Application of Targeted Optical Coherence Tomography in Oral Cancer: A Cross-Sectional Preliminary Study
Abstract
:1. Introduction
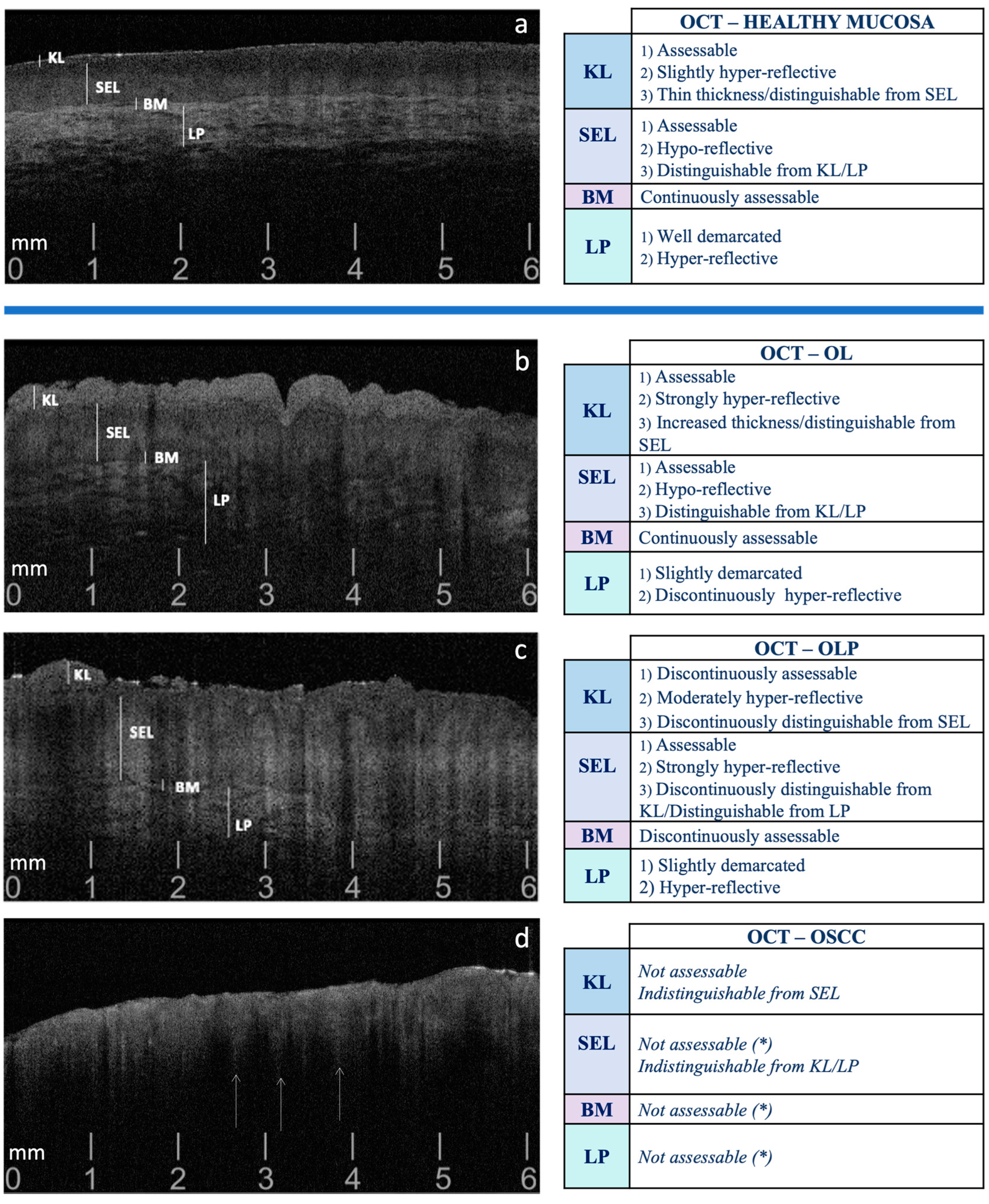
2. Materials and Methods
2.1. Sample Selection
- Age ≥ 18 years;
- Ability to provide informed consent;
2.2. Phase 1: OCT Evaluation Pre-Target Site Registration
2.3. Phase 2: OCT Evaluation Post-Target Site Registration
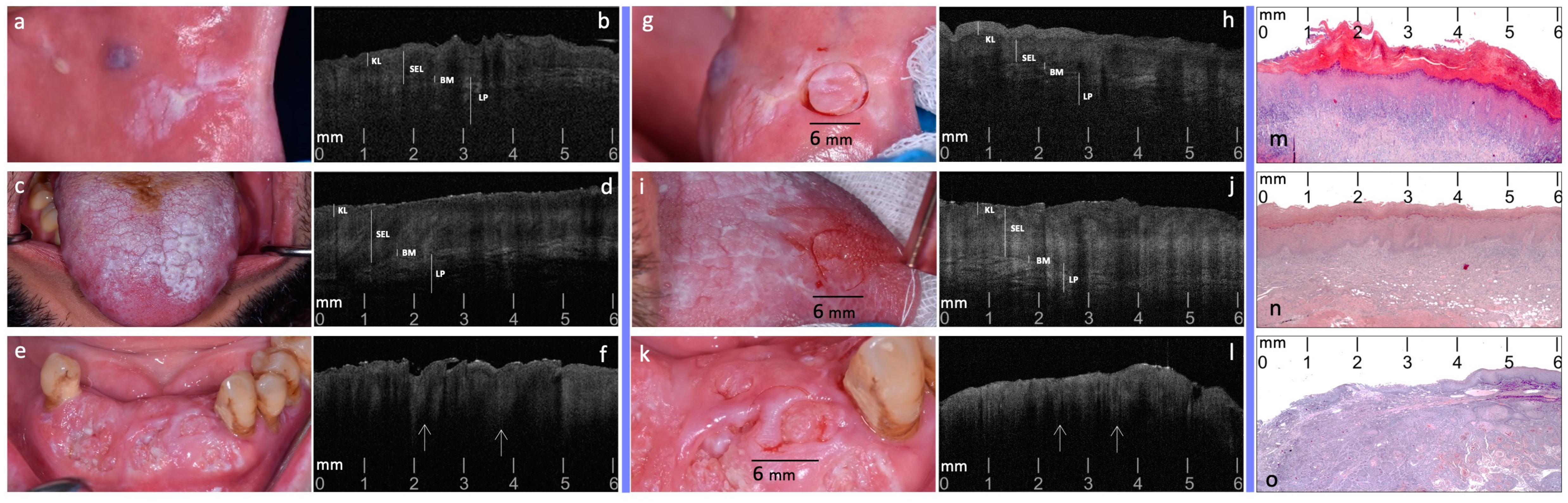
2.4. Phase 3: Targeted Biopsy and Histological Evaluation
2.5. Phase 4: Blinded Pre- and Post-Site Registration OCT Inter-Comparison to Histological Diagnosis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conway, D.I.; Purkayastha, M.; Chestnutt, I.G. The Changing Epidemiology of Oral Cancer: Definitions, Trends, and Risk Factors. Br. Dent. J. 2018, 225, 867–873. [Google Scholar] [CrossRef]
- Su, Y.-F.; Chen, Y.-J.; Tsai, F.-T.; Li, W.-C.; Hsu, M.-L.; Wang, D.-H.; Yang, C.-C. Current Insights into Oral Cancer Diagnostics. Diagnostics 2021, 11, 1287. [Google Scholar] [CrossRef]
- Abdul, N.S. Role of Advanced Diagnostic Aids in the Detection of Potentially Malignant Disorders and Oral Cancer at an Early Stage. Cureus 2023, 15, e34113. [Google Scholar] [CrossRef]
- de Kleijn, B.J.; Heldens, G.T.N.; Herruer, J.M.; Sier, C.F.M.; Piazza, C.; de Bree, R.; Guntinas-Lichius, O.; Kowalski, L.P.; Vander Poorten, V.; Rodrigo, J.P.; et al. Intraoperative Imaging Techniques to Improve Surgical Resection Margins of Oropharyngeal Squamous Cell Cancer: A Comprehensive Review of Current Literature. Cancers 2023, 15, 896. [Google Scholar] [CrossRef] [PubMed]
- Di Fede, O.; Panzarella, V.; Buttacavoli, F.; La Mantia, G.; Campisi, G. Doctoral: A Smartphone-Based Decision Support Tool for the Early Detection of Oral Potentially Malignant Disorders. Digit. Health 2023, 9, 205520762311771. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mauceri, R.; Bazzano, M.; Coppini, M.; Tozzo, P.; Panzarella, V.; Campisi, G. Diagnostic Delay of Oral Squamous Cell Carcinoma and the Fear of Diagnosis: A Scoping Review. Front. Psychol. 2022, 13, 1009080. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Boppart, S.A. Review of Optical Coherence Tomography in Oncology. J. Biomed. Opt. 2017, 22, 1. [Google Scholar] [CrossRef]
- Mauceri, R.; Coppini, M.; Vacca, D.; Bertolazzi, G.; Panzarella, V.; Di Fede, O.; Tripodo, C.; Campisi, G. Salivary Microbiota Composition in Patients with Oral Squamous Cell Carcinoma: A Systematic Review. Cancers 2022, 14, 5441. [Google Scholar] [CrossRef]
- Lee, C.-K.; Chi, T.-T.; Wu, C.-T.; Tsai, M.-T.; Chiang, C.-P.; Yang, C.-C. Diagnosis of Oral Precancer with Optical Coherence Tomography. Biomed. Opt. Express 2012, 3, 1632. [Google Scholar] [CrossRef]
- Hamdoon, Z.; Jerjes, W.; Al-Delayme, R.; McKenzie, G.; Jay, A.; Hopper, C. Structural Validation of Oral Mucosal Tissue Using Optical Coherence Tomography. Head. Neck Oncol. 2012, 4, 29. [Google Scholar] [CrossRef]
- Ghosh, B.; Bhandari, A.; Mandal, M.; Paul, R.R.; Pal, M.; Mitra, P.; Chatterjee, J. Quantitative in Situ Imaging and Grading of Oral Precancer with Attenuation Corrected-Optical Coherence Tomography. Oral. Oncol. 2021, 117, 105216. [Google Scholar] [CrossRef]
- Capocasale, G.; Panzarella, V.; Rodolico, V.; Di Fede, O.; Campisi, G. In Vivo Optical Coherence Tomography Imaging in a Case of Mucous Membrane Pemphigoid and a Negative Nikolsky’s Sign. J. Dermatol. 2018, 45, 603–605. [Google Scholar] [CrossRef]
- Ilhan, B.; Lin, K.; Guneri, P.; Wilder-Smith, P. Improving Oral Cancer Outcomes with Imaging and Artificial Intelligence. J. Dent. Res. 2020, 99, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Jerjes, W.; Upile, T.; Conn, B.; Hamdoon, Z.; Betz, C.S.; McKenzie, G.; Radhi, H.; Vourvachis, M.; El Maaytah, M.; Sandison, A.; et al. In Vitro Examination of Suspicious Oral Lesions Using Optical Coherence Tomography. Br. J. Oral. Maxillofac. Surg. 2010, 48, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; McKenzie, G.; Jay, A.; Hopper, C. Optical Coherence Tomography in the Assessment of Oral Squamous Cell Carcinoma Resection Margins. Photodiagn. Photodyn. Ther. 2016, 13, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; Upile, T.; McKenzie, G.; Jay, A.; Hopper, C. Optical Coherence Tomography in the Assessment of Suspicious Oral Lesions: An Immediate Ex Vivo Study. Photodiagn. Photodyn. Ther. 2013, 10, 17–27. [Google Scholar] [CrossRef]
- Obade, A.Y.; Pandarathodiyil, A.K.; Oo, A.L.; Warnakulasuriya, S.; Ramanathan, A. Application of Optical Coherence Tomography to Study the Structural Features of Oral Mucosa in Biopsy Tissues of Oral Dysplasia and Carcinomas. Clin. Oral. Investig. 2021, 25, 5411–5419. [Google Scholar] [CrossRef]
- Panzarella, V.; Buttacavoli, F.; Gambino, A.; Capocasale, G.; Di Fede, O.; Mauceri, R.; Rodolico, V.; Campisi, G. Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study. Cancers 2022, 14, 5916. [Google Scholar] [CrossRef]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of Oral Precancerous and Cancerous Tissue by Swept Source Optical Coherence Tomography. Lasers Surg. Med. 2022, 54, 320–328. [Google Scholar] [CrossRef]
- Yang, E.C.; Tan, M.T.; Schwarz, R.A.; Richards-Kortum, R.R.; Gillenwater, A.M.; Vigneswaran, N. Noninvasive Diagnostic Adjuncts for the Evaluation of Potentially Premalignant Oral Epithelial Lesions: Current Limitations and Future Directions. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2018, 125, 670–681. [Google Scholar] [CrossRef]
- Sunny, S.P.; Agarwal, S.; James, B.L.; Heidari, E.; Muralidharan, A.; Yadav, V.; Pillai, V.; Shetty, V.; Chen, Z.; Hedne, N.; et al. Intra-Operative Point-of-Procedure Delineation of Oral Cancer Margins Using Optical Coherence Tomography. Oral. Oncol. 2019, 92, 12–19. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Efficacy of Optical Coherence Tomography in the Diagnosing of Oral Cancerous Lesion: Systematic Review and Meta-analysis. Head. Neck 2023, 45, 473–481. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; Yang, J.; Yin, B.; Fan, X.; Yang, J.; Li, S.; Zhong, J.; Huang, X. Noninvasive Oral Cancer Screening Based on Local Residual Adaptation Network Using Optical Coherence Tomography. Med. Biol. Eng. Comput. 2022, 60, 1363–1375. [Google Scholar] [CrossRef]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of Oral Cancer in OCT Images Based on an Optical Attenuation Model. Lasers Med. Sci. 2020, 35, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Deng, X.; Sun, Y.; Wang, X.; Xiao, Y.; Li, Y.; Chen, Q.; Jiang, L. Optical Imaging in the Diagnosis of OPMDs Malignant Transformation. J. Dent. Res. 2022, 101, 749–758. [Google Scholar] [CrossRef]
- Ramezani, K.; Tofangchiha, M. Oral Cancer Screening by Artificial Intelligence-Oriented Interpretation of Optical Coherence Tomography Images. Radiol. Res. Pract. 2022, 2022, 1614838. [Google Scholar] [CrossRef]
- Jerjes, W.; Hamdoon, Z.; Yousif, A.A.; Al-Rawi, N.H.; Hopper, C. Epithelial Tissue Thickness Improves Optical Coherence Tomography’s Ability in Detecting Oral Cancer. Photodiagn. Photodyn. Ther. 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Heidari, A.E.; Suresh, A.; Kuriakose, M.A.; Chen, Z.; Wilder-Smith, P.; Sunny, S.P.; James, B.L.; Lam, T.M.; Tran, A.V.; Yu, J.; et al. Optical Coherence Tomography as an Oral Cancer Screening Adjunct in a Low Resource Settings. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Lee, C.-K.; Lee, H.-C.; Chen, H.-M.; Chiang, C.-P.; Wang, Y.-M.; Yang, C.-C. Differentiating Oral Lesions in Different Carcinogenesis Stages with Optical Coherence Tomography. J. Biomed. Opt. 2009, 14, 044028. [Google Scholar] [CrossRef]
- Yang, C.C.; Tsai, M.-T.; Lee, H.-C.; Lee, C.-K.; Yu, C.-H.; Chen, H.-M.; Chiang, C.-P.; Chang, C.-C.; Wang, Y.-M.; Yang, C.C. Effective Indicators for Diagnosis of Oral Cancer Using Optical Coherence Tomography. Opt. Express 2008, 16, 15847. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; Beamishaj; Bashashati, M.; Millham, F.H.; Orgill, D.P.; et al. STROCSS 2019 Guideline: Strengthening the Reporting of Cohort Studies in Surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral. Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head. Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Essat, M.; Cooper, K.; Bessey, A.; Clowes, M.; Chilcott, J.B.; Hunter, K.D. Diagnostic Accuracy of Conventional Oral Examination for Detecting Oral Cavity Cancer and Potentially Malignant Disorders in Patients with Clinically Evident Oral Lesions: Systematic Review and Meta-analysis. Head. Neck 2022, 44, 998–1013. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, M.; Whelan, S. ICD-O International Classification of Diseases for Oncology; First Revision; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- National Cancer Institute. Surveillance, Epidemiology, and E.R.P.-(NIH/SEER). Head and Neck Equivalent Terms and Definitions C000-C148, C300-C339, C410, C411, C442, C479 Excludes Lymphoma and Leukemia M9590–M9992 and Kaposi Sarcoma M9140. 2021; pp. 1–50. Available online: https://seer.cancer.gov/tools/solidtumor/Head_Neck_STM.pdf (accessed on 15 August 2024).
- Gambino, A.; Cabras, M.; Cafaro, A.; Broccoletti, R.; Carossa, S.; Hopper, C.; Chiusa, L.; El Haddad, G.; Porter, S.R.; Arduino, P.G. In-Vivo Usefulness of Optical Coherence Tomography in Atrophic-Erosive Oral Lichen Planus: Comparison between Histopathological and Ultrastructural Findings. J. Photochem. Photobiol. B 2020, 211, 112009. [Google Scholar] [CrossRef]
- Gambino, A.; Martina, E.; Panzarella, V.; Ruggiero, T.; El Haddad, G.; Broccoletti, R.; Arduino, P.G. Potential Use of Optical Coherence Tomography in Oral Potentially Malignant Disorders: In-Vivo Case Series Study. BMC Oral. Health 2023, 23, 540. [Google Scholar] [CrossRef]
- Wilder-Smith, P.; Lee, K.; Guo, S.; Zhang, J.; Osann, K.; Chen, Z.; Messadi, D. In Vivo Diagnosis of Oral Dysplasia and Malignancy Using Optical Coherence Tomography: Preliminary Studies in 50 Patients. Lasers Surg. Med. 2009, 41, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.M.; Armstrong, W.B.; Guo, S.; Mahmood, U.; Su, J.; Jackson, R.P.; Shibuya, T.; Crumley, R.L.; Gu, M.; Chen, Z.; et al. In Vivo Optical Coherence Tomography of the Human Oral Cavity and Oropharynx. Arch. Otolaryngol. Head. Neck Surg. 2006, 132, 1074. [Google Scholar] [CrossRef]
- Volgger, V.; Stepp, H.; Ihrler, S.; Kraft, M.; Leunig, A.; Patel, P.M.; Susarla, M.; Jackson, K.; Betz, C.S. Evaluation of Optical Coherence Tomography to Discriminate Lesions of the Upper Aerodigestive Tract. Head. Neck 2013, 35, 1558–1566. [Google Scholar] [CrossRef]
- Panzarella, V.; Bartolone, A.; Coniglio, R.; Rodolico, V.; Maniscalco, L.; Capocasale, G.; Iurato Carbone, M.; Campisi, G. Diagnostic Concordance between Optical Coherence Tomography and Histological Investigations for Immune-Mediated Desquamative Gingivitis: Observational Study. Int. J. Environ. Res. Public Health 2021, 18, 9095. [Google Scholar] [CrossRef]
- Gruda, Y.; Albrecht, M.; Buckova, M.; Haim, D.; Lauer, G.; Koch, E.; Joehrens, K.; Schnabel, C.; Golde, J.; Li, J.; et al. Characteristics of Clinically Classified Oral Lichen Planus in Optical Coherence Tomography: A Descriptive Case-Series Study. Diagnostics 2023, 13, 2642. [Google Scholar] [CrossRef]
- Gambino, A.; Cafaro, A.; Broccoletti, R.; Turotti, L.; Karimi, D.; El Haddad, G.; Hopper, C.; Porter, S.R.; Chiusa, L.; Arduino, P.G. In Vivo Evaluation of Traumatic and Malignant Oral Ulcers with Optical Coherence Tomography: A Comparison between Histopathological and Ultrastructural Findings. Photodiagn. Photodyn. Ther. 2022, 39, 103019. [Google Scholar] [CrossRef]
- Mercaldo, N.D.; Lau, K.F.; Zhou, X.H. Confidence intervals for predictive values with an emphasis to case-control studies. Stat. Med. 2007, 26, 2170–2183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Kim, B.G.; Hwang, S.H. Efficacy of Artificial Intelligence-Assisted Discrimination of Oral Cancerous Lesions from Normal Mucosa Based on the Oral Mucosal Image: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3499. [Google Scholar] [CrossRef]
- James, B.L.; Sunny, S.P.; Heidari, A.E.; Ramanjinappa, R.D.; Lam, T.; Tran, A.V.; Kankanala, S.; Sil, S.; Tiwari, V.; Patrick, S.; et al. Validation of a Point-of-Care Optical Coherence Tomography Device with Machine Learning Algorithm for Detection of Oral Potentially Malignant and Malignant Lesions. Cancers 2021, 13, 3583. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, H.; Shang, J.; Zhang, J.; Liang, Y. Deep-Learning-Based Automated Identification and Visualization of Oral Cancer in Optical Coherence Tomography Images. Biomedicines 2023, 11, 802. [Google Scholar] [CrossRef]
| Predictive Value % (95% CI) | ||||
|---|---|---|---|---|
| Observer 1 | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive | Negative |
| Pre-target | ||||
| OSCC | 82.86, (71.97–90.82) | 97.86, (93.87–99.56) | 95.08, (86.26–98.35) | 91.95, (87.21–95.03) |
| OL | 70.00, (57.87–80.38) | 85.00, (77.99–90.47) | 70.00, (60.45–78.08) | 85.00, (79.74–89.08) |
| OLP | 68.57, (56.37–79.15) | 77.86, (70.07–84.43) | 60.76, (52.21–68.70) | 83.21, (77.61–87.63) |
| Post-target | ||||
| OSCC | 98.57, (92.30–99.96) | 100.00, (97.40–100.00) | 100.00, (94.79–100.00) | 99.29, (95.24–99.90) |
| OL | 98.57, (92.30–99.96) | 98.57, (94.93–99.83) | 97.18, (89.70–99.27) | 99.28, (95.17–99.90) |
| OLP | 97.14, (90.06–99.65) | 98.57, (94.93–99.83) | 97.14, (89.56–99.26) | 98.57, (94.62–99.63) |
| Observer 2 | ||||
| Pre-target | ||||
| OSCC | 82.86, (71.97–90.82) | 98.57, (94.93–99.83) | 96.67, (87.94–99.14) | 92.00, (87.29–95.06) |
| OL | 70.00, (57.87–80.38) | 84.29, (77.18–89.88) | 69.01, (59.57–77.10) | 84.89, (79.60–89.00) |
| OLP | 68.57, (56.37–79.15) | 78.57, (70.84–85.05) | 61.54, (52.88–69.52) | 83.33, (77.78–87.72) |
| Post-target | ||||
| OSCC | 98.57, (92.30–99.96) | 100.00, (97.40–100.00) | 100.00, (94.79–100.00) | 99.29, (95.24–99.90) |
| OL | 98.57, (92.30–99.96) | 98.57, (94.93–99.83) | 97.18, (89.70–99.27) | 99.28, (95.17–99.90) |
| OLP | 97.14, (90.06–99.65) | 98.57, (94.93–99.83) | 97.14, (89.56–99.26) | 98.57, (94.62–99.63) |
| Observer 1 | Pre-Target n. (%) | Post-Target n. (%) | Difference | 95% CI, % * | p-Value | |
|---|---|---|---|---|---|---|
| OSCC | 58 (82.9) | 69 (98.6) | 15.71% | 7.19 | 24.24 | 0.001 |
| OL | 49 (70) | 69 (98.6) | 28.57% | 17.99 | 39.15 | <0.001 |
| OLP | 48 (68.6) | 68 (97.1) | 28.57% | 17.99 | 39.15 | <0.001 |
| Observer 2 | ||||||
| OSCC | 57 (81.4) | 69 (98.6) | 17.14% | 8.31 | 25.97 | <0.001 |
| OL | 50 (71.4) | 69 (98.6) | 27.14% | 16.73 | 37.56 | <0.001 |
| OLP | 49 (70) | 68 (97.1) | 27.14% | 16.73 | 37.56 | <0.001 |
| Author (Year) | Study Design | N. Cases | Clinical Appearance | Pathological Diagnosis | Oral Sites | OCT Patterns Parameters/Evaluation | |
|---|---|---|---|---|---|---|---|
| 1 | Ridgway (2006) [41] | Case series | 41 | Not specified benign and malignant lesions | Begnin lesions, OL, CIS, OSCC | Buccal mucosa, Floor of mouth, Gingiva, Hard palate, Lip, Tongue | SS, BM/LP |
| 2 | Wilder-Smith (2009) [40] | Preliminary Study | 50 | Leukoplakia, erythroplakia | Dysplasia (mild, moderate, severe) CIS, OSCC | Tongue, Buccal mucosa, Floor of mouth | SEL, BM, LP |
| 3 | Volgger (2012) [42] | Prospective diagnostic trial | 100 | Leukoplakia, erythroplakia | OSCC, dysplasia, OLP | Buccal mucosa, Floor of mouth, Gingiva, Palate, Lip, Tongue (detailed only for healthy mucosa) | KL, EP, ET, BM, LP |
| 4 | Gambino (2020) [38] | Case control study | 20 | Atrophic-erosive OLP | OLP | Buccal mucosa | EP, BM, LP |
| 5 | Panzarella (2021) [43] | Observational study | 43 | DG | OLP, PV, MMP | Gingiva | SEL, BM, LP |
| 6 | Panzarella (2022) [19] | Descriptive pilot Study | 30 | Suspected OSCC | OSCC | Tongue, Gingiva, Buccal mucosa | KL, SEL, BM, LP |
| 7 | Gambino 2022 [45] | Case/control study | 50 | Non-healing Ulcerations | Traumatic lesions, OSCC | Buccal mucosa, Gingiva, Tongue | EP, BM, LP |
| 8 | Gambino (2023) [39] | Case series | 11 | White, red-white lesions, ulcers | PVL, OLP, OL, GVHD, K-MICRO | Tongue, Gingiva, Buccal mucosa | KL, EP, BM, LP |
| 9 | Gruda (2023) [44] | Case series | 15 | OLP, leukoplakia | OLP, OL, OSCC | Buccal mucosa, Tongue | EP, EP Re, LP Re, BM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzarella, V.; Buttacavoli, F.; Rodolico, V.; Maniscalco, L.; Firenze, A.; De Caro, V.; Mauceri, R.; Rombo, S.E.; Campisi, G. Application of Targeted Optical Coherence Tomography in Oral Cancer: A Cross-Sectional Preliminary Study. Diagnostics 2024, 14, 2247. https://doi.org/10.3390/diagnostics14192247
Panzarella V, Buttacavoli F, Rodolico V, Maniscalco L, Firenze A, De Caro V, Mauceri R, Rombo SE, Campisi G. Application of Targeted Optical Coherence Tomography in Oral Cancer: A Cross-Sectional Preliminary Study. Diagnostics. 2024; 14(19):2247. https://doi.org/10.3390/diagnostics14192247
Chicago/Turabian StylePanzarella, Vera, Fortunato Buttacavoli, Vito Rodolico, Laura Maniscalco, Alberto Firenze, Viviana De Caro, Rodolfo Mauceri, Simona E. Rombo, and Giuseppina Campisi. 2024. "Application of Targeted Optical Coherence Tomography in Oral Cancer: A Cross-Sectional Preliminary Study" Diagnostics 14, no. 19: 2247. https://doi.org/10.3390/diagnostics14192247











