Can Artificial Intelligence Revolutionize the Diagnosis and Management of the Atrial Septal Defect in Children?
Abstract
:1. Introduction
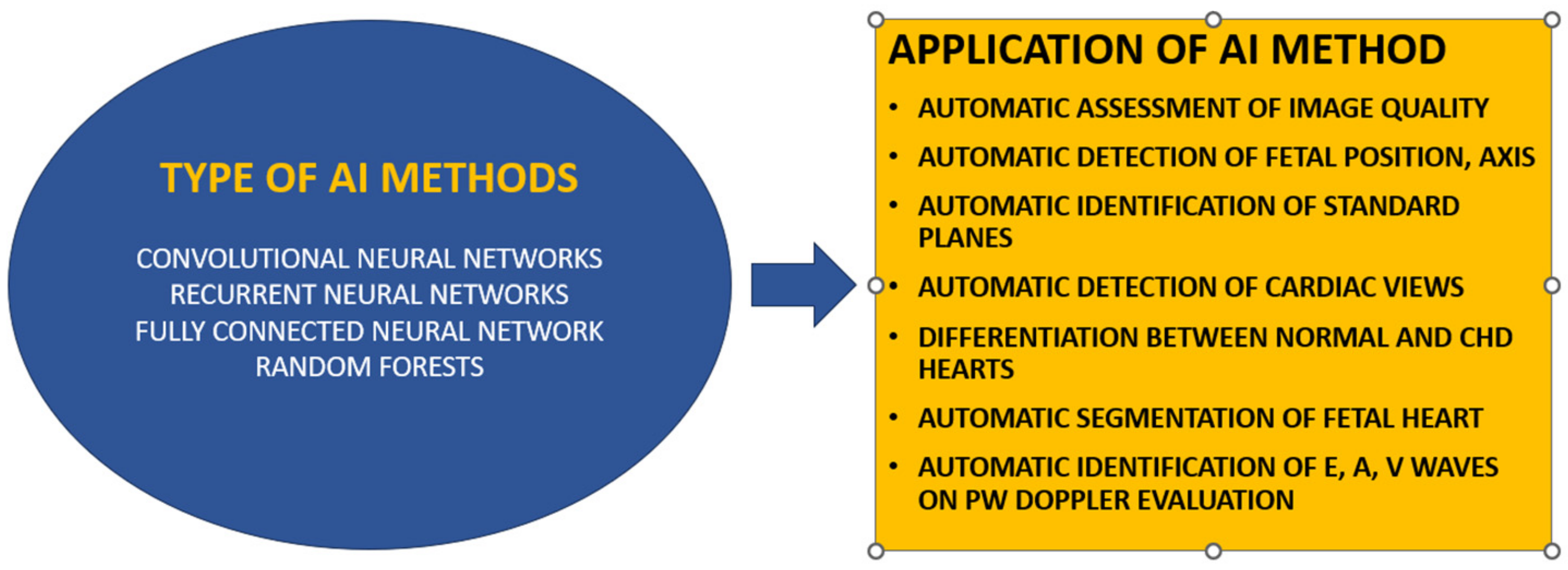
2. The Evolution of AI in Echocardiography
3. Potential for AI in ASD Diagnosis
4. Challenges and Limitations
| Study | Challenge or Limitation | Description |
|---|---|---|
| Contreras [62], Kijima [63] | Posteroinferior rim deficiency | Associated with both closure failure and concurrent adverse events. Patients with this deficiency have a lower success rate compared to those with sufficient rims. |
| Cao [64], Huang [65] | Device selection and measurement | Correct device size selection is crucial. In cases of rim deficiency, measurements need adjustment, and slight oversizing is permissible to avoid migration. |
| Carlson [80], Harikrishnan [81] | Complications of balloon sizing | Balloon sizing can cause overstretching of defect rims, leading to the use of oversized devices and potential complications such as atrial septum tear, stroke, and cardiac perforation. |
| Rana [85], Mor-Avi [86], Taniguchi [87] | Use of transesophageal echocardiography (TEE) | TEE, including 3D TEE, provides a better understanding of ASD anatomy and plays a vital role in determining device size. It has been found to be as effective as balloon sizing. |
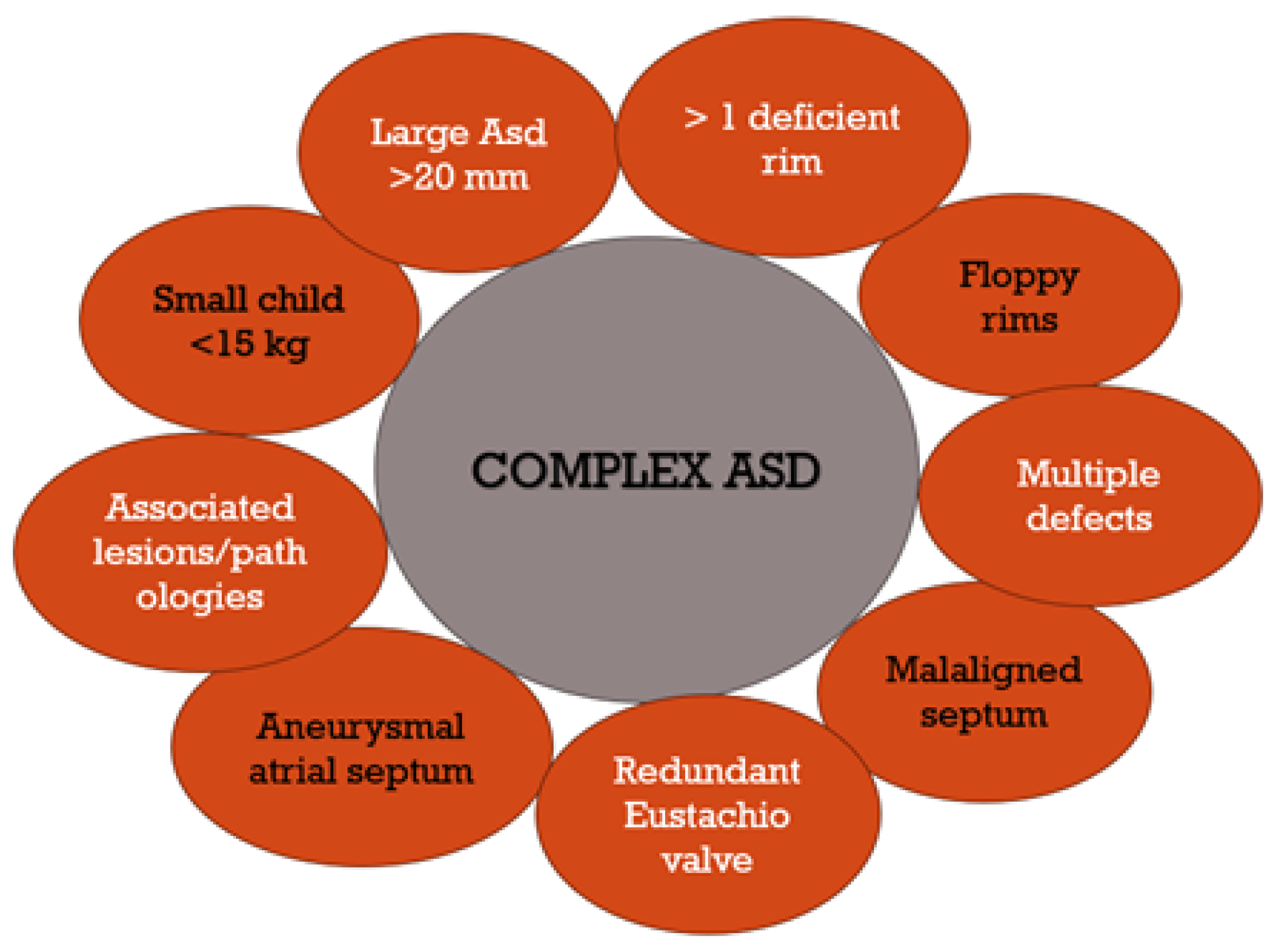
| Mahmoud [14], Shuler [90], Bartakian [8] | Complex ASD cases | Complications in complex ASD cases where traditional methods are insufficient. Includes factors like location, size, number of fenestrations, associated lesions, and patient age. |
| Butera [26], Amin [71], Awad [75] | Multi-fenestrated ASDs | Challenges in treating ASDs with multiple fenestrations, requiring consideration of aneurysmal septums, floppy rims, heart dilatation, and potentially multiple devices. |
| Faccini [93], Kim [94] | Ethical, legal, and privacy concerns in AI use | Ensuring patient autonomy, consent, and confidentiality in AI use. Legal concerns about liability in diagnostic errors or mismanagement. Privacy issues in handling patient data. |
| Tal [74], Amin [95] | Limitations of AI technology | AI model accuracy depends on training data quality and diversity. Challenges in integrating AI into existing healthcare systems and potential for bias in underrepresented populations. |
5. Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, T.G.; Kainz, B.; Hajnal, J.; Razavi, R.; Simpson, J.M. Artificial intelligence, fetal echocardiography, and congenital heart disease. Prenat. Diagn. 2021, 41, 733–742. [Google Scholar] [CrossRef]
- Vasile, C.M.; Iriart, X. Embracing AI: The Imperative Tool for Echo Labs to Stay Ahead of the Curve. Diagnostics 2023, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.S.; Smith, A.G.C.; A Sable, C.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; A Bhutta, Z.; Boucher, J.L.; et al. Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Roberson, D.A.; Cui, W.; Patel, D.; Tsang, W.; Sugeng, L.; Weinert, L.; Bharati, S.; Lang, R.M. Three-Dimensional transesophageal echocardiography of atrial septal defect: A qualitative and quantitative anatomic study. J. Am. Soc. Echocardiogr. 2011, 24, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M. 2020 ESC guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhu, H.; Zhu, J.; Li, Y.; Yu, Y.; Lei, L.; Lin, F.; Zhou, M.; Cui, L.; Zhu, T.; et al. Artificial intelligence-enabled 8-lead ECG detection of atrial septal defect among adults: A novel diagnostic tool. Front. Cardiovasc. Med. 2023, 10, 1279324. [Google Scholar] [CrossRef] [PubMed]
- Deaconu, S.; Deaconu, A.; Marascu, G.; Stanculescu, M.O.; Cozma, D.; Cinteza, E.; Vatasescu, R. Arrhythmic Risk and Treatment after Transcatheter Atrial Septal Defect Closure. Diagnostics 2024, 14, 33. [Google Scholar] [CrossRef]
- Bartakian, S.; El-Said, H.G.; Printz, B.; Moore, J.W. Prospective randomized trial of transthoracic echocardiography versus transesophageal echocardiography for assessment and guidance of transcatheter closure of atrial septal defects in children using the amplatzer septal occluder. JACC Cardiovasc. Interv. 2013, 6, 974–980. [Google Scholar] [CrossRef]
- Silvestry, F.E.; Cohen, M.S.; Armsby, L.B.; Burkule, N.J.; Fleishman, C.E.; Hijazi, Z.M.; Lang, R.M.; Rome, J.J.; Wang, Y. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen Ovale: From the American society of echocardiography and society for cardiac angiography and interventions. J. Am. Soc. Echocardiogr. 2015, 28, 910–958. [Google Scholar] [CrossRef]
- Unçalp, Ö.; Pena-Rosas, J.P.; Lawrie, T.; Bucagu, M.; Oladapo, O.T.; Portela, A.; Gülmezoglu, A.M. Who recommendations on antenatal care for a positive pregnancy experience-going beyond survival. BJOG 2017, 124, 860–862. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Feltes, T.F.; Bacha, E.; Beekman, R.H., 3rd; Cheatham, J.P.; Feinstein, J.A.; Gomes, A.S.; Hijazi, Z.M.; Ing, F.F.; de Moor, M.; Morrow, W.R.; et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2607–2652. [Google Scholar] [CrossRef] [PubMed]
- Radzik, D.; Davignon, A.; van Doesburg, N.; Fournier, A.; Marchand, T.; Ducharme, G. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J. Am. Coll Cardiol. 1993, 22, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.; Nicolescu, A.M.; Filip, C.; Nicolae, G.; Duică, G.; Bălgrădean, M.; Cinteză, E.E. Complex atrial septal defect closure in children. Rom. J. Morphol. Embryol. 2019, 60, 49–57. [Google Scholar]
- Bartakian, S.; Fagan, T.E.; Schaffer, M.S.; Darst, J.R. Device closure of secundum atrial septal defects in children < 15 kg: Complication rates and indications for referral. JACC Cardiovasc. Interv. 2012, 5, 1178–1184. [Google Scholar] [PubMed]
- Tanghöj, G.; Odermarsky, M.; Naumburg, E.; Liuba, P. Early complications after percutaneous closure of atrial septal defect in infants with procedural weight less than 15 kg. Pediatr. Cardiol. 2017, 38, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Pinto, R.; Dalvi, B. Transcatheter closure of atrial septal defect in symptomatic children weighing ≤10 kg: Addressing unanswered issues from a decade of experience. Ann. Pediatr. Cardiol. 2020, 13, 4–10. [Google Scholar] [CrossRef]
- Bedford, D.E. The anatomical types of atrial septal defect. Their incidence and clinical diagnosis. Am. J. Cardiol. 1960, 6, 568–574. [Google Scholar] [CrossRef]
- Rao, P.S.; Harris, A.D. Recent advances in managing septal defects: Atrial septal defects. F1000Research 2017, 6, 2042. [Google Scholar] [CrossRef]
- Garg, G.; Tyagi, H.; Radha, A.S. Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein into superior vena cava–an innovative technique. Catheter. Cardiovasc. Interv. 2014, 84, 473–477. [Google Scholar] [CrossRef]
- King, T.D.; Thompson, S.L.; Steiner, C.; Mills, N.L. Secundum atrial septal defect. Nonoperative closure during cardiac catheterisation. JAMA 1976, 235, 2506–2509. [Google Scholar] [CrossRef] [PubMed]
- Rashkind, W.J.; Cuaso, C.E. Transcatheter closure of atrial septal defects in children. Eur. J. Cardiol. 1977, 8, 119–120. [Google Scholar]
- Ewert, P.; Eicken, A. Do we need another device for catheter interventional closure of septum secundum atrial septal defects? EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2023, 19, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, R.; Luxenberg, D.M.; Khalid, O.; Abdulla, R. Atrial septal defect: Spectrum of care. Pediatr. Cardiol. 2008, 29, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Ramachandran, P.; Kadavigere, R.; Chacko, B. Transcatheter closure of an atrial septal defect with single coronary artery and retro-aortic right coronary artery. Hellenic. J. Cardiol. 2013, 54, 221–223. [Google Scholar] [PubMed]
- Butera, G.; Romagnoli, E.; Saliba, Z.; Chessa, M.; Sangiorgi, G.; Giamberti, A.; Cappato, R.; Bussadori, C.; Abella, R.; Pelissero, G.; et al. Percutaneous closure of multiple defects of the atrial septum: Procedural results and long-term follow-up. Catheter. Cardiovasc. Interv. 2010, 76, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Radtke, W.; Berger, F.; Zhu, W.; Hijazi, Z.M. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transoesophageal echocardiography. Eur. Heart J. 2000, 21, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Acar, P.; Saliba, Z.; Bonhoeffer, P.; Aggoun, Y.; Bonnet, D.; Sidi, D.; Kachaner, J. Influence of atrial septal defect anatomy in patient selection and assessment of closure with the Cardioseal device; a three-dimensional transoesophageal echocardiographic reconstruction. Eur. Heart J. 2000, 21, 573–581. [Google Scholar] [CrossRef]
- Feng, R.; Saraf, R.; Shapeton, A.; Matyal, R.; Laham, R.; Mahmood, F. A complex atrial septal defect and three-dimensional echocardiography: A question and an answer. J. Cardiothorac. Vasc. Anesth 2016, 30, 1050–1052. [Google Scholar] [CrossRef]
- Bartel, T.; Konorza, T.; Arjumand, J.; Ebradlidze, T.; Eggebrecht, H.; Caspari, G.; Neudorf, U.; Erbel, R. Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation 2003, 107, 795–797. [Google Scholar] [CrossRef]
- Rigatelli, G.; Dell’Avvocata, F.; Cardaioli, P.; Giordan, M.; Vassiliev, D.; Nghia, N.T.; Chen, J.P. Five-year follow-up of intracardiac echocardiography-assisted transcatheter closure of complex ostium secundum atrial septal defect. Congenit. Heart Dis. 2012, 7, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.; Scarpini, S.; Moreo, A.; Ciliberto, G.R.; Austoni, P.; Donatelli, F.; Gordini, V. Color Doppler echocardiographic assessment of atrial septal defect size: Correlation with surgical measurements. J. Am. Soc. Echocardiogr. 1991, 4, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gajjala, S.; Agrawal, P.; Tison, G.H.; Hallock, L.A.; Beussink-Nelson, L.; Lassen, M.H.; Fan, E.; Aras, M.A.; Jordan, C.; et al. Fully automated echocardiogram interpretation in clinical practice. Circulation 2018, 138, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Abe, T.; Haga, A.; Fukuda, D.; Yamada, H.; Harada, M.; Sata, M. A deep learning approach for assessment of regional wall motion abnormality from echocardiographic images. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.S.; Wang, C.S.; Chiang, J.H.; Liu, P.Y.; Tsai, W.C. Automated recognition of regional wall motion abnormalities through deep neural network interpretation of transthoracic echocardiography. Circulation 2020, 142, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.P.; Heidenreich, P.A.; Harrington, R.A.; Liang, D.H.; Ashley, E.A.; et al. Video-Based ai for beat-to-beat assessment of cardiac function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef]
- Yang, F.; Chen, X.; Lin, X.; Chen, X.; Wang, W.; Liu, B.; Li, Y.; Pu, H.; Zhang, L.; Huang, D.; et al. Automated analysis of Doppler echocardiographic videos as a screening tool for valvular heart diseases. JACC Cardiovasc. Imaging 2022, 15, 551–563. [Google Scholar] [CrossRef]
- Karatzia, L.; Aung, N.; Aksentijevic, D. Artificial intelligence in cardiology: Hope for the future and power for the present. Front. Cardiovasc. Med. 2022, 9, 945726. [Google Scholar] [CrossRef]
- Alsharqi, M.; Woodward, W.J.; Mumith, J.A.; Markham, D.C.; Upton, R.; Leeson, P. Artificial intelligence and echocardiography. Echo Res. Pract. 2018, 5, R115–R125. [Google Scholar] [CrossRef]
- Cannesson, M.; Tanabe, M.; Suffoletto, M.S.; McNamara, D.M.; Madan, S.; Lacomis, J.M.; Gorcsan, J. A novel two-dimensional echocardiographic image analysis system using artificial intelligence-learned pattern recognition for rapid automated ejection fraction. J. Am. Coll Cardiol. 2007, 49, 217–226. [Google Scholar] [CrossRef]
- Knackstedt, C.; Bekkers, S.C.; Schummers, G.; Schreckenberg, M.; Muraru, D.; Badano, L.P.; Franke, A.; Bavishi, C.; Omar, A.M.S.; Sengupta, P.P. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: The FAST-EFs multicenter study. J. Am. Coll Cardiol. 2015, 66, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Arnaout, R.; Mofrad, M.; Arnaout, R. Fast and accurate view classification of echocardiograms using deep learning. NPJ Digit Med. 2018, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Huang, Y.-M.; Bansal, M.; Ashrafi, A.; Fisher, M.; Shameer, K.; Gall, W.; Dudley, J.T. Cognitive machine-learning algorithm for cardiac imaging: A pilot study for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ Cardiovasc. Imaging 2016, 9, e004330. [Google Scholar] [CrossRef] [PubMed]
- Narula, S.; Shameer, K.; Salem Omar, A.M.; Dudley, J.T.; Sengupta, P.P. Machine learning algorithms to automate morphological and functional assessments in 2D echocardiography. J. Am. Coll Cardiol. 2016, 68, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Moghaddasi, H.; Nourian, S. Automatic assessment of mitral regurgitation severity based on extensive textural features on 2D echocardiography videos. Comput. Biol. Med. 2016, 73, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.M.; Bouteiller, X.P.; Avesani, M.; Velly, C.; Chan, C.; Jalal, Z.; Thambo, J.-B.; Iriart, X. Exploring the Potential of Artificial Intelligence in Pediatric Echocardiography—Preliminary Results from the First Pediatric Study Using AI Software Developed for Adults. J. Clin. Med. 2023, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Thalappillil, R.; Datta, P.; Datta, S.; Zhan, Y.; Wells, S.; Mahmood, F.; Cobey, F.C. Artificial intelligence for the measurement of the aortic valve annulus. J. Cardiothorac. Vasc. Anesth 2020, 34, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.; Luewan, S.; Romero, R. Fetal intelligent navigation echocardiography (FINE) detects 98% of congenital heart disease. J. Ultrasound Med. 2018, 37, 2577–2593. [Google Scholar] [CrossRef]
- Yeo, L.; Romero, R. Fetal Intelligent Navigation Echocardiography (FINE): A novel method for rapid, simple, and automatic examination of the fetal heart. Ultrasound Obstet. Gynecol. 2013, 42, 268–284. [Google Scholar] [CrossRef]
- Yeo, L.; Romero, R. New and advanced features of fetal intelligent navigation echocardiography (FINE) or 5D heart. J. Matern. -Fetal Neonatal Med. 2022, 35, 1498–1516. [Google Scholar] [CrossRef]
- Baumgartner, C.F.; Kamnitsas, K.; Matthew, J.; Fletcher, T.P.; Smith, S.; Koch, L.M.; Rueckert, D. SonoNet: Real-time detection and localisation of fetal standard scan planes in freehand ultrasound. IEEE Trans. Med. Imaging 2017, 36, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.; Truong, V.; Nguyen-Vo, T.-H.; Nguyen, B.P.; Ngo, T.N.; Bui, Q.V.; Pham, T.K.; Tretter, J.; Taylor, M.; Levy, P.; et al. Application of machine learning in screening of congenital heart diseases using fetal echocardiography. J. Am. Coll Cardiol. 2020, 75, 648. [Google Scholar] [CrossRef]
- Sulas, E.; Ortu, E.; Raffo, L.; Urru, M.; Tumbarello, R.; Pani, D. Automatic recognition of complete atrioventricular activity in fetal pulsed-wave Doppler signals. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, R.; Curran, L.; Zhao, Y.; Levine, J.C.; Chinn, E.; Moon-Grady, A.J. An ensemble of neural networks provides expert-level prenatal detection of complex congenital heart disease. Nat. Med. 2021, 27, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Gearhart, A.; Dwork, N.; Jone, P.-N. Artificial intelligence in echocardiography to diagnose congenital heart disease and fetal echocardiography. Intell-Based Med. 2022, 6, 100082. [Google Scholar] [CrossRef]
- Kusunose, K. Steps to use artificial intelligence in echocardiography. J. Echocardiogr. 2021, 19, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Orwat, S.; Vahle, J.; Bauer, U.M.M.; Urban, A.; Sarikouch, S.; Berger, F.; Beerbaum, P.; Baumgartner, H. Prediction of prognosis in patients with tetralogy of Fallot based on deep learning imaging analysis. Heart 2020, 106, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wei, Y.; Lu, Y.; Wong, K.K.L. A novel U-net approach to segment the cardiac chamber in magnetic resonance images with ghost artifacts. Comput. Methods Programs Biomed. 2020, 196, 105623. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Wang, F.; Zheng, L.; Gao, F.; Zhang, H.; Zhang, X.; Xie, W.; Wang, B. Automated interpretation of congenital heart disease from multi-view echocardiograms. Med. Image Anal. 2021, 69, 101942. [Google Scholar] [CrossRef]
- Hong, W.; Sheng, Q.; Dong, B.; Wu, L.; Chen, L.; Zhao, L.; Liu, Y.; Zhu, J.; Liu, Y.; Xie, Y.; et al. Automatic Detection of Secundum Atrial Septal Defect in Children Based on Color Doppler Echocardiographic Images Using Convolutional Neural Networks. Front. Cardiovasc. Med. 2022, 9, 834285. [Google Scholar] [CrossRef]
- Lin, X.; Yang, F.; Chen, Y.; Chen, X.; Wang, W.; Li, W.; Wang, Q.; Zhang, L.; Li, X.; Deng, Y.; et al. Echocardiography-based AI for detection and quantification of atrial septal defect. Front. Cardiovasc. Med. 2023, 10, 985657. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.E.; Ledesma, F.; Peirone, A.R.; Juaneda, E.; Defago, V.; Cuestas, E. Sufficient versus deficient rims during percutaneous closure of ostium secundum type atrial septal defect: A systematic review and meta-analysis. Indian Heart J. 2023, 75, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kijima, Y.; Akagi, T.; Takaya, Y.; Taniguchi, M.; Nakagawa, K.; Kusano, K.; Sano, S.; Ito, H. Deficient surrounding rims in patients undergoing transcatheter atrial septal defect closure. J. Am. Soc. Echocardiogr. 2016, 29, 768–776. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Z.; Huang, J.; Fan, L.; Li, R.; Wang, S.; Li, Y.; Zhang, Z. Feasibility, safety and long-term follow-up of transcatheter closure of secundum atrial septal defects with deficient rims. Cardiology 2016, 134, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Wu, J.; Chen, M.; Jiang, C.L.; Zeng, C.; Su, C.X.; Zheng, B.S. Transcatheter closure of atrial septal defect with deficient posterior-inferior or inferior vena cava rim under echocardiography only: A feasibility and safety analysis. Cardiol. Young 2022, 32, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, C.M.; Bogati, A.; Prajapati, D.; Dhungel, S.; Najmy, S.; Acharya, K.; Shahi, R.; Subedi, C.; Adhikari, J.; Sharma, D. Atrial septal defect size and rims on transesophageal echocardiogram. Maedica 2019, 14, 81–85. [Google Scholar] [PubMed]
- Pedra, C.A.; Pedra, S.R.; Esteves, C.A.; Cassar, R.; Pontes, S.C., Jr.; Braga, S.L.; Fontes, V.F. Transcatheter closure of secundum atrial septal defects with complex anatomy. J. Invasive Cardiol. 2004, 16, 117–122. [Google Scholar]
- Baruteau, A.E.; Petit, J.; Lambert, V.; Gouton, M.; Piot, D.; Brenot, P.; Angel, C.Y.; Houyel, L.; Le Bret, E.; Roussin, R.; et al. Transcatheter closure of large atrial septal defects: Feasibility and safety in a large adult and pediatric population. Circ. Cardiovasc. Interv. 2014, 7, 837–843. [Google Scholar] [CrossRef]
- Pillai, A.A.; Satheesh, S.; Pakkirisamy, G.; Selvaraj, R.; Jayaraman, B. Techniques and outcomes of transcatheter closure of complex atrial septal defects–single center experience. Indian Heart J. 2014, 66, 38–44. [Google Scholar] [CrossRef]
- Lahiri, S.; Qureshi, A.M.; Pignatelli, R.H.; Eilers, L.; Khan, A.; Bansal, M.; Webb, M.K.; Stapleton, G.; Gowda, S.T. Single tertiary center experience using Gore Cardioform Atrial Septal Defect Occluder for secundum atrial septal defect closure with a focus on deficient rims. J. Invasive Cardiol. 2023, 35, 37992333. [Google Scholar]
- Amin, Z. Transcatheter closure of secundum atrial septal defects. Catheter. Cardiovasc. Interv. 2006, 68, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.R.; Koranne, K.P.; Barker, C.M. Amplatzer Septal Occluder failure resulting in paradoxical cerebral embolism. Tex. Heart Inst. J. 2012, 39, 647–652. [Google Scholar] [PubMed]
- Panneerselvam, A. Device closure of complex ASD–safe and feasible. Indian Heart J. 2014, 66, 485. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Dahud, Q.; Lorber, A. Fenestrated atrial septal defect percutaneously occluded by a single device: Procedural and financial considerations. Cardiol. Ther. 2013, 2, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.M.; Garay, F.F.; Cao, Q.L.; Hijazi, Z.M. Multiple Amplatzer septal occluder devices for multiple atrial communications: Immediate and long-term follow-up results. Catheter. Cardiovasc. Interv. 2007, 70, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Bramlet, M.T.; Hoyer, M.H. Single pediatric center experience with multiple device implantations for complex secundum atrial septal defect. Catheter. Cardiovasc. Interv. 2008, 72, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Masseli, J.; Bertog, S.; Stanczak, L.; Blankenbach, K.; Majunke, N.; Reiffenstein, I.; Renkhoff, K.; Lehn, K.; Wunderlich, N.; Sievert, H. Transcatheter closure of multiple interatrial communications. Catheter. Cardiovasc. Interv. 2013, 81, 825–836. [Google Scholar] [CrossRef]
- Santoro, G.; Bigazzi, M.C.; Lacono, C.; Gaio, G.; Caputo, S.; Pisacane, C.; Caianiello, G.; Russo, M.G.; Calabrò, R. Transcatheter closure of complex atrial septal defects: Feasibility and mid-term results. J. Cardiovasc. Med. 2006, 7, 176–181. [Google Scholar] [CrossRef]
- Numan, M.; El Sisi, A.; Tofeig, M.; Gendi, S.; Tohami, T.; El-Said, H.G. Cribriform Amplatzer device closure of fenestrated atrial septal defects: Feasibility and technical aspects. Pediatr. Cardiol. 2008, 29, 530–535. [Google Scholar] [CrossRef]
- Carlson, K.M.; Justino, H.; O’Brien, R.E.; Dimas, V.V.; Leonard, G.T.; Pignatelli, R.H.; Mullins, C.E.; Smith, E.O.; Grifka, R.G. Transcatheter atrial septal defect closure: Modified balloon sizing technique to avoid overstretching the defect and oversizing the Amplatzer septal occluder. Catheter Cardiovasc Interv. 2005, 66, 390–396. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Narayanan, N.K.; Sivasubramonian, S. Sizing balloon-induced tear of the atrial septum. J. Invasive Cardiol. 2005, 17, 546–547. [Google Scholar] [PubMed]
- Szkutnik, M.; Masura, J.; Bialkowski, J.; Gavora, P.; Banaszak, P.; Kusa, J.; Zembala, M. Transcatheter closure of double atrial septal defects with a single Amplatzer device. Catheter. Cardiovasc. Interv. 2004, 61, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Carano, N.; Hagler, D.J.; Agnetti, A.; Squarcia, U. Device closure of fenestrated atrial septal defects: Use of a single Amplatz atrial septal occluder after balloon atrial septostomy to create a single defect. Catheter. Cardiovasc. Interv. 2001, 52, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sivasankaran, S.; Bijulal, S.; Tharakan, J.M.; Harikrishnan, S.; Ajit, K. Trans-catheter closure of atrial septal defect: Balloon sizing or no balloon sizing-single centre experience. Ann. Pediatr. Cardiol. 2011, 4, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.S. Echocardiography guidance of atrial septal defect closure. J. Thorac. Dis. 2018, 10 (Suppl. 24), S2899–S2908. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Sugeng, L.; Lang, R.M. Real-time 3-dimensional echocardiography: An integral component of the routine echocardiographic examination in adult patients? Circulation 2009, 119, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Akagi, T.; Watanabe, N.; Okamoto, Y.; Nakagawa, K.; Kijima, Y.; Toh, N.; Ohtsuki, S.; Kusano, K.; Sano, S. Application of real-time three-dimensional transesophageal echocardiography using a matrix array probe for transcatheter closure of atrial septal defect. J. Am. Soc. Echocardiogr. 2009, 22, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Heo, R.; Cho, M.S.; Bae, J.; Hong, J.A.; Lee, S.; Ahn, J.-M.; Park, D.-W.; Kim, D.-H.; Kang, D.-H.; et al. Efficacy of 3D transoesophageal echocardiography for transcatheter device closure of atrial septal defect without balloon sizing. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 684–689. [Google Scholar] [CrossRef]
- Hascoet, S.; Hadeed, K.; Marchal, P.; Dulac, Y.; Alacoque, X.; Heitz, F.; Acar, P. The relation between atrial septal defect shape, diameter, and area using three-dimensional transoesophageal echocardiography and balloon sizing during percutaneous closure in children. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 747–755. [Google Scholar] [CrossRef]
- Shuler, C.O.; Tripathi, A.; Black, G.B.; Park, Y.M.; Jerrell, J.M. Prevalence of treatment, risk factors, and management of atrial septal defects in a pediatric Medicaid cohort. Pediatr. Cardiol. 2013, 34, 1723–1728. [Google Scholar] [CrossRef]
- Abdelkarim, A.; Levi, D.S.; Tran, B.; Ghobrial, J.; Aboulhosn, J. Fenestrated transcatheter ASD closure in adults with diastolic dysfunction and/or pulmonary hypertension: Case series and review of the literature. Congenit. Heart Dis. 2016, 11, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Harikrishnan, S.; Sasidharan, B.; Ganapathi, S.; Valaparambil, A.K. Utility of 3D Echocardiography for Device Sizing During Transcatheter ASD Closure: A Comparative Study. J. Cardiovasc. Imaging 2023, 31, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Faccini, A.; Butera, G. Atrial septal defect (ASD) device transcatheter closure: Limitations. J. Thorac. Dis. 2018, 10 (Suppl. S24), S2923–S2930. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yeom, S.Y.; Kim, S.H.; Choi, J.W.; Kim, K.H. Delayed left atrial perforation associated with erosion after device closure of an atrial septal defect. Korean J. Thorac. Cardiovasc. Surg 2017, 50, 110–113. [Google Scholar] [CrossRef]
- Amin, Z.; Hijazi, Z.M.; Bass, J.L.; Cheatham, J.P.; Hellenbrand, W.E.; Kleinman, C.S. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: Review of registry of complications and recommendations to minimize future risk. Catheter. Cardiovasc. Interv. 2004, 63, 496–502. [Google Scholar] [CrossRef]
- Santini, F.; Morjan, M.; Onorati, F.; Morando, G.; Faggian, G.; Mazzucco, A. Life-threatening isometric-exertion related cardiac perforation 5 years after Amplatzer atrial septal defect closure: Should isometric activity be limited in septal occluder holders? Ann. Thorac. Surg 2012, 93, 671. [Google Scholar] [CrossRef]
- Thomson, J.D.R.; Qureshi, S.A. Device closure of secundum atrial septal defect’s and the risk of cardiac erosion. Echo. Res. Pract. 2015, 2, R73–R78. [Google Scholar] [CrossRef]
- Divekar, A.; Gaamangwe, T.; Shaikh, N.; Raabe, M.; Ducas, J. Cardiac perforation after device closure of atrial septal defects with the Amplatzer septal occluder. J. Am. Coll Cardiol. 2005, 45, 1213–1218. [Google Scholar] [CrossRef]
- Oflaz, M.B.; Pac, F.A.; Kibar, A.E.; Balli, S.; Ece, I. Evaluation of morphological characteristics of septal rims affecting successful transcatheter atrial septal defect closure in children and adults. Postep. Kardiol Interwencyjnej 2013, 9, 205–211. [Google Scholar] [CrossRef]
- Seo, J.S.; Song, J.M.; Kim, Y.H.; Park, D.W.; Lee, S.W.; Kim, W.J.; Song, J.K. Effect of atrial septal defect shape evaluated using three-dimensional transesophageal echocardiography on size measurements for percutaneous closure. J. Am. Soc. Echocardiogr. 2012, 25, 1031–1040. [Google Scholar] [CrossRef]
- Roushdy, A.; El Sayegh, A.; Ali, Y.A.; Attia, H.; El Fiky, A.; El Sayed, M. A novel three-dimensional echocardiographic method for device size selection in patients undergoing ASD trans-catheter closure. Egypt Heart J. 2020, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Method | Traditional TTE | AI-Enhanced Echocardiography |
|---|---|---|
| Description | Primary noninvasive technique for ASD detection. | Reduces variability between operators, reveals subtle details, and automates diagnostics and patient prognostication. |
| Dependency on expertise | Efficacy depends on the expertise of physicians. | Less dependent on operator expertise. |
| Accuracy and efficiency | Varies based on physician skill and experience. | Consistently high accuracy and efficiency. |
| Nr | Formulas for Predicted Device Size | Author |
|---|---|---|
| 1 | (0.964 × 3D Maximum Diameter) − (2.622 × Circular Index) + 7.084 | Seo et al. [100] |
| 2 | 0.03199 (3D Defect Area) + 0.01238 (3D Defect Circumference) + 17.39961 | Mani et al. [92] |
| 3 | 10.8 + (3.95 × 3D ASD Area) (3.85 × 3D ASD Circumference) − 1.02 | Roushdy et al. [101] |
| 4 | 1.07 × 3D-TEE(max) − 3.1 × ASDshape + 3; ASDshape circular 0; ASDoval 1 4.5 × ASDarea + 11.5 | Hascoet et al. [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinteza, E.; Vasile, C.M.; Busnatu, S.; Armat, I.; Spinu, A.D.; Vatasescu, R.; Duica, G.; Nicolescu, A. Can Artificial Intelligence Revolutionize the Diagnosis and Management of the Atrial Septal Defect in Children? Diagnostics 2024, 14, 132. https://doi.org/10.3390/diagnostics14020132
Cinteza E, Vasile CM, Busnatu S, Armat I, Spinu AD, Vatasescu R, Duica G, Nicolescu A. Can Artificial Intelligence Revolutionize the Diagnosis and Management of the Atrial Septal Defect in Children? Diagnostics. 2024; 14(2):132. https://doi.org/10.3390/diagnostics14020132
Chicago/Turabian StyleCinteza, Eliza, Corina Maria Vasile, Stefan Busnatu, Ionel Armat, Arsenie Dan Spinu, Radu Vatasescu, Gabriela Duica, and Alin Nicolescu. 2024. "Can Artificial Intelligence Revolutionize the Diagnosis and Management of the Atrial Septal Defect in Children?" Diagnostics 14, no. 2: 132. https://doi.org/10.3390/diagnostics14020132
APA StyleCinteza, E., Vasile, C. M., Busnatu, S., Armat, I., Spinu, A. D., Vatasescu, R., Duica, G., & Nicolescu, A. (2024). Can Artificial Intelligence Revolutionize the Diagnosis and Management of the Atrial Septal Defect in Children? Diagnostics, 14(2), 132. https://doi.org/10.3390/diagnostics14020132










