Intraoperative Facial Nerve Monitoring during Parotidectomy: The Current Practices and Patterns of the Korean Society of Head and Neck Surgery (KSHNS)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Settings and the Level of Experience of the Respondents
3.2. Usage, Indications, and Purpose
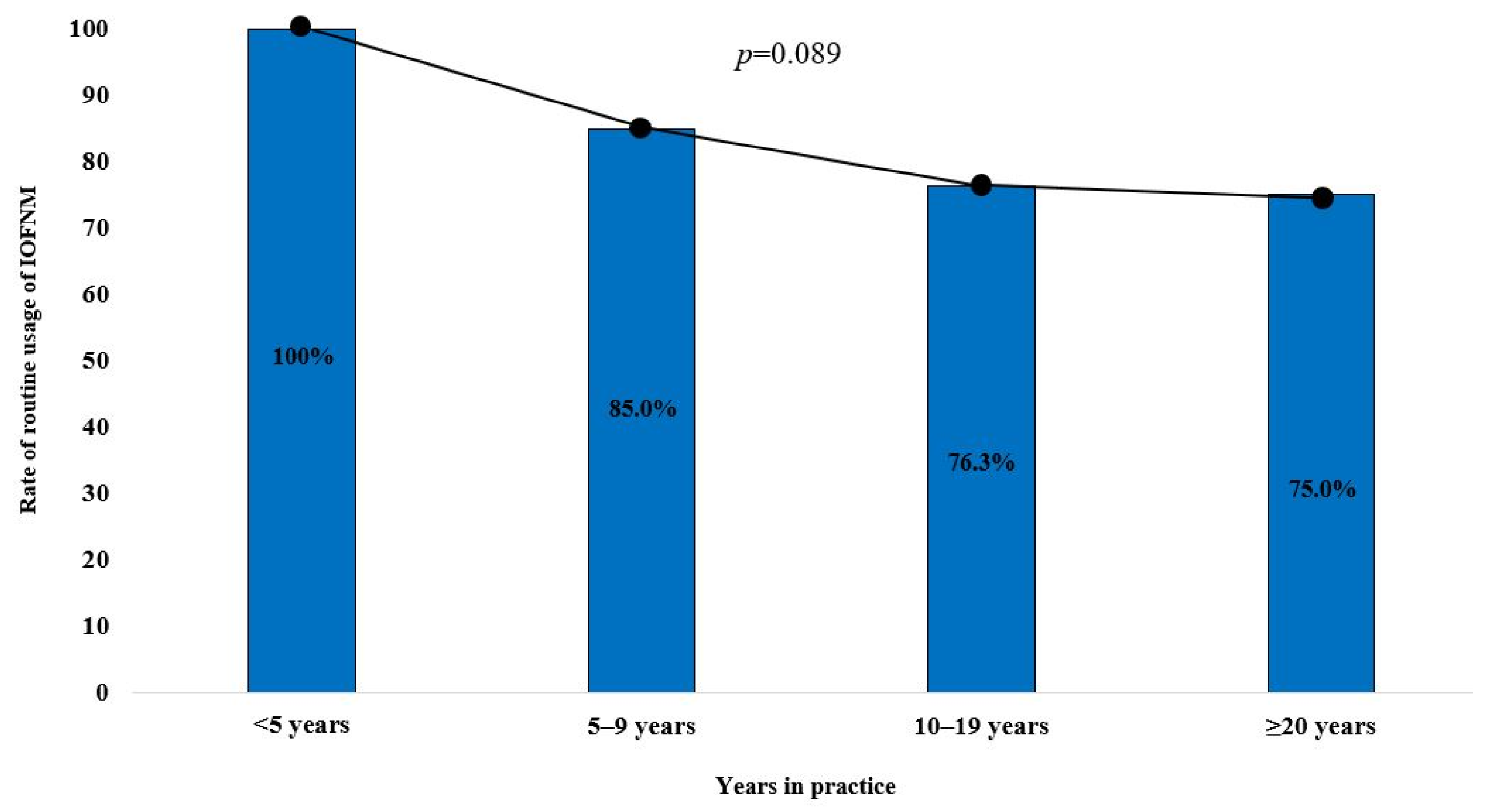
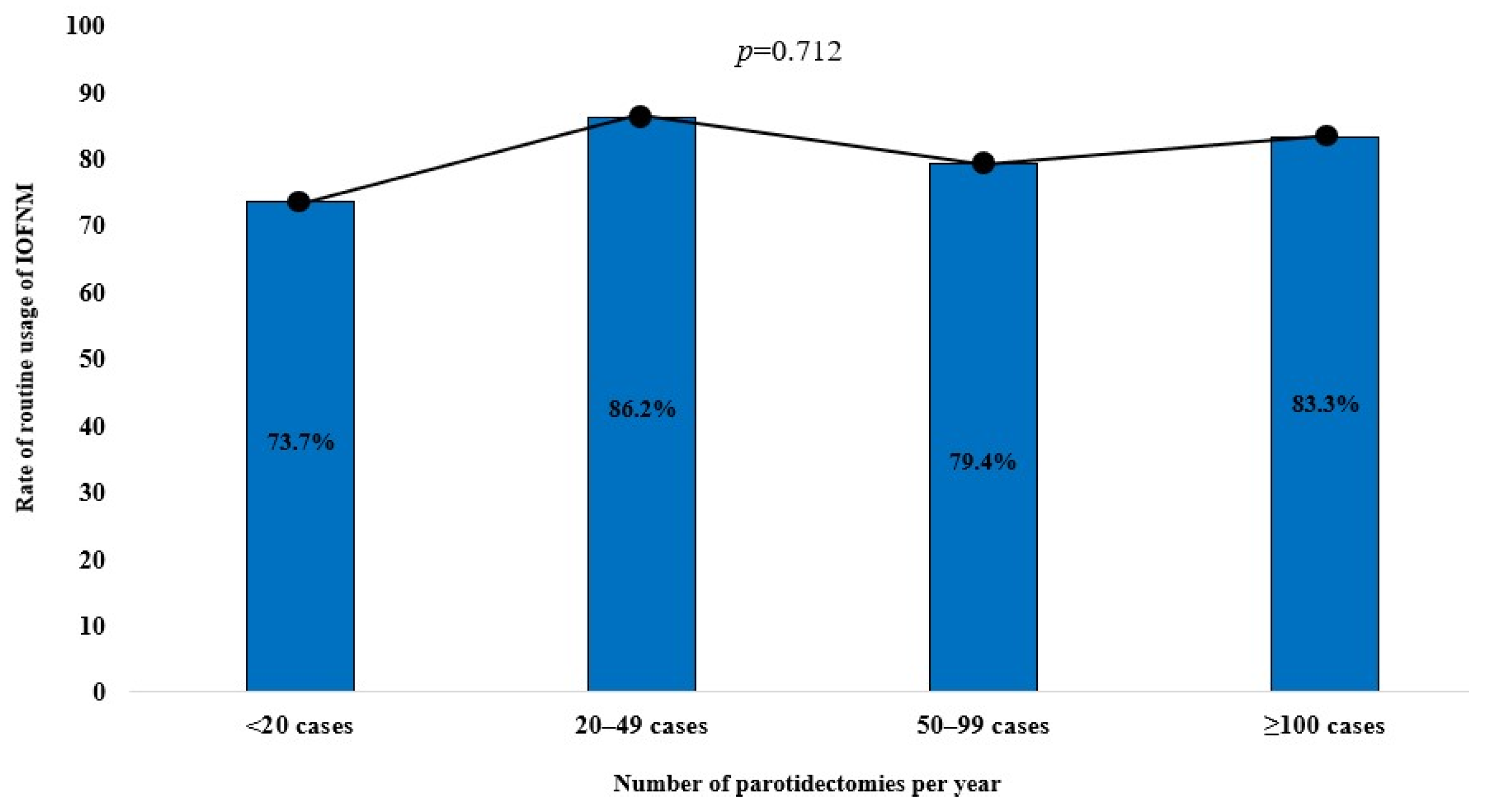
3.3. Trends of IOFNM Usage by Level of Experience and Number of Parotidectomy Procedures Performed
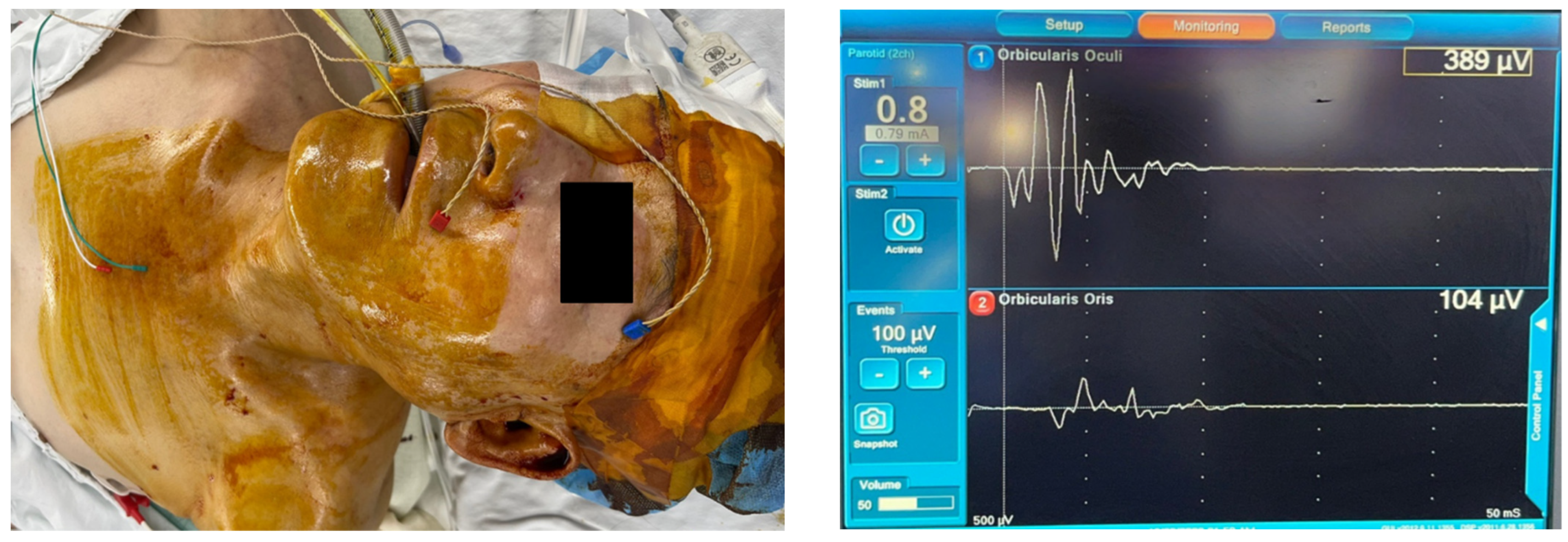
3.4. IOFNM Settings and Techniques
3.5. Management of LOS
3.6. Anesthetic Considerations When Using IOFNM
3.7. The Surgeons’ Perceptions of the Usefulness of IOFNM
3.8. Presumed Incidence of FN Injury during Parotidectomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fliss, E.; Yanko, R.; Zaretski, A.; Tulchinsky, R.; Arad, E.; Kedar, D.J.; Fliss, D.M.; Gur, E. Facial Nerve Repair following Acute Nerve Injury. Arch. Plast. Surg. 2022, 49, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kim, B.Y.; Kim, H.; Lee, E.; Park, W.; Choi, S.; Chung, M.K.; Son, Y.-I.; Baek, C.-H.; Jeong, H.-S. Incidence of postoperative facial weakness in parotid tumor surgery: A tumor subsite analysis of 794 parotidectomies. BMC Surg. 2019, 19, 199. [Google Scholar] [CrossRef]
- Salih, A.M.; Baba, H.O.; Saeed, Y.A.; Muhialdeen, A.S.; Kakamad, F.H.; Mohammed, S.H.; Hammood, Z.D.; Salih, K.M.; Salih, R.Q.; Hussein, D.A. Pattern of facial nerve palsy during parotidectomy: A single-center experience. J. Int. Med. Res. 2022, 50, 03000605221108930. [Google Scholar] [CrossRef] [PubMed]
- Chiang, F.-Y.; Lien, C.-F.; Wang, C.-C.; Wang, C.-C.; Hwang, T.-Z.; Shih, Y.-C.; Tseng, H.-Y.; Wu, C.-W.; Huang, Y.-C.; Huang, T.-Y. Proposals for standardization of intraoperative facial nerve monitoring during parotid surgery. Diagnostics 2022, 12, 2387. [Google Scholar] [CrossRef] [PubMed]
- Chiesa-Estomba, C.M.; Larruscain-Sarasola, E.; Lechien, J.R.; Mouawad, F.; Calvo-Henriquez, C.; Diom, E.S.; Ramirez, A.; Ayad, T. Facial nerve monitoring during parotid gland surgery: A systematic review and meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 933–943. [Google Scholar] [CrossRef]
- HIRA Bigdata Open Potal. Removal of Benign and Malignant Parotid Tumors (2010–2022). Available online: https://opendata.hira.or.kr/op/opc/olapDiagBhvInfoTab1.do (accessed on 31 August 2023).
- Subramaniam, N.; Gao, K.; Gupta, R.; Clark, J.R.; Low, T.H. Trends in parotidectomy over 30 years in an Australian tertiary care center. Head Neck 2020, 42, 2905–2911. [Google Scholar] [CrossRef]
- Franzen, A.M.; Kaup Franzen, C.; Guenzel, T.; Lieder, A. Increased incidence of Warthin tumours of the parotid gland: A 42-year evaluation. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2593–2598. [Google Scholar] [CrossRef]
- Czerninski, R.; Zini, A.; Sgan-Cohen, H.D. Risk of parotid malignant tumors in Israel (1970–2006). Epidemiology 2011, 22, 130–131. [Google Scholar] [CrossRef]
- De Vocht, F. Cell phones and parotid cancer trends in England. Epidemiology 2011, 22, 608–609. [Google Scholar] [CrossRef]
- Krauze, F. Surgery of the Brain and Spinal Cord; Rebman: New York, NY, USA, 1912. [Google Scholar]
- Minahan, R.E.; Mandir, A.S. Neurophysiologic intraoperative monitoring of trigeminal and facial nerves. J. Clin. Neurophysiol. 2011, 28, 551–565. [Google Scholar] [CrossRef]
- Sood, A.J.; Houlton, J.J.; Nguyen, S.A.; Gillespie, M.B. Facial nerve monitoring during parotidectomy: A systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2015, 152, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Delgado, T.E.; Buchheit, W.A.; Rosenholtz, H.R.; Chrissian, S. Intraoperative monitoring of facial muscle evoked responses obtained by intracranial stimulation of the facial nerve: A more accurate technique for facial nerve dissection. Neurosurgery 1979, 4, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Dionigi, G.; Barczynski, M.; Chiang, F.Y.; Dralle, H.; Schneider, R.; Al-Quaryshi, Z.; Angelos, P.; Brauckhoff, K.; Brooks, J.A. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer—Incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018, 128, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Randolph, G.W.; Dionigi, G.; Wu, C.W.; Barczynski, M.; Chiang, F.Y.; Al-Quaryshi, Z.; Angelos, P.; Brauckhoff, K.; Cernea, C.R. International neural monitoring study group guideline 2018 part I: Staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope 2018, 128, S1–S17. [Google Scholar] [CrossRef]
- Serpell, J.; Sidhu, S.; Vallance, N.; Panizza, B.; Randolph, G. Consensus statement on intra-operative electrophysiological recurrent laryngeal nerve monitoring during thyroid surgery. ANZ J. Surg. 2014, 84, 603–604. [Google Scholar] [CrossRef]
- Wu, C.-W.; Randolph, G.W.; Barczyński, M.; Schneider, R.; Chiang, F.-Y.; Huang, T.-Y.; Karcioglu, A.S.; Konturek, A.; Frattini, F.; Weber, F. Training courses in laryngeal nerve monitoring in thyroid and parathyroid surgery-the INMSG consensus statement. Front. Endocrinol. 2021, 12, 705346. [Google Scholar] [CrossRef]
- Zhang, D.; Pino, A.; Caruso, E.; Dionigi, G.; Sun, H. Neural monitoring in thyroid surgery is here to stay. Gland Surg. 2020, 9, S43. [Google Scholar] [CrossRef]
- Preuss, S.; Guntinas-Lichius, O. On the diagnosis and treatment of parotid gland tumors: Results of a nationwide survey of ENT hospitals in Germany. HNO 2006, 54, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Khemani, S.; Terry, R.; Golding-Wood, D. How we do it: Nerve monitoring in ENT surgery: Current UK practice. Clin. Otolaryngol. 2005, 30, 195–198. [Google Scholar] [CrossRef]
- Lowry, T.R.; Gal, T.J.; Brennan, J.A. Patterns of use of facial nerve monitoring during parotid gland surgery. Otolaryngol.—Head Neck Surg. 2005, 133, 313–318. [Google Scholar] [CrossRef]
- Chiesa-Estomba, C.M.; Saga-Gutiérrez, C.; González-García, J.Á.; Calvo-Henríquez, C.; Larruscain, E.; Sistiaga-Suárez, J.A.; de Cerio-Canduela, P.D.; Parente-Arias, P.; Quer, M. Intraoperative monitoring of the facial nerve during parotid gland surgery in Otolaryngology services–Head and Neck Surgery. Acta Otorrinolaringol. 2021, 72, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.; Moore, E.J.; Shuler, K.J. Prospective analysis of the efficacy of continuous intraoperative nerve monitoring during thyroidectomy, parathyroidectomy, and parotidectomy. Otolaryngol.—Head Neck Surg. 2001, 124, 537–543. [Google Scholar] [CrossRef]
- Duque, C.S.; Londoño, A.F.; Duque, A.M.; Zuleta, J.J.; Marulanda, M.; Otálvaro, L.M.; Agudelo, M.; Dueñas, J.P.; Palacio, M.F.; Dionigi, G. Facial nerve monitoring in parotid gland surgery: Design and feasibility assessment of a potential standardized technique. World J. Otorhinolaryngol.-Head Neck Surg. 2023, 9, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Kartush, J.M.; Rice, K.S.; Minahan, R.E.; Balzer, G.K.; Yingling, C.D.; Seubert, C.N. Best practices in facial nerve monitoring. Laryngoscope 2021, 131, S1–S42. [Google Scholar] [CrossRef]
- Zieliński, M.; Sowa, P.; Adamczyk-Sowa, M.; Szlęzak, M.; Misiołek, M. Prospective assessment of intraoperative facial nerve monitoring in patients undergoing partial parotidectomy. BioMed Res. Int. 2022, 2022. [Google Scholar] [CrossRef]
- Graciano, A.J.; Fischer, C.A.; Coelho, G.V.; Steck, J.H.; Paschoal, J.R.; Chone, C.T. Facial nerve dysfunction after superficial parotidectomy with or without continuous intraoperative electromyographic neuromonitoring: A prospective randomized pilot study. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.T.; Ellsperman, S.E.; Edwards, B.M.; Kileny, P.; Kovatch, D.; Mannarelli, G.R.; Meloch, M.A.; Miller, C.; Pitts, C.; Prince, M.E. Assessment of intraoperative nerve monitoring parameters associated with facial nerve outcome in parotidectomy for benign disease. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 1137–1143. [Google Scholar] [CrossRef]
- Ozturk, K.; Akyildiz, S.; Gode, S.; Turhal, G.; Gursan, G.; Kirazli, T. The effect of partial superficial parotidectomy on amplitude, latency and threshold of facial nerve stimulation. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1527–1531. [Google Scholar] [CrossRef]
- Chiang, F.-Y.; Wang, C.-C.; Wu, C.-W.; Lu, I.-C.; Chang, P.-Y.; Lin, Y.-C.; Lien, C.-F.; Wang, C.-C.; Huang, T.-Y.; Hwang, T.-Z. Correlation between Electrophysiological Change and Facial Function in Parotid Surgery Patients. J. Clin. Med. 2021, 10, 5730. [Google Scholar] [CrossRef]
- Randolph, G.W.; Dralle, H.; with the International Intraoperative Monitoring Study Group; Abdullah, H.; Barczynski, M.; Bellantone, R.; Brauckhoff, M.; Carnaille, B.; Cherenko, S.; Chiang, F.Y. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: International standards guideline statement. Laryngoscope 2011, 121, S1–S16. [Google Scholar] [CrossRef]
- Dombrowski, M.E.; Morales-Restrepo, A.; Fourman, M.S.; Vaudreuil, N.; Lee, J.Y. Prophylactic perioperative dexamethasone decreases the incidence of postoperative C5 palsies after a posterior cervical laminectomy and fusion. Spine J. 2019, 19, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Gokaslan, Z.L.; Bydon, M.; De la Garza-Ramos, R.; Smith, Z.A.; Hsu, W.K.; Qureshi, S.A.; Cho, S.K.; Baird, E.O.; Mroz, T.E.; Fehlings, M. Recurrent laryngeal nerve palsy after cervical spine surgery: A multicenter AOSpine clinical research network study. Glob. Spine J. 2017, 7, 53S–57S. [Google Scholar] [CrossRef] [PubMed]
- Galloway III, E.B.; Jensen, R.L.; Dailey, A.T.; Gregory Thompson, B.; Shelton, C. Role of topical steroids in reducing dysfunction after nerve injury. Laryngoscope 2000, 110, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, E.; Ballabio, M.; Consoli, A.; Roglio, I.; Magnaghi, V.; Melcangi, R.C. Neuroactive steroids: A therapeutic approach to maintain peripheral nerve integrity during neurodegenerative events. J. Mol. Neurosci. 2006, 28, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Randolph, G.; Dionigi, G.; Barczyński, M.; Chiang, F.Y.; Triponez, F.; Vamvakidis, K.; Brauckhoff, K.; Musholt, T.J.; Almquist, M. Prospective study of vocal fold function after loss of the neuromonitoring signal in thyroid surgery: The I nternational N eural M onitoring S tudy G roup’s POLT study. Laryngoscope 2016, 126, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Graceffa, G.; Vieni, S.; Mannino, V.; Gennari, V.; Genova, P.; Cipolla, C. Effectiveness of early administration of a single dose of steroids and escin after loss of signal on electromyographic signal recovery during neuromonitored thyroidectomy. Am. J. Surg. 2022, 223, 923–926. [Google Scholar] [CrossRef]
- Donatini, G.; Danion, J.; Zerrweck, C.; Etienne, P.; Lacoste, L.; Kraimps, J.-L. Single dose steroid injection after loss of signal (LOS) during thyroid surgery is effective to recover electric signal avoiding vocal cord palsy and the need of staged thyroidectomy: Prospective evaluation on 702 patients. World J. Surg. 2020, 44, 417–425. [Google Scholar] [CrossRef]
| Respondents (N = 89) | |
|---|---|
| Clinical setting | |
| Private | 5 (5.6%) |
| Hospital-based | 2 (2.3%) |
| Academic | 82 (92.1%) |
| Years in practice | |
| <5 years | 10 (11.2%) |
| 5–10 years | 20 (22.5%) |
| 10–20 years | 38 (42.7%) |
| ≥20 years | 21 (23.6%) |
| Number of parotidectomies per year | |
| None | 1 (1.1%) |
| <20 | 19 (21.3%) |
| 20–49 | 29 (32.5%) |
| 50–99 | 34 (38.2%) |
| ≥100 | 6 (6.7%) |
| Respondents (n = 88) | |
|---|---|
| Type of IOFNM usage | |
| Never | 3 (3.4%) |
| due to no equipment | 2 |
| due to time and cost burden | 1 |
| Selective | 14 (15.9%) |
| Personal indications * | |
| Revision | 13 |
| Possible adhesion | 13 |
| Malignancy | 12 |
| Deep lobe location | 9 |
| Retrograde dissection | 4 |
| Large tumor | 3 |
| Routine | 71 (80.7%) |
| Purpose of using IOFNM * (for 85 IOFNM users) | |
| Prevention of inadvertent FN injury | 79 (92.9%) |
| Facilitation of identification and mapping FN | 61 (71.8%) |
| Intraoperative assessment of FN function | 28 (62.9%) |
| Education | 17 (20.0%) |
| Prevention of medico-legal issues | 1 (1.2%) |
| Respondents (n = 85) | |
|---|---|
| Channel and electrode | |
| 2-channel recording with needle electrode | 52 (61.2%) |
| 4-channel recording with needle electrode | 33 (38.8%) |
| 2- or 4-channel recording with surface electrode | 0 (0.0%) |
| Event threshold setting | |
| 50–99 μV | 37 (43.5%) |
| 100–149 μV | 44 (51.8%) |
| 150–199 μV | 3 (3.5%) |
| ≥200 μV | 1 (1.2%) |
| Neural mapping of FN before visual identification of FN main trunk | |
| No | 32 (37.6%) |
| Yes | 53 (62.4%) |
| Stimulation intensity | |
| 0.5–0.9 mA | 2 |
| 1–1.4 mA | 26 |
| 1.5–1.9 mA | 14 |
| 2.0–2.4 mA | 6 |
| ≥2.5 mA | 5 |
| Assessment of initial FN function after visual identification of FN | |
| No | 7 (8.2%) |
| Yes | 78 (91.8%) |
| Stimulation intensity | |
| 0.5–0.9 mA | 49 |
| 1–1.4 mA | 24 |
| 1.5–1.9 mA | 5 |
| ≥2.0 mA | 0 |
| Assessment of final FN function after tumor dissection | |
| No | 4 (4.6%) |
| Yes | 81 (95.4%) |
| Technique | |
| Check generation of EMG signal | 73 |
| Compare initial and final stimulation thresholds | 3 |
| Compare initial and final maximal response amplitudes | 5 |
| Respondents (n = 85) | |
|---|---|
| Initial action for LOS * | |
| Checking the IOFNM system | 64 (75.3%) |
| Confirmation of the timing and dosage of the muscle relaxant | 64 (75.3%) |
| Visual and tactile identification of facial twitch during stimulation | 50 (58.8%) |
| Exploration of adjacent regions to identify other FN branches | 50 (58.8%) |
| None | 1 (1.2%) |
| Management of persistent LOS after initial action * | |
| Proceeding with surgery | 69 (81.2%) |
| Re-stimulation using a higher intensity or lower event threshold | 34 (40.0%) |
| Re-stimulation after waiting for 20–30 min | 15 (17.6%) |
| Re-stimulation after administration of muscle relaxant antagonists | 13 (15.3%) |
| Discontinuing surgery | 0 (0.0%) |
| Administration of steroid for true LOS | |
| Never | 23 (27.1%) |
| Sometimes | 45 (52.9%) |
| All the time | 17 (20.0%) |
| Inadvertent FN injury due to false LOS | |
| No | 53 (62.4%) |
| Yes | 32 (37.6%) |
| Transient injury | 30 |
| Permanent injury | 2 |
| Respondents (n = 85) | |
|---|---|
| Perception of usefulness of IOFNM | |
| Useful for preventing both transient and permanent FN injury | 66 (77.6%) |
| Useful for preventing transient FN injury only | 0 (0.0%) |
| Useful for preventing permanent FN injury only | 5 (6.0%) |
| Useful in selective cases | 13 (15.2%) |
| Not useful | 1 (1.2%) |
| Is IOFNM generally beneficial for safety parotidectomy? | |
| No | 1 (1.2%) |
| Yes | 84 (98.9%) |
| Would you use IONM if you undergo parotidectomy as a patient? | |
| No | 1 (1.2%) |
| Yes | 81 (95.3%) |
| Uncertain | 3 (3.5%) |
| Selective Use (n = 14) | Routine Use (n = 71) | p-Value | |
|---|---|---|---|
| Transient FN injury | |||
| <5.0% | 11 (78.6%) | 45 (63.4%) | 0.590 |
| 5.0–9.9% | 3 (21.4%) | 19 (26.8%) | |
| 10.0–19.9% | 0 (0.0%) | 6 (8.5%) | |
| 20.0–29.9% | 0 (0.0%) | 1 (1.4%) | |
| Permanent FN injury | |||
| <1.0% | 13 (92.9%) | 58 (81.7%) | 0.448 |
| 1.0–4.9% | 1 (7.1%) | 13 (18.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, D.; Kwak, J.H.; Kim, G.-J.; Kim, H.; Lee, D.W.; Cho, K.J. Intraoperative Facial Nerve Monitoring during Parotidectomy: The Current Practices and Patterns of the Korean Society of Head and Neck Surgery (KSHNS). Diagnostics 2024, 14, 2277. https://doi.org/10.3390/diagnostics14202277
Ahn D, Kwak JH, Kim G-J, Kim H, Lee DW, Cho KJ. Intraoperative Facial Nerve Monitoring during Parotidectomy: The Current Practices and Patterns of the Korean Society of Head and Neck Surgery (KSHNS). Diagnostics. 2024; 14(20):2277. https://doi.org/10.3390/diagnostics14202277
Chicago/Turabian StyleAhn, Dongbin, Ji Hye Kwak, Geun-Jeon Kim, Heejin Kim, Dong Won Lee, and Kwang Jae Cho. 2024. "Intraoperative Facial Nerve Monitoring during Parotidectomy: The Current Practices and Patterns of the Korean Society of Head and Neck Surgery (KSHNS)" Diagnostics 14, no. 20: 2277. https://doi.org/10.3390/diagnostics14202277
APA StyleAhn, D., Kwak, J. H., Kim, G.-J., Kim, H., Lee, D. W., & Cho, K. J. (2024). Intraoperative Facial Nerve Monitoring during Parotidectomy: The Current Practices and Patterns of the Korean Society of Head and Neck Surgery (KSHNS). Diagnostics, 14(20), 2277. https://doi.org/10.3390/diagnostics14202277







