Analysis of Predictors and Risk Factors of Postpolypectomy Syndrome
Abstract
1. Introduction
2. Methods
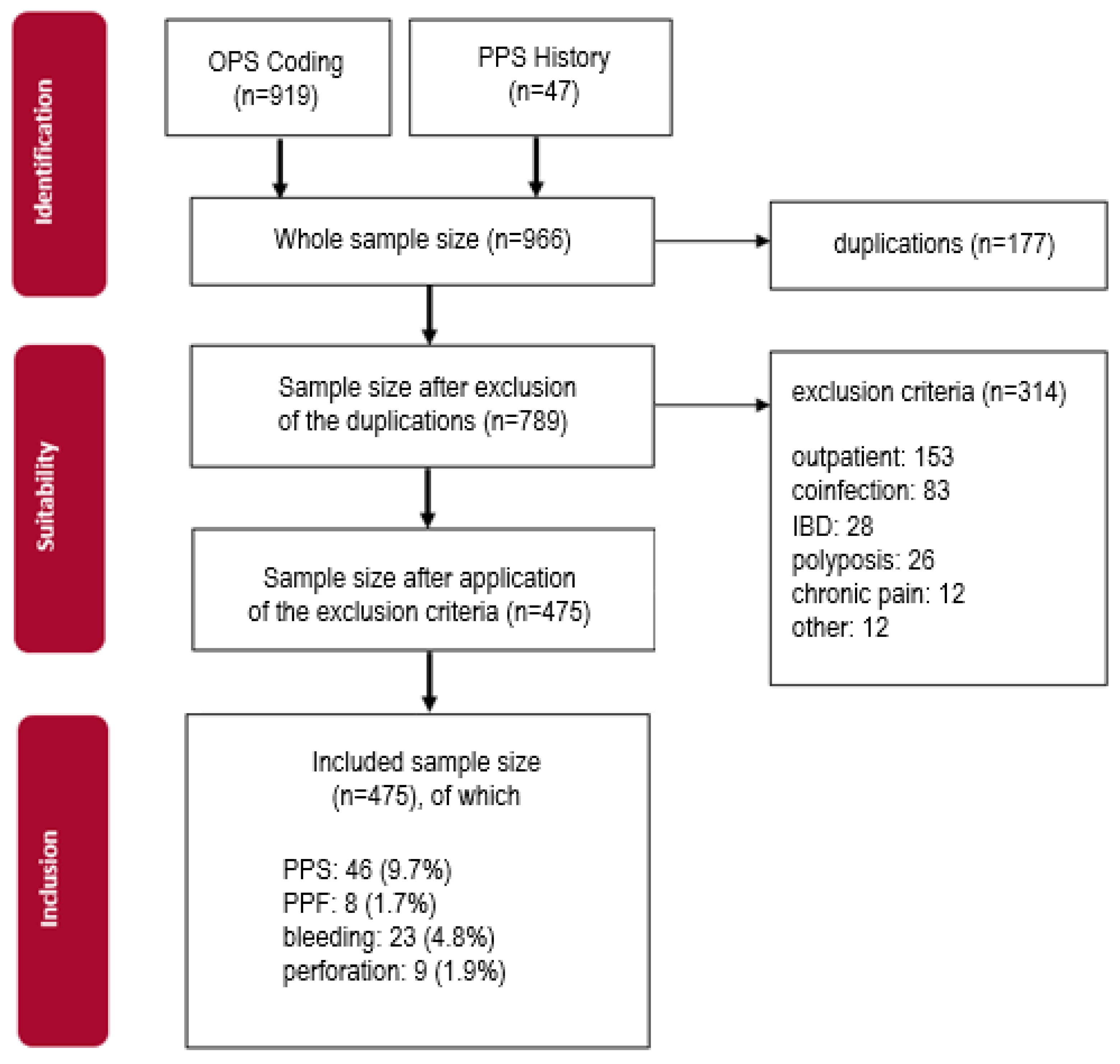
2.1. Study Setting and Patient Selection
2.2. Statistical Analysis
3. Results
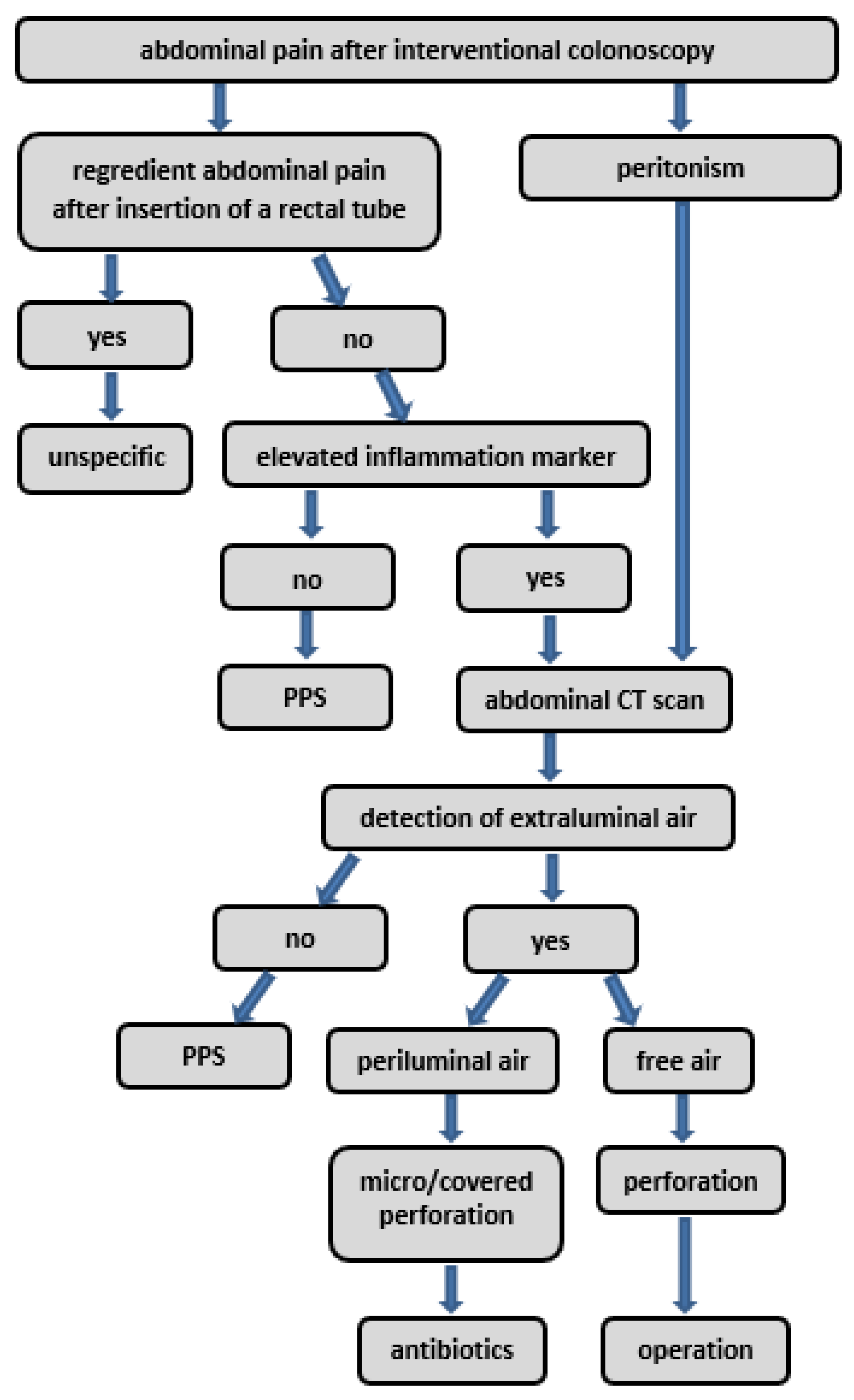
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waye, J.D.; Lewis, B.S.; Yessayan, S. Colonoscopy: A prospective report of complications. J. Clin. Gastroenterol. 1992, 15, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Dafnis, G.; Ekbom, A.; Pahlman, L.; Blomqvist, P. Complications of diagnostic and therapeutic colonoscopy within a defined population in Sweden. Gastrointest. Endosc. 2001, 54, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Viiala, C.H.; Zimmerman, M.; Cullen, D.J.E.; Hoffman, N.E. Complication rates of colonoscopy in an Australian teaching hospital environment. Intern. Med. J. 2003, 33, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cheoi, K.S.; Chung, H.; Park, J.C.; Shin, S.K.; Lee, S.K.; Lee, Y.C. Clinical features and predictive factors of coagulation syndrome after endoscopic submucosal dissection for early gastric neoplasm. Gastric Cancer 2012, 15, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hotta, K.; Imai, K.; Yamaguchi, Y.; Kishida, Y.; Takizawa, K.; Kakushima, N.; Tanaka, M.; Kawata, N.; Yoshida, M.; et al. Risk factors of post-endoscopic submucosal dissection electrocoagulation syndrome for colorectal neoplasm. J. Gastroenterol. Hepatol. 2018, 33, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Kus, J.; Haque, S.; Kazan-Tannus, J.; Jawahar, A. Postpolypectomy coagulation syndrome—An uncommon complication of colonoscopy. Clin. Imaging 2021, 79, 133–135. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, M.G.; Jeong, S.H.; Yang, H.J.; Jung, Y.S.; Choi, K.Y.; Kim, H.; Kim, H.O.; Jeong, K.U.; Chun, H.K.; et al. Prospective Analysis of Minor Adverse Events after Colon Polypectomy. Dig. Dis. Sci. 2017, 62, 2113–2119. [Google Scholar] [CrossRef]
- Jung, D.; Youn, Y.H.; Jahng, J.; Kim, J.H.; Park, H. Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy 2013, 45, 714–717. [Google Scholar] [CrossRef]
- Rogers, B.H.; Silvis, S.E.; Nebel, O.T.; Sugawa, C.; Mandelstam, P. Complications of flexible fiberoptic colonoscopy and polypectomy. Gastrointest. Endosc. 1975, 22, 73–77. [Google Scholar] [CrossRef]
- Waye, J.D. Selected papers from the Second International Congress on Colonoscopy and Diseases of the Large Bowel, March 6 to 8, 1980. Gastrointest. Endosc. 1981, 27, 184–187. [Google Scholar] [CrossRef]
- Zachou, M.; Pikramenos, K.; Mpetsios, G.; Lalla, E.; Panoutsakou, M.; Varytimiadis, K.; Karantanos, P. Post-polypectomy coagulation syndrome: A tricky to diagnose hot snare problem that can be eliminated thanks to cold snare revolution. Arch. Clin. Cases 2022, 9, 170–172. [Google Scholar] [CrossRef]
- Christie, J.P.; Marrazzo, J., 3rd. “Mini-perforation” of the colon--not all postpolypectomy perforations require laparotomy. Dis. Colon. Rectum 1991, 34, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.W.; Riffle, S.; Michaels, L.; Morris, C.; Holub, J.; Shapiro, J.A.; Ciol, M.A.; Kimmey, M.B.; Seeff, L.C.; Lieberman, D. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin. Gastroenterol. Hepatol. 2010, 8, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Shimura, T.; Katano, T.; Iwai, T.; Mizuno, Y.; Yamada, T.; Ebi, M.; Hirata, Y.; Nishie, H.; Mizushima, T.; et al. A multicenter, single-blind randomized controlled trial of endoscopic clipping closure for preventing coagulation syndrome after colorectal endoscopic submucosal dissection. Gastrointest. Endosc. 2020, 91, 859–867.e851. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Ho, Y.H.; Leong, A.P.; Seow-Choen, F. Clinical behavior of complicated right-sided and left-sided diverticulosis. Dis. Colon. Rectum 1997, 40, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.K.; Subhani, J. Complication rates of colonic polypectomy in relation to polyp characteristics and techniques: A district hospital experience. J. Interv. Gastroenterol. 2012, 2, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, T.; Takeuchi, Y.; Uedo, N.; Hamada, K.; Aoi, K.; Yamasaki, Y.; Matsuura, N.; Kanesaka, T.; Akasaka, T.; Yamamoto, S.; et al. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J. Gastroenterol. Hepatol. 2016, 31, 615–620. [Google Scholar] [CrossRef]
- Lee, T.H.; Hsueh, P.R.; Yeh, W.C.; Wang, H.P.; Wang, T.H.; Lin, J.T. Low frequency of bacteremia after endoscopic mucosal resection. Gastrointest. Endosc. 2000, 52, 223–225. [Google Scholar] [CrossRef]
- Min, B.H.; Chang, D.K.; Kim, D.U.; Kim, Y.H.; Rhee, P.L.; Kim, J.J.; Rhee, J.C. Low frequency of bacteremia after an endoscopic resection for large colorectal tumors in spite of extensive submucosal exposure. Gastrointest. Endosc. 2008, 68, 105–110. [Google Scholar] [CrossRef]
- Muro, T.; Higuchi, N.; Imamura, M.; Nakagawa, H.; Honda, M.; Nakao, K.; Izumikawa, K.; Sasaki, H.; Kitahara, T. Post-operative infection of endoscopic submucosal dissection of early colorectal neoplasms: A case-controlled study using a Japanese database. J. Clin. Pharm. Ther. 2015, 40, 573–577. [Google Scholar] [CrossRef][Green Version]
- Shi, Z.; Qiu, H.; Liu, H.; Yu, H. Should antibiotics be administered after endoscopic mucosalresection in patients with colon polyps? Turk. J. Med. Sci. 2016, 46, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Carr, N.J.; Mahajan, H.; Tan, K.L.; Hawkins, N.J.; Ward, R.L. Serrated and non-serrated polyps of the colorectum: Their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J. Clin. Pathol. 2009, 62, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Spring, K.J.; Zhao, Z.Z.; Karamatic, R.; Walsh, M.D.; Whitehall, V.L.; Pike, T.; Simms, L.A.; Young, J.; James, M.; Montgomery, G.W.; et al. High prevalence of sessile serrated adenomas with BRAF mutations: A prospective study of patients undergoing colonoscopy. Gastroenterology 2006, 131, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.I.; van Niekerk de, W.; Owen, D.; Tha, S.P.; Turbin, D.A.; Webber, D.L. Longitudinal outcome study of sessile serrated adenomas of the colorectum: An increased risk for subsequent right-sided colorectal carcinoma. Am. J. Surg. Pathol. 2010, 34, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Sugihara, K.; Jass, J.R. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005, 47, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Ahnen, D.J.; Baron, J.A.; Batts, K.P.; Burke, C.A.; Burt, R.W.; Goldblum, J.R.; Guillem, J.G.; Kahi, C.J.; Kalady, M.F.; et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am. J. Gastroenterol. 2012, 107, 1315–1329, quiz 1314, 1330. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Tanaka, K.; Katsurahara, M.; Horiki, N.; Yamada, R.; Yamada, T.; Takei, Y. Utility of the narrow-band imaging international colorectal endoscopic classification for optical diagnosis of colorectal polyp histology in clinical practice: A retrospective study. BMC Gastroenterol. 2021, 21, 336. [Google Scholar] [CrossRef]
- Nivatvongs, S. Complications in colonoscopic polypectomy. An experience with 1555 polypectomies. Dis. Colon. Rectum 1986, 29, 825–830. [Google Scholar] [CrossRef]
- Waye, J.D. Pitfalls in polypectomy: From gene to cure. Eur. J. Cancer 1995, 31A, 1133–1137. [Google Scholar] [CrossRef]
- Arimoto, J.; Higurashi, T.; Kato, S.; Fuyuki, A.; Ohkubo, H.; Nonaka, T.; Yamaguchi, Y.; Ashikari, K.; Chiba, H.; Goto, S.; et al. Risk factors for post-colorectal endoscopic submucosal dissection (ESD) coagulation syndrome: A multicenter, prospective, observational study. Endosc. Int. Open 2018, 6, E342–E349. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Takeuchi, Y.; Iwatsubo, T.; Kato, M.; Hamada, K.; Tonai, Y.; Matsuura, N.; Kanesaka, T.; Yamashina, T.; Arao, M.; et al. Line-assisted complete closure for a large mucosal defect after colorectal endoscopic submucosal dissection decreased post-electrocoagulation syndrome. Dig. Endosc. 2018, 30, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.M.; Lim, K.S.; Lee, S.H.; Joo, Y.E.; Hong, S.P.; Kim, T.I.; Kim, H.G.; Park, D.I.; Kim, S.E.; Yang, D.H.; et al. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: A multicenter, retrospective, case-control study. Endoscopy 2013, 45, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Tariq, T.; Gilmore, A.; Kim, H.N.; Tang, J.; Ghabril, M.; Abdeljawad, K. Bleeding Risk with Cold Snare Polypectomy of ≤10 mm Pedunculated Colon Polyps. J. Clin. Gastroenterol. 2022, 57, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.M.; Zhu, W.Q. Cold or Hot Snare with Endoscopic Mucosal Resection for 6–9 mm Colorectal Polyps: A Propensity Score Matching Analysis. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 158–164. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Sugita, A.; Yamauchi, M.; Moriyama, M. Width and depth of resection for small colorectal polyps: Hot versus cold snare polypectomy. Gastrointest. Endosc. 2018, 87, 1095–1103. [Google Scholar] [CrossRef]
- La Regina, D.; Mongelli, F.; Fasoli, A.; Lollo, G.; Ceppi, M.; Saporito, A.; Garofalo, F.; Di Giuseppe, M.; Ferrario di Tor Vajana, A. Clinical Adverse Events after Endoscopic Resection for Colorectal Lesions: A Meta-Analysis on the Antibiotic Prophylaxis. Dig. Dis. 2020, 38, 15–22. [Google Scholar] [CrossRef]
- Sethi, A.; Song, L.M. Adverse events related to colonic endoscopic mucosal resection and polypectomy. Gastrointest. Endosc. Clin. N. Am. 2015, 25, 55–69. [Google Scholar] [CrossRef]


| Control | PPS | p Values | |
|---|---|---|---|
| Cohort Size, n | n1 = 429 | n2 = 46 | |
| patient characteristics | |||
| sex, n (%) | |||
| female | 154 (35.9) | 20 (43.5) | 0.28 |
| male | 275 (64.1) | 26 (56.5) | 0.42 |
| age, mean ± SD | 68.8 ± 11.1 | 65.7 ± 11.1 | 0.68 |
| BMI | |||
| n1: median (IQR) | 26.1 (24.0–29.4) | ||
| n2: mean ± SD | 27.6 ± 4.9 | ||
| pre-existing diseases, n (%) | 279 (65.0) | 28 (60.9) | 0.36 |
| coronary heart disease | 63 (14.7) | 5 (10.9) | 0.29 |
| diabetes mellitus | 76 (17.7) | 7 (15.2) | 0.71 |
| arterial hypertension | 205 (47.8) | 25 (54.3) | 0.32 |
| kidney insufficiency | 46 (10.7) | 2 (4.3) | 0.18 |
| liver insufficiency | 29 (6.8) | 4 (8.7) | 0.45 |
| COPD | 19 (4.4) | 3 (6.5) | 0.51 |
| autoimmune disorder | 17 (4.0) | 1 (2.2) | 0.34 |
| immunosuppressive disease | 9 (2.1) | 2 (4.3) | 0.29 |
| multimorbidity | 126 (29.4) | 13 (28.3) | 0.75 |
| long-term analgesic medication, n (%) | 56 (13.1) | 7 (15.2) | 0.21 |
| nicotine abuse, n (%) | 136 (31.7) | 8 (17.4) | 0.09 |
| yes | 89 (65.4) | 6 (75.0) | 0.23 |
| no | 47 (34.6) | 2 (25.0) | 0.17 |
| peri-procedural aspects | |||
| antibiotic pretreatment, n (%) | 15 (3.5) | 1 (2.2) | 0.34 |
| antibiotic treatment, n (%) | 35 (8.2) | 11 (23.9) | 0.18 |
| blood cultures, n (%) | 8 (1.9) | 4 (8.7) | 0.22 |
| negative | 6 (1.4) | 4 (8.7) | 0.30 |
| positive | 2 (0.5) | 0 | 0.56 |
| periluminal air, n (%) | |||
| negative | 19 (4.4) | 6 (13.0) | 0.16 |
| positive | 0 | 1 (2.2) | 0.43 |
| adverse events, n (%) | |||
| perforation | 9 (2.1) | ||
| periinterventional bleeding | 59 (13.8) | ||
| postinterventional bleeding | 23 (5.4) | ||
| PPF | 8 (1.8) | ||
| other | 2 (0.5) | ||
| (a) | |||
|---|---|---|---|
| Control | PPS | p Value | |
| sample size, n | 429 | 46 | |
| duration of intervention (min) | |||
| median (IQR) | 45 (31.3–61.5) | 52 (40–70) | 0.24 |
| antibiotic during inpatient care, n (%) | 35 (8.2) | 11 (23.9) | 0.12 |
| particularities, n (%) | 52 (12.1) | 6 (13) | 0.67 |
| APC, n (%) | 84 (19.6) | 8 (17.4) | 0.54 |
| clip application, n (%) | 193 (45.0) | 20 (43.5) | 0.72 |
| relevant diverticulosis, n (%) | 143 (33.3) | 18 (39.1) | 0.34 |
| (b) | |||
| number of polyps, n | 1156 | 133 | |
| median (IQR) | 2.84 (1–4) | 2.98 (1–4) | 0.53 |
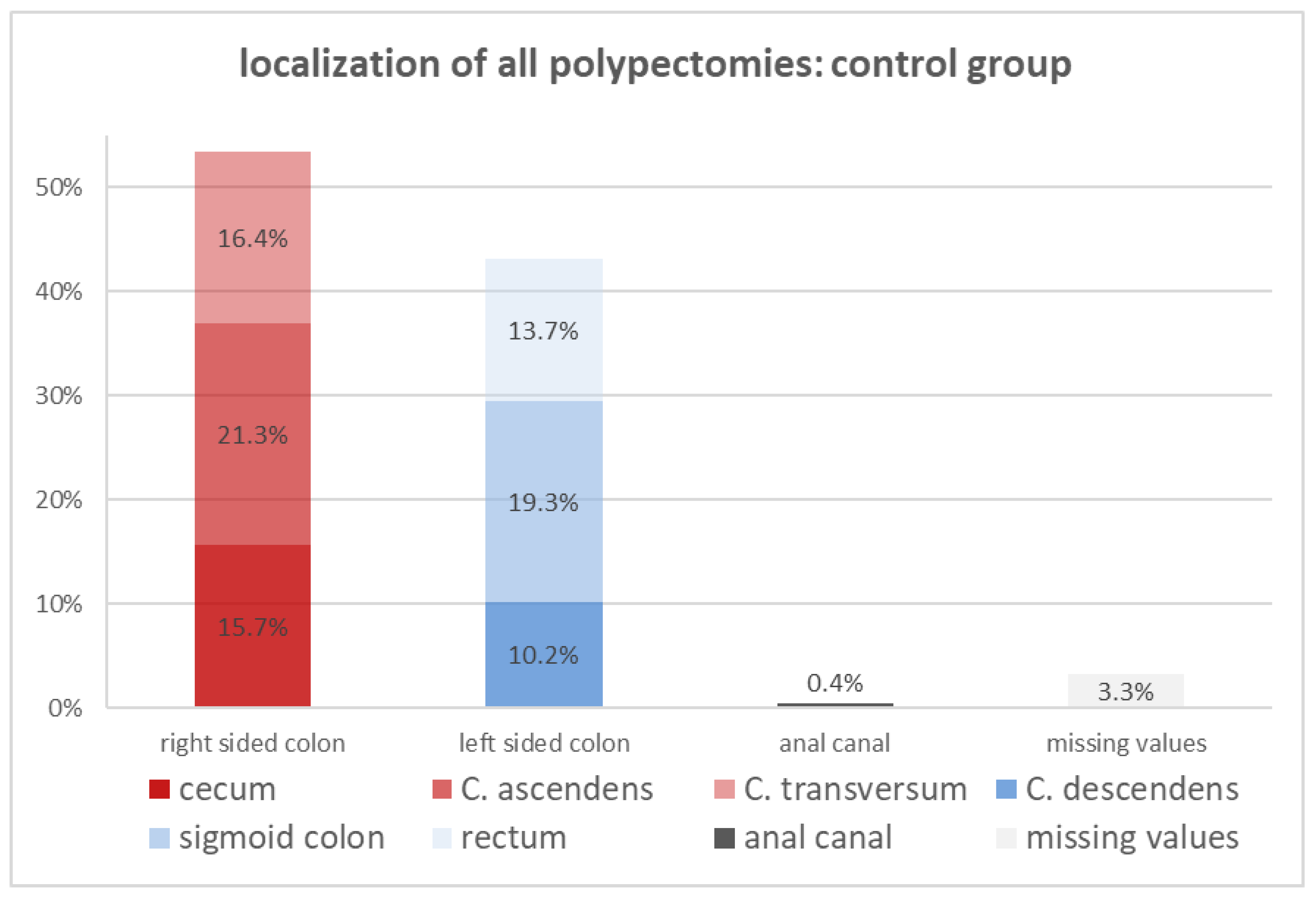
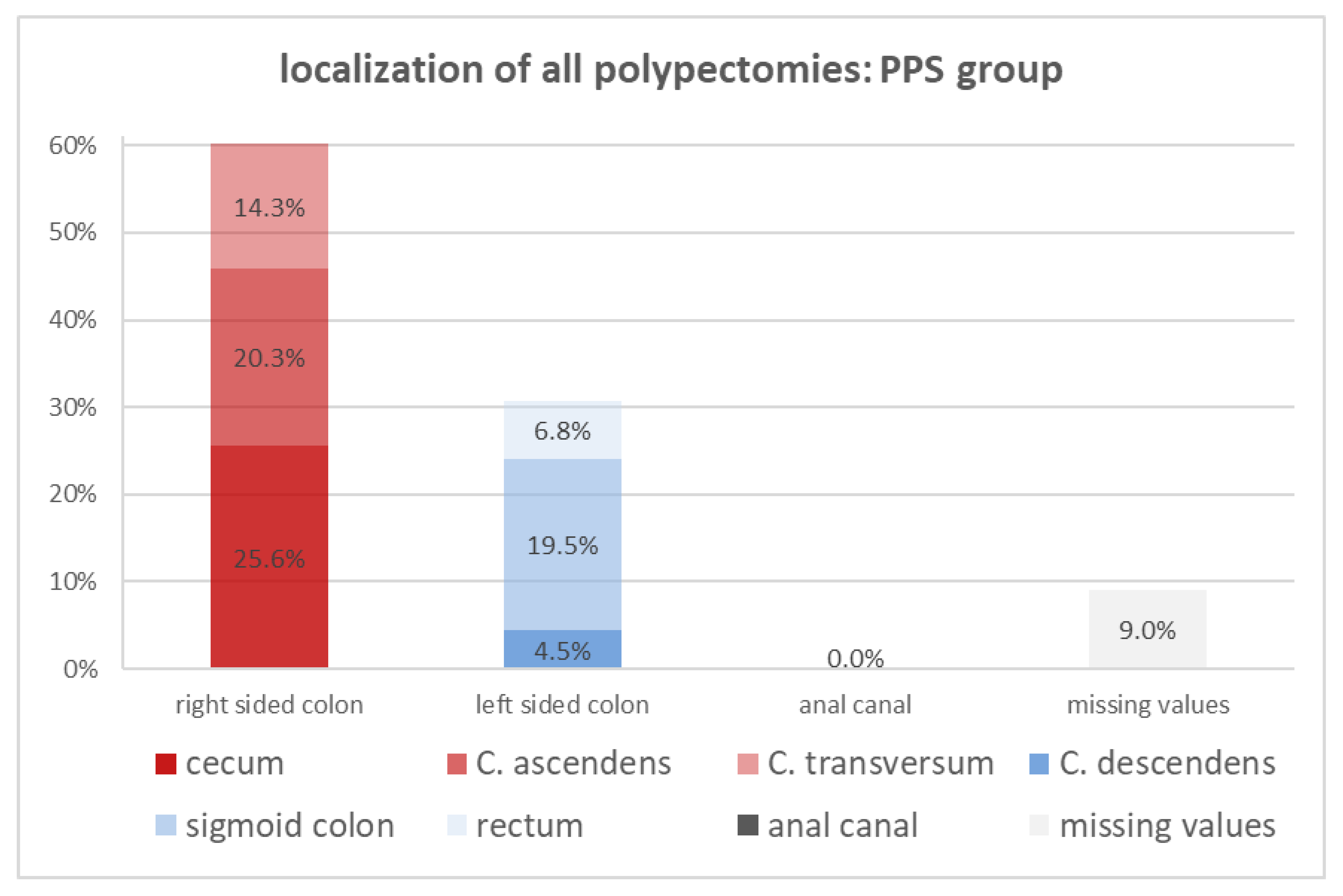
| localization, n (%) | 1122 (97.0) | 121 (91) | |
| cecum | 182 (15.7) | 34 (25.6) | 0.45 |
| colon ascendens | 246 (21.3) | 27 (20.3) | 0.51 |
| colon transversum | 190 (16.4) | 19 (14.3) | 0.34 |
| colon descendens | 118 (10.2) | 6 (4.5) | 0.29 |
| sigmoid colon | 223 (19.3) | 26 (19.5) | 0.75 |
| rectum | 158 (13.7) | 9 (6.8) | 0.21 |
| anal canal | 5 (0.4) | 0 | 0.69 |
| result of resection, n (%) | 1067 (92.3) | 121 (91) | |
| failure | 1 (0.1) | 0 (0) | 0.76 |
| en bloc | 827 (71.5) | 94 (70.7) | 0.82 |
| piecemeal | 202 (17.5) | 25 (18.8) | 0.53 |
| incomplete | 30 (2.6) | 2 (1.5) | 0.47 |
| method of intervention, n (%) | 1052 (91) | 121 (91) | |
| forceps | 381 (33.0) | 37 (27.8) | 0.26 |
| EMR | 716 (61.9) | 88 (66.2) | 0.24 |
| EMR combined | 46 (6.4% of EMR) | 8 (10) | |
| ESD | 0 | 0 | 1.00 |
| EFTR | 15 (1.3) | 1 (2.2) | 0.64 |
| EFTR combined | 9 (60% of EFTR) | 0 | |
| Odds Ratio (95% CI) | p-Value ≤ 0.05 | |
|---|---|---|
| female sex, n (%) | 1.374 (0.742–2.542) | 0.310 |
| pre-existing diseases, n (%) | ||
| 1.301 (0.707–2.395) | 0.397 |
| duration of the procedure | ||
| 1.723 (0.925–3.208) | 0.083 |
| relevant diverticulosis | 1.277 (0.683–2.386) | 0.443 |
| localization, n (%) | ||
| 1.653 (0.774–3.536) | 0.190 |
| 2.021 (1.096–3.727) | 0.022 |
| 1.207 (0.653–2.231) | 0.548 |
| piecemeal resection, n (%) | 1.356 (0.737–2.496) | 0.326 |
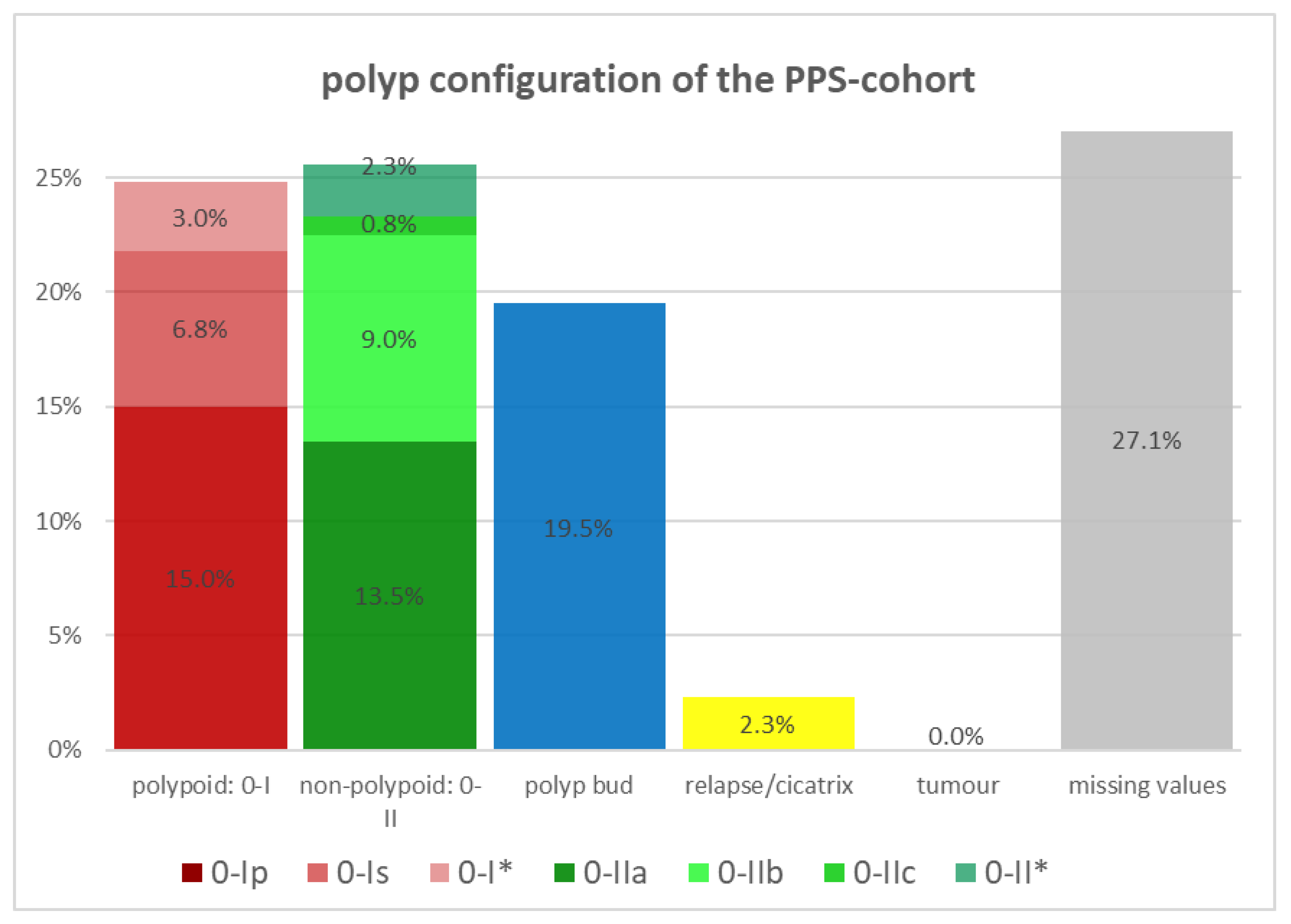
| polyp configuration, n (%) | ||
| 2.470 (1.229–4.966) | 0.009 |
| 1.527 (0.809–2.881) | 0.189 |
| 2.233 (0.870–5.731) | 0.087 |
| polyp morphology, n (%) | ||
| 1.568 (0.852–2.2885) | 0.146 |
| 3.716 (1.556–8.875) | 0.002 |
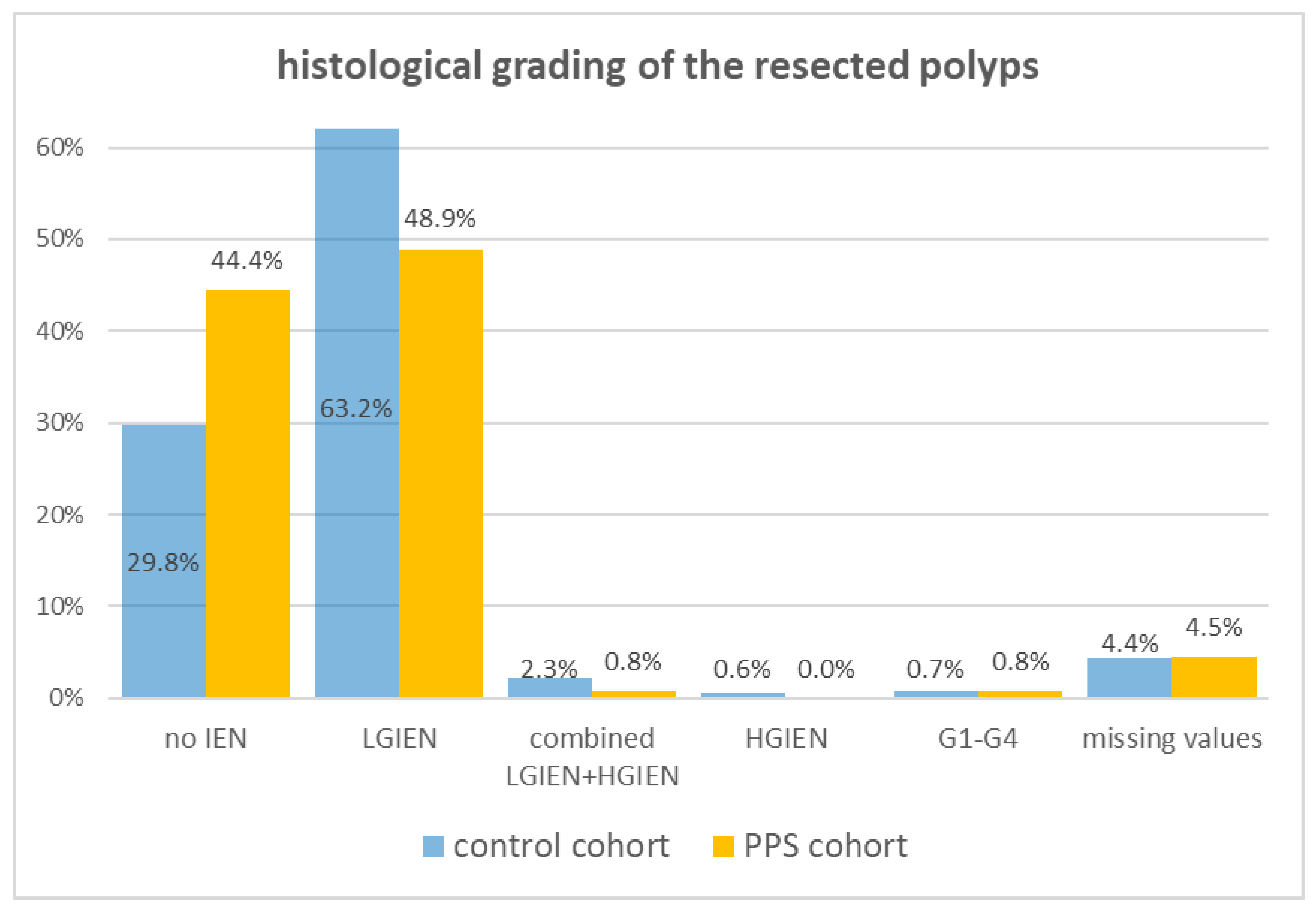
| grading, n (%) | ||
| 2.700 (1.416–5.148) | 0.002 |
| submucosal fibrosis, n (%) | 0.430 (0.101–1.840) | 0.242 |
| Coefficients a | |||||||
|---|---|---|---|---|---|---|---|
| Unstandardized Coefficients | Standardized Coefficients | 95% Confidence Interval for B | |||||
| Model | B | Std. Error | Beta | t | Sig. | Lower Bound | Upper Bound |
| (Constant) | 0.016 | 0.021 | 0.759 | 0.448 | −0.026 | 0.058 | |
| no EIN | 0.072 | 0.028 | 0.121 | 2.615 | 0.009 | 0.018 | 0.126 |
| pedunculated | 0.112 | 0.037 | 0.136 | 3.025 | 0.003 | 0.039 | 0.185 |
| serrated | 0.123 | 0.056 | 0.102 | 2.206 | 0.028 | 0.013 | 0.232 |
| cecum | 0.065 | 0.028 | 0.105 | 2.324 | 0.021 | 0.010 | 0.120 |
| Sample Size, n | n1 = 429 | n2 = 46 |
|---|---|---|
| body temperature (°C), | ||
| n1 = median + IQR (min; max) | 36.9 (36.5–37.3) | |
| n2 = mean ± SD | 37.2 ± 0.8 | |
| leukocytes (cells/µL), median (IQR) | 9200 (7115–11,288) | 10,885 (8163–13,708) |
| thrombocytes, (103/µL), mean ± SD | 210,415 ± 70,290 | 199,262 + 77,848 |
| CRP (mg/dL), median (IQR) | 1.9 (1; 4.3) | 9.4 (3.2–14.8) |
| Abdominal Pain ≤ 6 h | Abdominal Pain ≥ 6 h | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| CRP (≥0.5 mg/dL) | 83.3% | 33.3% | 94.7% | 35.3% |
| Leukocytosis | 51.1% | 62.3% | 59.5% | 63% |
| ≥37.5 °C | 26.9% | 84.2% | 31.1% | 84.5% |
| CRP + ≥37.5 °C | 13.6% | 91.5% | 22.5% | 92.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, S.; Bauer, M.E.; Schempf, U.; Stüker, D.; Blumenstock, G.; Malek, N.P.; Werner, C.R.; Wichmann, D. Analysis of Predictors and Risk Factors of Postpolypectomy Syndrome. Diagnostics 2024, 14, 127. https://doi.org/10.3390/diagnostics14020127
Fusco S, Bauer ME, Schempf U, Stüker D, Blumenstock G, Malek NP, Werner CR, Wichmann D. Analysis of Predictors and Risk Factors of Postpolypectomy Syndrome. Diagnostics. 2024; 14(2):127. https://doi.org/10.3390/diagnostics14020127
Chicago/Turabian StyleFusco, Stefano, Michelle E. Bauer, Ulrike Schempf, Dietmar Stüker, Gunnar Blumenstock, Nisar P. Malek, Christoph R. Werner, and Dörte Wichmann. 2024. "Analysis of Predictors and Risk Factors of Postpolypectomy Syndrome" Diagnostics 14, no. 2: 127. https://doi.org/10.3390/diagnostics14020127
APA StyleFusco, S., Bauer, M. E., Schempf, U., Stüker, D., Blumenstock, G., Malek, N. P., Werner, C. R., & Wichmann, D. (2024). Analysis of Predictors and Risk Factors of Postpolypectomy Syndrome. Diagnostics, 14(2), 127. https://doi.org/10.3390/diagnostics14020127







