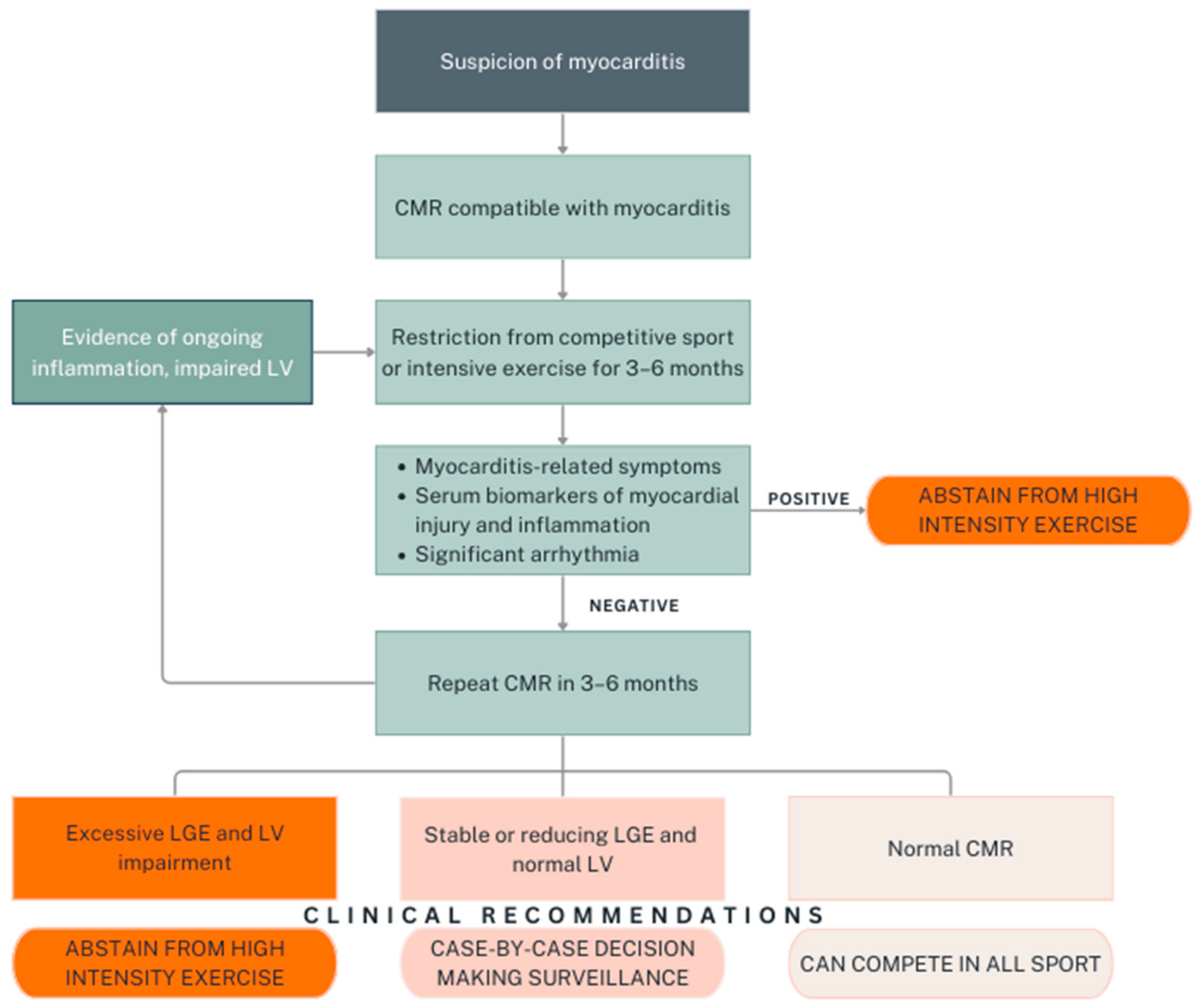
4. Return-to-Play
5. Shared Decision-Making Approach
6. Clinical Recommendations and Future Research
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, M.; Chippa, V.; An, J. Viral Myocarditis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Al-Akchar, M.; Shams, P.; Kiel, J. Acute Myocarditis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rroku, A.; Kottwitz, J.; Heidecker, B. Update on myocarditis—What we know so far and where we may be heading. Eur. Heart J. Acute Cardiovasc. Care 2020, 10, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Lynge, T.H.; Nielsen, T.S.; Gregers Winkel, B.; Tfelt-Hansen, J.; Banner, J. Sudden cardiac death caused by myocarditis in persons aged 1–49 years: A nationwide study of 14 294 deaths in Denmark. Forensic Sci. Res. 2019, 4, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, R.D.; Weintraub, R.G.; Ingles, J.; Duflou, J.; Yeates, L.; Lam, L.; Davis, A.M.; Thompson, T.; Connell, V.; Wallace, J.; et al. A prospective study of sudden cardiac death among children and young adults. N. Engl. J. Med. 2016, 374, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Sudden death in young athletes. N. Engl. J. Med. 2003, 349, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef]
- Harmon, K.G.; Asif, I.M.; Maleszewski, J.J.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J.; et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation 2015, 132, 10–19. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of sudden death in sports: Insights from a United Kingdom regional registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef]
- Thiene, G. Sudden cardiac death in the young: A genetic destiny? Clin. Med. 2018, 18, s17. [Google Scholar] [CrossRef]
- Pelliccia, A.; Solberg, E.E.; Papadakis, M.; Adami, P.E.; Biffi, A.; Caselli, S.; La Gerche, A.; Niebauer, J.; Pressler, A.; Schmied, C.M.; et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2019, 40, 19–33. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef]
- Maron, B.J.; Udelson, J.E.; Bonow, R.O.; Nishimura, R.A.; Ackerman, M.J.; Estes, N.A.M.; Cooper, L.T.; Link, M.S.; Maron, M.S. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 3: Hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis. Circulation 2015, 132, e273–e280. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Modica, G.; Bianco, M.; Sollazzo, F.; Di Murro, E.; Monti, R.; Cammarano, M.; Morra, L.; Nifosì, F.M.; Gervasi, S.F.; Gravina, E.M.; et al. Myocarditis in athletes recovering from COVID-19: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 4279. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.T.; Finocchiaro, G.; Westaby, J.; Chatrath, N.; Behr, E.R.; Papadakis, M.; Sharma, S.; Sheppard, M.N. Myocarditis and sudden cardiac death in the community: Clinical and pathological insights from a national registry in the United Kingdom. Circ. Arrhythm. Electrophysiol. 2023, 16, e012129. [Google Scholar] [CrossRef]
- Vio, R.; Zorzi, A.; Corrado, D. Myocarditis in the athlete: Arrhythmogenic substrates, clinical manifestations, management, and eligibility decisions. J. Cardiovasc. Transl. Res. 2020, 13, 284–295. [Google Scholar] [CrossRef]
- Eichhorn, C.; Bière, L.; Schnell, F.; Schmied, C.; Wilhelm, M.; Kwong, R.Y.; Gräni, C. Myocarditis in athletes is a challenge: Diagnosis, risk stratification, and uncertainties. JACC Cardiovasc. Imaging 2020, 13, 494–507. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Nieman, D.C. Is infection risk linked to exercise workload? Med. Sci. Sports Exerc. 2000, 32, S406–S411. [Google Scholar] [CrossRef]
- Suzui, M.; Kawai, T.; Kimura, H.; Takeda, K.; Yagita, H.; Okumura, K.; Shek, P.N.; Shephard, R.J. Natural killer cell lytic activity and CD56dim and CD56bright cell distributions during and after intensive training. J. Appl. Physiol. 2004, 96, 2167–2173. [Google Scholar] [CrossRef]
- Prieto-Hinojosa, A.; Knight, A.; Compton, C.; Gleeson, M.; Travers, P.J. Reduced thymic output in elite athletes. Brain Behav. Immun. 2014, 39, 75–79. [Google Scholar] [CrossRef]
- Keaney, L.C.; Kilding, A.E.; Merien, F.; Dulson, D.K. The impact of sport related stressors on immunity and illness risk in team-sport athletes. J. Sci. Med. Sport. 2018, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Davie, A.; Su, Q. Effects of moderate and high intensity exercise on T1/T2 balance. Exerc. Immunol. Rev. 2012, 18, 98–114. [Google Scholar] [PubMed]
- Mont, L.; Elosua, R.; Brugada, J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. EP Eur. 2009, 11, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Miguel-dos-Santos, R.; Moreira, J.B.N.; Loennechen, J.P.; Wisløff, U.; Mesquita, T. Exercising immune cells: The immunomodulatory role of exercise on atrial fibrillation. Prog. Cardiovasc. Dis. 2021, 68, 52–59. [Google Scholar] [CrossRef]
- Halle, M.; Binzenhöfer, L.; Mahrholdt, H.; Schindler, M.J.; Esefeld, K.; Tschöpe, C. Myocarditis in athletes: A clinical perspective. Eur. J. Prev. Cardiol. 2021, 28, 1050–1057. [Google Scholar] [CrossRef]
- Asif, I.M.; Price, D.E.; Ewing, A.; Rao, A.L.; Harmon, K.G.; Drezner, J.A. The impact of diagnosis: Measuring the psychological response to being diagnosed with serious or potentially lethal cardiac disease in young competitive athletes. Br. J. Sports Med. 2016, 50, 163. [Google Scholar] [CrossRef]
- Halle, M. Myocarditis in athletes. In The ESC Textbook of Sports Cardiology; Pelliccia, A., Heidbuchel, H., Corrado, D., Borjesson, M., Sharma, S., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 201–209. [Google Scholar]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Drezner, J.A.; Sharma, S.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International criteria for electrocardiographic interpretation in athletes: Consensus statement. Br. J. Sports Med. 2017, 51, 704. [Google Scholar] [CrossRef]
- Baker, P.; Leckie, T.; Harrington, D.; Richardson, A. Exercise-induced cardiac troponin elevation: An update on the evidence, mechanism and implications. IJC Heart Vasc. 2019, 22, 181–186. [Google Scholar] [CrossRef]
- Kindermann, W. Creatine kinase levels after exercise. Dtsch. Arztebl. Int. 2016, 113, 344. [Google Scholar] [CrossRef]
- Polte, C.L.; Bobbio, E.; Bollano, E.; Bergh, N.; Polte, C.; Himmelman, J.; Lagerstrand, K.M.; Gao, S.A. Cardiovascular magnetic resonance in myocarditis. Diagnostics 2022, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Meier, C.; Eisenblätter, M.; Gielen, S. Myocardial late gadolinium enhancement (LGE) in cardiac magnetic resonance imaging (CMR)- an important risk marker for cardiac disease. J. Cardiovasc. Dev. Dis. 2024, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Tymińska, A.; Ozierański, K.; Caforio, A.; Marcolongo, R.; Marchel, M.; Kapłon-Cieślicka, A.; Baritussio, A.; Filipiak, K.J.; Opolski, G.; Grabowski, M. Myocarditis and inflammatory cardiomyopathy in 2021: An update. Pol. Arch. Intern. Med. 2021, 131, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Lasica, R.; Djukanovic, L.; Savic, L.; Krljanac, G.; Zdravkovic, M.; Ristic, M.; Lasica, A.; Asanin, M.; Ristic, A. Update on myocarditis: From etiology and clinical picture to modern diagnostics and methods of treatment. Diagnostics 2023, 13, 3073. [Google Scholar] [CrossRef]
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-term follow-up of biopsy-proven viral myocarditis: Predictors of mortality and incomplete recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.T.; et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: Multicenter Lombardy registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- McKinney, J.; Connelly, K.A.; Dorian, P.; Fournier, A.; Goodman, J.M.; Grubic, N.; Isserow, S.; Moulson, N.; Philippon, F.; Pipe, A.; et al. COVID-19–myocarditis and return to play: Reflections and recommendations from a Canadian Working Group. Can. J. Cardiol. 2021, 37, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; Harmon, K.G.; et al. ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: Myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic impact of late gadolinium enhancement by cardiovascular magnetic resonance in myocarditis. Circ. Cardiovasc. Imaging 2021, 14, e011492. [Google Scholar] [CrossRef]
- Zorzi, A.; Perazzolo Marra, M.; Rigato, I.; De Lazzari, M.; Susana, A.; Niero, A.; Pilichou, K.; Migliore, F.; Rizzo, S.; Giorgi, B.; et al. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ. Arrhythm. Electrophysiol. 2016, 9, e004229. [Google Scholar] [CrossRef]
- Bohbot, Y.; Sanguineti, F.; Renard, C.; Hovasse, T.; Limouzineau, I.; Unterseeh, T.; Di Lena, C.; Boukefoussa, W.; Tawa, C.; Duhamel, S.; et al. Associated factors and clinical implications of dynamic changes in late gadolinium enhancement after acute myocarditis. JACC Cardiovasc. Imaging 2023, 16, 859–861. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Ghebru Habtemicael, Y.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lanzillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; et al. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J. Am. Coll. Cardiol. 2019, 74, 2439–2448. [Google Scholar] [CrossRef]
- Claessen, G.; La Gerche, A.; De Bosscher, R. Return to play after myocarditis: Time to abandon the one-size-fits-all approach? Br. J. Sports Med. 2023, 57, 1282. [Google Scholar] [CrossRef]
- Lota, A.S.; Hazebroek, M.R.; Theotokis, P.; Wassall, R.; Salmi, S.; Halliday, B.P.; Tayal, U.; Verdonschot, J.; Meena, D.; Owen, R.; et al. Genetic architecture of acute myocarditis and the overlap with inherited cardiomyopathy. Circulation 2022, 146, 1123–1134. [Google Scholar] [CrossRef]
- McNally, E.M.; Selgrade, D.F. Genetic testing for myocarditis. JACC Heart Fail. 2022, 10, 728–730. [Google Scholar] [CrossRef]
- Ammirati, E.; Raimondi, F.; Piriou, N.; Sardo Infirri, L.; Mohiddin, S.A.; Mazzanti, A.; Shenoy, C.; Cavallari, U.A.; Imazio, M.; Aquaro, G.D.; et al. Acute myocarditis associated with desmosomal gene variants. JACC Heart Fail. 2022, 10, 714–727. [Google Scholar] [CrossRef]
- Ollitrault, P.; Al Khoury, M.; Troadec, Y.; Calcagno, Y.; Champ-Rigot, L.; Ferchaud, V.; Pellissier, A.; Legallois, D.; Milliez, P.; Labombarda, F. Recurrent acute myocarditis: An under-recognized clinical entity associated with the later diagnosis of a genetic arrhythmogenic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 998883. [Google Scholar] [CrossRef] [PubMed]
- Monda, E.; Bakalakos, A.; Cannie, D.; O’Mahony, C.; Syrris, P.; Kaski, J.P.; Limongelli, G.; Elliott, P.M. Prevalence of pathogenic variants in cardiomyopathy-associated genes in acute myocarditis: A systematic review and meta-analysis. JACC Heart Fail. 2024, 12, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
| Topic | AHA/ACC Scientific Statement (2015) [13] | EAPC Position Statement (2019) [11] | ESC Guidelines on Sports Cardiology (2021) [14] |
|---|---|---|---|
| Initial Evaluation |
|
|
|
| (Class I; Level of Evidence C). | (Class IIb; Level of Evidence C). | (Class I; Level of Evidence B). | |
| Risk Assessment Post-Recovery | Resuming training and competition is reasonable if all of the following criteria are satisfied:
| Resume training and competition if all of the following criteria are satisfied:
| RTP in 3–6 months can be considered if all of the following criteria are satisfied:
|
| (Class IIa; Level of Evidence C). | (Class IIa; Level of Evidence C). | (Class IIa; Level of Evidence C). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamagata, K.; Malhotra, A. Return-to-Play Post-Myocarditis for Athletes: To Play or Not to Play? Diagnostics 2024, 14, 2236. https://doi.org/10.3390/diagnostics14192236
Yamagata K, Malhotra A. Return-to-Play Post-Myocarditis for Athletes: To Play or Not to Play? Diagnostics. 2024; 14(19):2236. https://doi.org/10.3390/diagnostics14192236
Chicago/Turabian StyleYamagata, Kentaro, and Aneil Malhotra. 2024. "Return-to-Play Post-Myocarditis for Athletes: To Play or Not to Play?" Diagnostics 14, no. 19: 2236. https://doi.org/10.3390/diagnostics14192236





