Deciphering the Role of Androgen in the Dermatologic Manifestations of Polycystic Ovary Syndrome Patients: A State-of-the-Art Review
Abstract
1. Introduction
- -
- Menstrual irregularities: these may include oligomenorrhea or amenorrhea.
- -
- Clinical or laboratory evidence of hyperandrogenism: Clinical evidence may manifest as hirsutism, acne, or male pattern baldness. Laboratory evidence involves elevated levels of androgens, such as testosterone, either measured directly or inferred through elevated levels of androgenic hormones like Dehydroepiandrosterone Sulfate (DHEAS).
- -
- Polycystic ovaries on ultrasound examination or enlargement of the ovary’s size or increased anti-Müllerian hormone (AMH) levels [3].
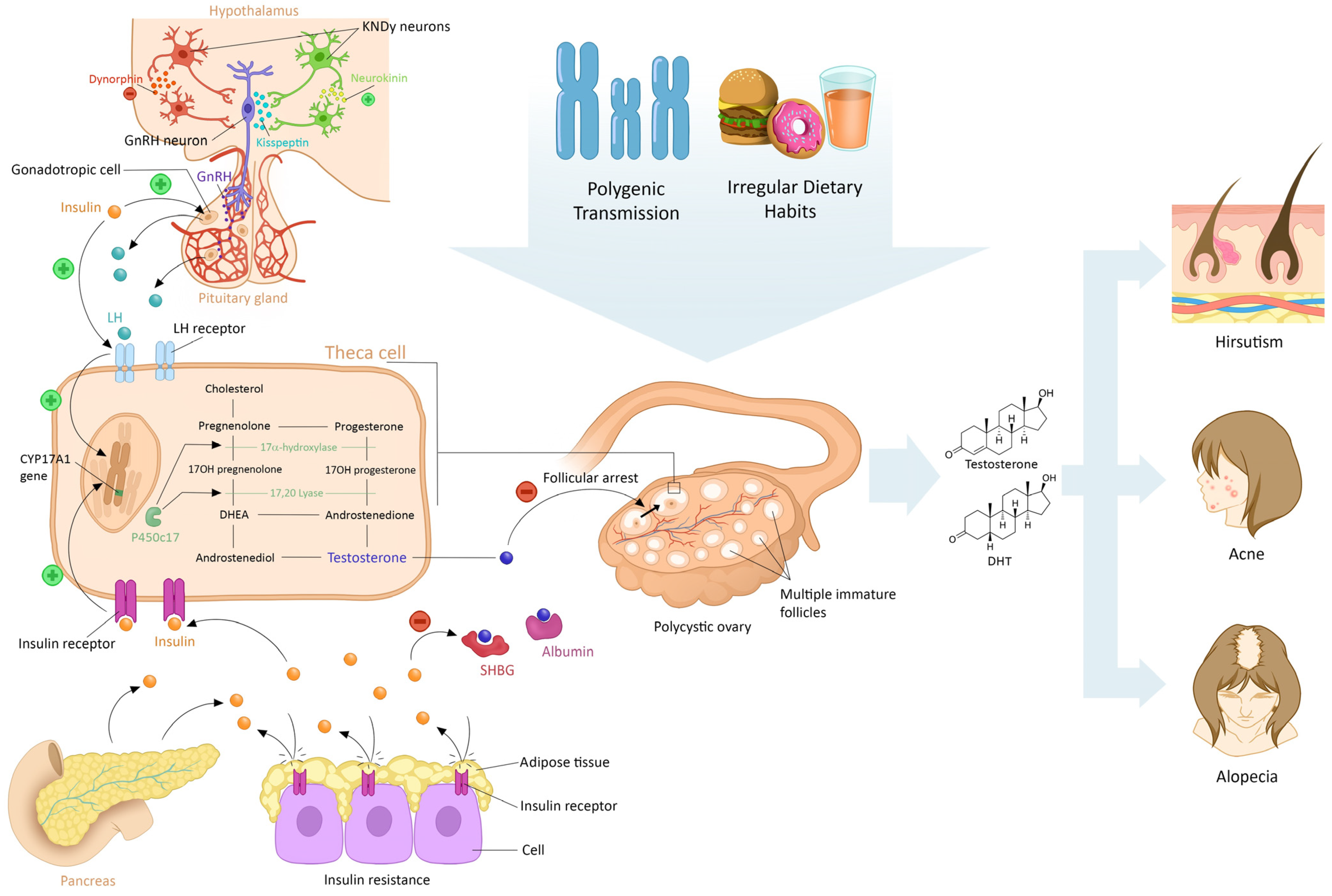
2. Main Pathophysiological Features of PCOS
2.1. Modifications During Fetal Conception
2.2. Abnormalities in the Hypothalamus–Pituitary–Ovarian Axis
2.3. Consequences of Hyperandrogenism
2.4. Peripheral Insulin Resistance
2.5. Effects of Lifestyle on PCOS
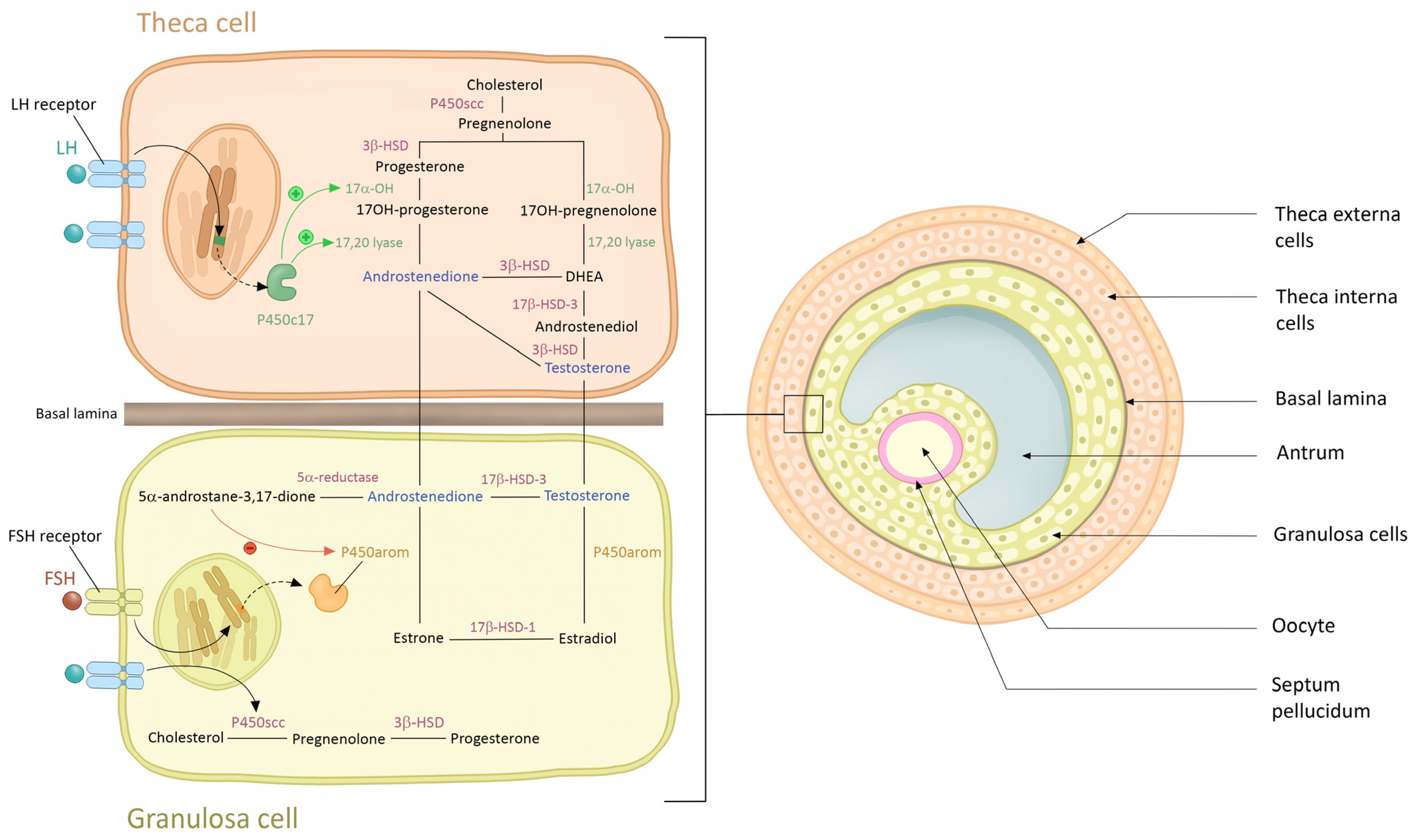
3. Origin of Androgen in PCO Syndrome
3.1. Ovarian Theca Cells
3.2. Adrenal Glands
3.3. Adipose Tissue
4. Physiological Effect of Androgen on Skin and Phaneres in Women
5. Hyperandrogenism: Criteria and Diagnostic Methods
6. Role of Skin Manifestations in the Diagnosis of PCOs
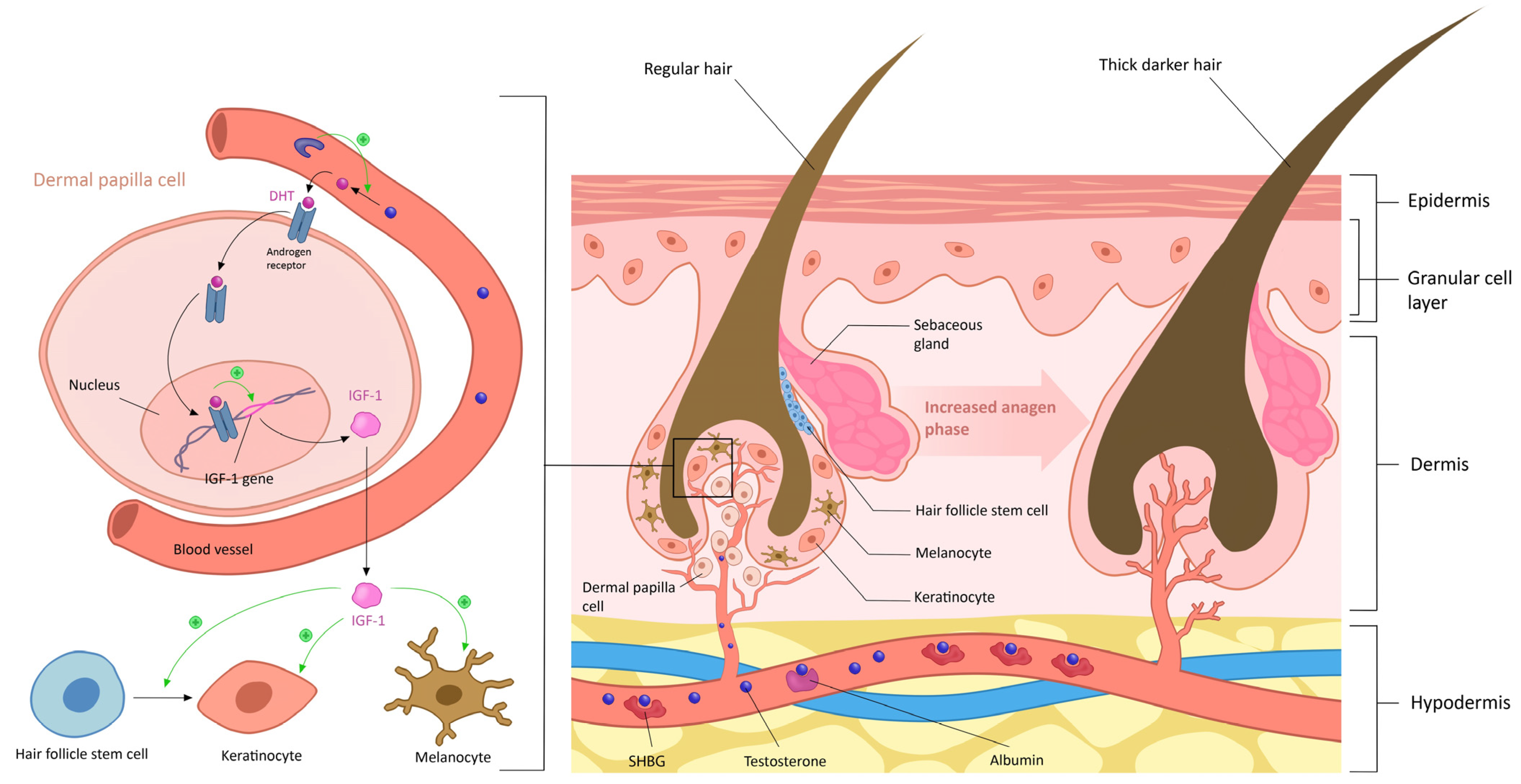
6.1. Hirsutism
6.1.1. Androgen Excess
6.1.2. Sensitivity of Hair Follicles to Androgens
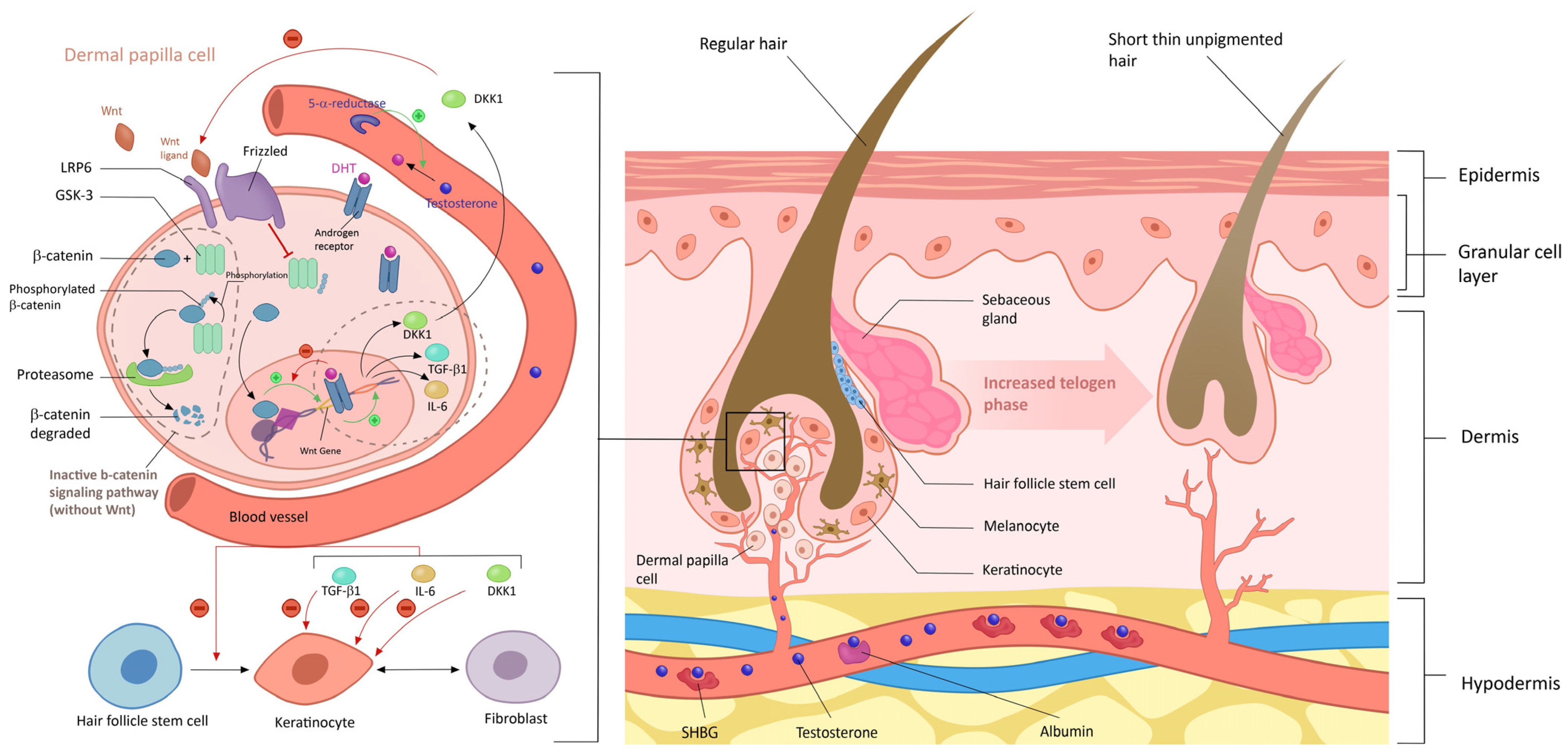
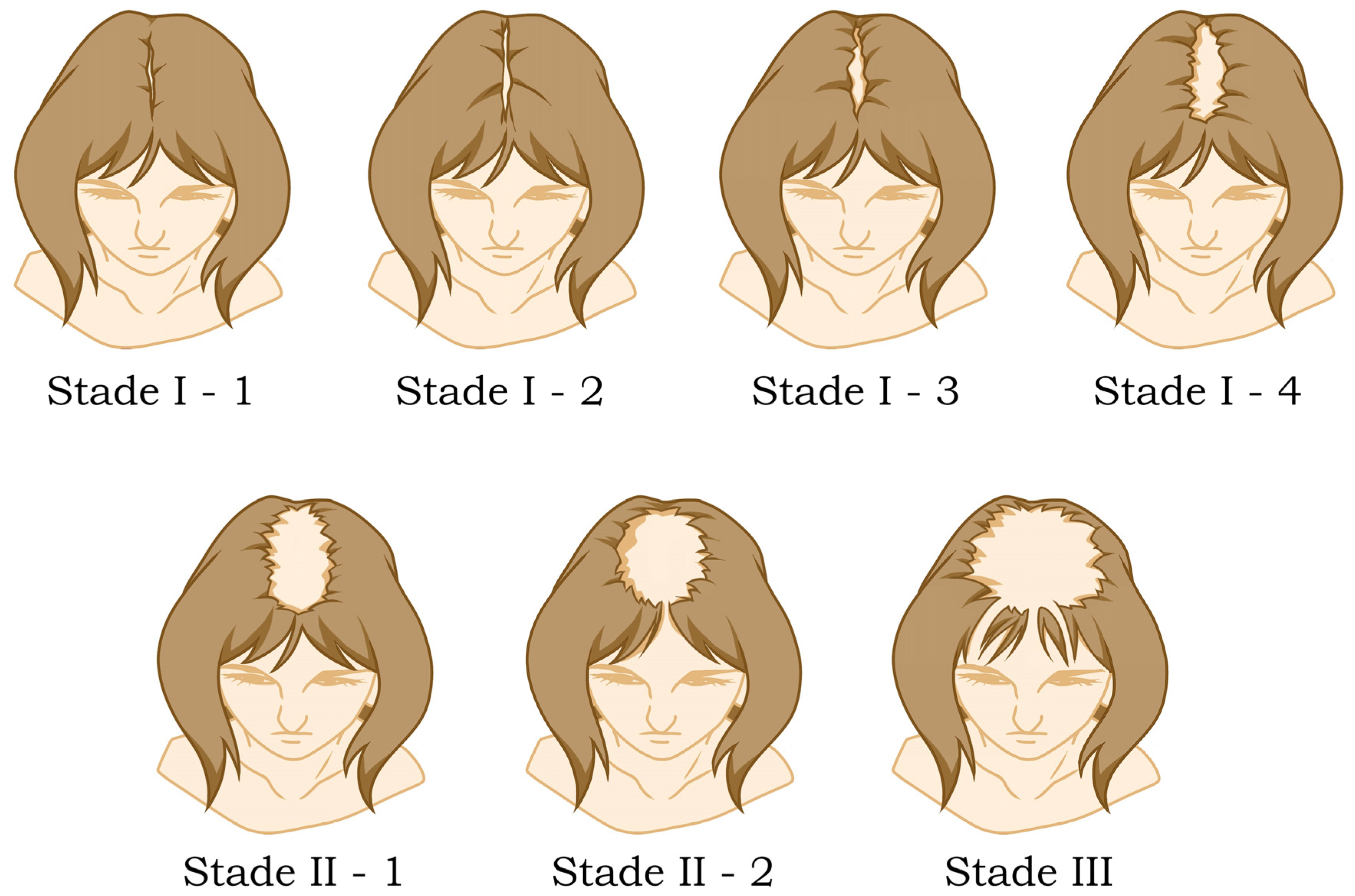
6.2. Female Pattern Hair Loss (FPHL)
6.2.1. Role of Androgens in Female Pattern Hair Loss
- -
- Androgen Excess
- -
- Genetic Susceptibility
- -
- Scalp Low-Grade Inflammation
6.2.2. Clinical Evaluation and Scoring
6.3. Acne and Seborrhea
- Is sebaceous gland hyperplasia leading to excessive sebum production?
- Irregularities in follicular growth and differentiation resulting in hyperkeratinization and obstruction of the pilosebaceous unit.
- Cutaneous dysbiosis, characterized by the selection of virulent subtypes of Cutibacterium acnes (C. acnes) and/or other bacterial agents.
- Inflammation and the activation of the innate immune response.
6.3.1. Androgen Excess
6.3.2. Receptor Sensitivity
6.3.3. Clinical Evaluation/Scoring
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef] [PubMed]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Zehravi, M.; Maqbool, M.; Ara, I. Depression and anxiety in women with polycystic ovarian syndrome: A literature survey. Int. J. Adolesc. Med. Health 2021, 33, 367–373. [Google Scholar] [CrossRef]
- Ramezani Tehrani, F.; Behboudi-Gandevani, S.; Bidhendi Yarandi, R.; Saei Ghare Naz, M.; Carmina, E. Prevalence of acne vulgaris among women with polycystic ovary syndrome: A systemic review and meta-analysis. Gynecol. Endocrinol. 2021, 37, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Spritzer, P.; Barone, C.; Oliveira, F. Hirsutism in Polycystic Ovary Syndrome: Pathophysiology and Management. Curr. Pharm. Des. 2016, 22, 5603–5613. [Google Scholar] [CrossRef]
- Ramos, P.M.; Miot, H.A. Female Pattern Hair Loss: A clinical and pathophysiological review. An. Bras. De Dermatol. 2015, 90, 529–543. [Google Scholar] [CrossRef]
- Schmidt, T.H.; Khanijow, K.; Cedars, M.I.; Huddleston, H.; Pasch, L.; Wang, E.T.; Lee, J.; Zane, L.T.; Shinkai, K. Cutaneous Findings and Systemic Associations in Women With Polycystic Ovary Syndrome. JAMA Dermatol. 2016, 152, 391. [Google Scholar] [CrossRef]
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100060. [Google Scholar] [CrossRef]
- Taieb, A.; Asma, G.; Jabeur, M.; Fatma, B.A.; Nassim, B.H.S.; Asma, B.A. Rethinking the Terminology: A Perspective on Renaming Polycystic Ovary Syndrome for an Enhanced Pathophysiological Understanding. Clin. Med. Insights Endocrinol. Diabetes 2024, 17, 11795514241296777. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.B.; Rosenfield, R.L.; Ehrmann, D.A.; Cara, J.F.; Cuttler, L.; Levitsky, L.L.; Rosenthal, I.M. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: Evidence for perinatal masculinization of neuroendocrine function in women. J. Clin. Endocrinol. Metab. 1994, 79, 1328–1333. [Google Scholar]
- Eisner, J.R.; Barnett, M.A.; Dumesic, D.A.; Abbott, D.H. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil. Steril. 2002, 77, 167–172. [Google Scholar] [CrossRef]
- Robinson, J.E.; Forsdike, R.A.; Taylor, J.A. In Utero Exposure of Female Lambs to Testosterone Reduces the Sensitivity of the GnRH Neuronal Network to Inhibition by Progesterone. Endocrinology 1999, 140, 5797–5805. [Google Scholar] [CrossRef]
- Eagleson, C.A.; Gingrich, M.B.; Pastor, C.L.; Arora, T.K.; Burt, C.M.; Evans, W.S.; Marshall, J.C. Polycystic Ovarian Syndrome: Evidence that Flutamide Restores Sensitivity of the Gonadotropin-Releasing Hormone Pulse Generator to Inhibition by Estradiol and Progesterone 1. J. Clin. Endocrinol. Metab. 2000, 85, 4047–4052. [Google Scholar]
- Waldstreicher, J.; Santoro, N.F.; Hall, J.E.; Filicori, M.; Crowley, W.F. Hyperfunction of the Hypothalamic-Pituitary Axis in Women with Polycystic Ovarian Disease: Indirect Evidence for Partial Gonadotroph Desensitization*. J. Clin. Endocrinol. Metab. 1988, 66, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, R.; Webster, N.J.G. GnRH Pulsatility, the Pituitary Response and Reproductive Dysfunction. Endocr. J. 2009, 56, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.K.; McCartney, C.R.; Chhabra, S.; Helm, K.D.; Eagleson, C.A.; Chang, R.J.; Marshall, J.C. Modulation of Gonadotropin-Releasing Hormone Pulse Generator Sensitivity to Progesterone Inhibition in Hyperandrogenic Adolescent Girls—Implications for Regulation of Pubertal Maturation. J. Clin. Endocrinol. Metab. 2009, 94, 2360–2366. [Google Scholar] [CrossRef]
- Willis, D.; Mason, H.; Gilling-Smith, C.; Franks, S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J. Clin. Endocrinol. Metab. 1996, 81, 302–309. [Google Scholar]
- Andrade, V.H.L.D.; Mata, A.M.O.F.D.; Borges, R.S.; Costa-Silva, D.R.; Martins, L.M.; Ferreira, P.M.P.; Cunha-Nunes, L.C.; DA Silva, B.B. Current aspects of polycystic ovary syndrome: A literature review. Rev. Assoc. Médica Bras. 2016, 62, 867–871. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Bhide, P.; Dilgil, M.; Gudi, A.; Shah, A.; Akwaa, C.; Homburg, R. Each small antral follicle in ovaries of women with polycystic ovary syndrome produces more antimüllerian hormone than its counterpart in a normal ovary: An observational cross-sectional study. Fertil. Steril. 2015, 103, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Van Hooff, M.H.A.; Voorhorst, F.J.; Kaptein, M.B.H.; Hirasing, R.A.; Koppenaal, C.; Schoemaker, J. Polycystic ovaries in adolescents and the relationship with menstrual cycle patterns, luteinizing hormone, androgens, and insulin. Fertil. Steril. 2000, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.E.; Flier, J.S. Detection of an Alteration in the Insulin-Receptor Gene in a Patient with Insulin Resistance, Acanthosis Nigricans, and the Polycystic Ovary Syndrome (Type A Insulin Resistance). N. Engl. J. Med. 1988, 319, 1526–1529. [Google Scholar] [CrossRef]
- Toulis, K.A.; Goulis, D.G.; Farmakiotis, D.; Georgopoulos, N.A.; Katsikis, I.; Tarlatzis, B.C.; Papadimas, I.; Panidis, D. Adiponectin levels in women with polycystic ovary syndrome: A systematic review and a meta-analysis. Hum. Reprod. Update 2009, 15, 297–307. [Google Scholar] [CrossRef]
- Aversa, A.; La Vignera, S.; Rago, R.; Gambineri, A.; Nappi, R.E.; Calogero, A.E.; Ferlin, A. Fundamental Concepts and Novel Aspects of Polycystic Ovarian Syndrome: Expert Consensus Resolutions. Front. Endocrinol. 2020, 11, 516. [Google Scholar] [CrossRef]
- Guo, F.; Gong, Z.; Fernando, T.; Zhang, L.; Zhu, X.; Shi, Y. The Lipid Profiles in Different Characteristics of Women with PCOS and the Interaction Between Dyslipidemia and Metabolic Disorder States: A Retrospective Study in Chinese Population. Front. Endocrinol. 2022, 13, 892125. [Google Scholar] [CrossRef]
- Garg, D.; Merhi, Z. Advanced Glycation End Products: Link between Diet and Ovulatory Dysfunction in PCOS? Nutrients 2015, 7, 10129–10144. [Google Scholar] [CrossRef] [PubMed]
- Ziech, D.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Reactive Oxygen Species (ROS)––Induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2011, 711, 167–173. [Google Scholar] [CrossRef]
- Notaro, A.L.G.; Neto, F.T.L. The use of metformin in women with polycystic ovary syndrome: An updated review. J. Assist. Reprod. Genet. 2022, 39, 573–579. [Google Scholar] [CrossRef]
- Palioura, E.; Diamanti-Kandarakis, E. Industrial endocrine disruptors and polycystic ovary syndrome. J. Endocrinol. Invest. 2013, 36, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, K.M.; Kochman, L.; Wixom, N.; Craig, K.; Miller, R.K.; Guzick, D.S. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: A pilot study. Fertil. Steril. 2004, 82, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Methnani, J.; Hajbelgacem, M.; Ach, T.; Chaieb, F.; Sellami, S.; Bouslama, A.; Zaouali, M.; Omezzine, A.; Bouhlel, E. Effect of Pre-Meal Metformin With or Without an Acute Exercise Bout on Postprandial Lipemic and Glycemic Responses in Metabolic Syndrome Patients: A Randomized, Open Label, Crossover Study. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231156318. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Brinkworth, G.; Noakes, M.; Norman, R.J. Effects of lifestyle modification in polycystic ovarian syndrome. Reprod. Biomed. Online 2006, 12, 569–578. [Google Scholar] [CrossRef]
- Ach, T.; Guesmi, A.; Kalboussi, M.; Ben Abdessalem, F.; Mraihi, E.; El Mhabrech, H. Validation of the follicular and ovarian thresholds by an 18-MHz ultrasound imaging in polycystic ovary syndrome: A pilot cutoff for North African patients. Ther. Adv. Reprod. Health 2024, 18, 26334941241270372. [Google Scholar] [CrossRef]
- Sharma, A.; Welt, C.K. Practical Approach to Hyperandrogenism in Women. Med. Clin. N. Am. 2021, 105, 1099–1116. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Chen, Y. Androgen excess: A hallmark of polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1273542. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Naamneh Elzenaty, R.; Du Toit, T.; Flück, C.E. Basics of androgen synthesis and action. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101665. [Google Scholar] [CrossRef]
- Wagner, I.V.; Savchuk, I.; Sahlin, L.; Kulle, A.; Klöting, N.; Dietrich, A.; Holterhus, P.-M.; Dötsch, J.; Blüher, M.; Söder, O. De Novo and Depot-Specific Androgen Production in Human Adipose Tissue: A Source of Hyperandrogenism in Women with Obesity. Obes. Facts 2022, 15, 281–291. [Google Scholar] [CrossRef]
- Wagner, I.V.; Sahlin, L.; Savchuk, I.; Klöting, N.; Svechnikov, K.; Söder, O. Adipose Tissue is a Potential Source of Hyperandrogenism in Obese Female Rats. Obesity 2018, 26, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Indran, I.R.; Lee, B.H.; Yong, E.-L. Cellular and Animal Studies: Insights into Pathophysiology and Therapy of PCOS. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.G. Androgen production in women. Fertil. Steril. 2002, 77, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.; Bell, R. Androgen Physiology. Semin. Reprod. Med. 2006, 24, 71–77. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Degitz, K. Androgen action on human skin—From basic research to clinical significance. Exp. Dermatol. 2004, 13, 5–10. [Google Scholar] [CrossRef]
- Randall, V.A. Androgens and hair growth. Dermatol. Ther. 2008, 21, 314–328. [Google Scholar] [CrossRef]
- Deplewski, D.; Rosenfield, R.L. Role of Hormones in Pilosebaceous Unit Development. Endocr. Rev. 2000, 21, 363–392. [Google Scholar] [CrossRef]
- Akamatsu, H.; Zouboulis, C.C.; Orfanos, C.E. Control of Human Sebocyte Proliferation In Vitro by Testosterone and 5-Alpha-Dihydrotestosterone Is Dependent on the Localization of the Sebaceous Glands. J. Investig. Dermatol. 1992, 99, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, C.-C.; Sheu, H.-M.; Seltmann, H.; Zouboulis, C.C. Expression of Peroxisome Proliferator-Activated Receptor and CCAAT/Enhancer Binding Protein Transcription Factors in Cultured Human Sebocytes. J. Investig. Dermatol. 2003, 121, 441–447. [Google Scholar] [CrossRef]
- Kao, J.S.; Garg, A.; Mao-Qiang, M.; Crumrine, D.; Ghadially, R.; Feingold, K.R.; Elias, P.M. Testosterone Perturbs Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2001, 116, 443–451. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Mills, S.J. Androgen receptor–mediated inhibition of cutaneous wound healing. J. Clin. Investig. 2002, 110, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Leerasiri, P.; Wongwananuruk, T.; Indhavivadhana, S.; Techatraisak, K.; Rattanachaiyanont, M.; Angsuwathana, S. Correlation of clinical and biochemical hyperandrogenism in THAI women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2016, 42, 678–683. [Google Scholar] [CrossRef]
- Artar, G.; Tas, B.; Turan, G.; Uckan, H.H. Evaluation of androgen-dependent skin findings of polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. 2022, 38, 1104–1108. [Google Scholar] [CrossRef]
- Rosner, W.; Auchus, R.J.; Azziz, R.; Sluss, P.M.; Raff, H. Utility, Limitations, and Pitfalls in Measuring Testosterone: An Endocrine Society Position Statement. J. Clin. Endocrinol. Metab. 2007, 92, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Barros, B.; Thiboutot, D. Hormonal therapies for acne. Clin. Dermatol. 2017, 35, 168–172. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Taylor, A.E.; Crabtree, N.J.; Hughes, B.A.; Capper, F.; Crowley, R.K.; Stewart, P.M.; Tomlinson, J.W.; Arlt, W. Hyperandrogenemia Predicts Metabolic Phenotype in Polycystic Ovary Syndrome: The Utility of Serum Androstenedione. J. Clin. Endocrinol. Metab. 2014, 99, 1027–1036. [Google Scholar] [CrossRef]
- Rosenfield, R.L. Ovarian and Adrenal Function in Polycystic Ovary Syndrome. Endocrinol. Metab. Clin. N. Am. 1999, 28, 265–293. [Google Scholar] [CrossRef]
- Unfer, V.; Dinicola, S.; Russo, M. A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes? Int. J. Mol. Sci. 2023, 24, 6213. [Google Scholar] [CrossRef]
- Zirkle, C. The discovery of sex-influenced, sex-limited, and sex-linked heredity. In Studies and Essays in the History of Service and Learning in Honour of George Sarton; Montagn, M.F.A., Ed.; Schuman: Brussels, Belgium, 1946; pp. 169–194. [Google Scholar]
- Yildiz, B.O.; Bolour, S.; Woods, K.; Moore, A.; Azziz, R. Visually scoring hirsutism. Hum. Reprod. Update 2010, 16, 51–64. [Google Scholar] [CrossRef]
- Legro, R.S.; Schlaff, W.D.; Diamond, M.P.; Coutifaris, C.; Casson, P.R.; Brzyski, R.G.; Christman, G.M.; Trussell, J.C.; Krawetz, S.A.; Snyder, P.J.; et al. Total Testosterone Assays in Women with Polycystic Ovary Syndrome: Precision and Correlation with Hirsutism. J. Clin. Endocrinol. Metab. 2010, 95, 5305–5313. [Google Scholar] [CrossRef] [PubMed]
- Ünlühizarci, K.; Karababa, Y.; Bayram, F.; Kelestimur, F. The Investigation of Insulin Resistance in Patients with Idiopathic Hirsutism. J. Clin. Endocrinol. Metab. 2004, 89, 2741–2744. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, J.M.; Leirós, G.J.; Balañá, M.E. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Yang, M.; Weng, T.; Zhang, W.; Zhang, M.; He, X.; Han, C.; Wang, X. The Roles of Non-coding RNA in the Development and Regeneration of Hair Follicles: Current Status and Further Perspectives. Front. Cell Dev. Biol. 2021, 9, 720879. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Anderson, R.R.; Chang, R.J.; Ehrmann, D.A.; Lobo, R.A.; Murad, M.H.; Pugeat, M.M.; Rosenfield, R.L. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1233–1257. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.A.; Vesely, S.; Beebe, L.; Whitsett, T.; Owen, W. Ferriman Gallwey Self-Scoring I: Performance Assessment in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 4112–4114. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018, 89, 251–268. [Google Scholar] [CrossRef]
- Afifi, L.; Saeed, L.; Pasch, L.A.; Huddleston, H.G.; Cedars, M.I.; Zane, L.T.; Shinkai, K. Association of ethnicity, Fitzpatrick skin type, and hirsutism: A retrospective cross-sectional study of women with polycystic ovarian syndrome. Int. J. Womens Dermatol. 2017, 3, 37–43. [Google Scholar] [CrossRef]
- Hsu, M.-I. Changes in the PCOS phenotype with age. Steroids 2013, 78, 761–766. [Google Scholar] [CrossRef]
- Starace, M.; Orlando, G.; Alessandrini, A.; Piraccini, B.M. Female Androgenetic Alopecia: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2020, 21, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Shinkai, K.; Pasch, L.; Kuzmich, L.; Cedars, M.; Huddleston, H. Prevalence of androgenic alopecia in patients with polycystic ovary syndrome and characterization of associated clinical and biochemical features. Fertil. Steril. 2014, 101, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Futterweit, W.; Dunaif, A.; Yeh, H.-C.; Kingsley, P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J. Am. Acad. Dermatol. 1988, 19, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.M.; Fino, E.; Mancini, C.; Mazzetti, M.; Starace, M.; Piraccini, B.M. HrQoL in hair loss-affected patients with alopecia areata, androgenetic alopecia and telogen effluvium: The role of personality traits and psychosocial anxiety. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Mirmirani, P. Hormonal changes in menopause: Do they contribute to a ‘midlife hair crisis’ in women?: Hormonal changes in menopause. Br. J. Dermatol. 2011, 165, 7–11. [Google Scholar] [CrossRef]
- Shimizu, H.; Morgan, B.A. Wnt Signaling through the β-Catenin Pathway Is Sufficient to Maintain, but Not Restore, Anagen-Phase Characteristics of Dermal Papilla Cells. J. Investig. Dermatol. 2004, 122, 239–245. [Google Scholar] [CrossRef]
- Redler, S.; Messenger, A.G.; Betz, R.C. Genetics and other factors in the aetiology of female pattern hair loss. Exp. Dermatol. 2017, 26, 510–517. [Google Scholar] [CrossRef]
- Cousen, P.; Messenger, A. Female pattern hair loss in complete androgen insensitivity syndrome: Female pattern hair loss. Br. J. Dermatol. 2010, 162, 1135–1137. [Google Scholar] [CrossRef]
- Ramos, P.M.; Brianezi, G.; Martins, A.C.P.; Da Silva, M.G.; Marques, M.E.A.; Miot, H.A. Apoptosis in follicles of individuals with female pattern hair loss is associated with perifollicular microinflammation. Int. J. Cosmet. Sci. 2016, 38, 651–654. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D 2 Inhibits Hair Growth and Is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Sci. Transl. Med. 2012, 4, 126ra34. [Google Scholar] [CrossRef]
- Olsen, E.A. The midline part: An important physical clue to the clinical diagnosis of androgenetic alopecia in women. J. Am. Acad. Dermatol. 1999, 40, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol. 1977, 97, 247–254. [Google Scholar] [CrossRef]
- Gupta, M.; Mysore, V. Classifications of patterned hair loss: A review. J. Cutan. Aesthetic Surg. 2016, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, A.; Slowinska, M.; Kowalska-Oledzka, E.; Olszewska, M.; Rudnicka, L. Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. Int. J. Trichology 2009, 1, 123. [Google Scholar] [CrossRef]
- Ben Abdessalem, F.; Ach, T.; Fetoui, N.G.; Mraihi, E.; Abdelkarim, A.B. Characterizing clinical and hormonal profiles of acne in north African women with polycystic ovary syndrome. Arch. Dermatol. Res. 2024, 316, 711. [Google Scholar] [CrossRef]
- Yazici, K.; Baz, K.; Yazici, A.; Köktürk, A.; Tot, S.; Demirseren, D.; Buturak, V. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Dreno, B.; Lucky, W.A.; Agak, W.G.; Dokras, A.; Kim, J.J.; Lobo, R.A.; Tehrani, F.R.; Dumesic, D. Female Adult Acne and Androgen Excess: A Report From the Multidisciplinary Androgen Excess and PCOS Committee. J. Endocr. Soc. 2022, 6, bvac003. [Google Scholar] [CrossRef]
- Thiboutot, D.; Harris, G.; Iles, V.; Cimis, G.; Gilliland, K.; Hagari, S. Activity of the Type 1 5α-Reductase Exhibits Regional Differences in Isolated Sebaceous Glands and Whole Skin. J. Investig. Dermatol. 1995, 105, 209–214. [Google Scholar] [CrossRef]
- Pretorius, E.; Arlt, W.; Storbeck, K.-H. A new dawn for androgens: Novel lessons from 11-oxygenated C19 steroids. Mol. Cell Endocrinol. 2017, 441, 76–85. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Deplewski, D.; Kentsis, A.; Ciletti, N. Mechanisms of Androgen Induction of Sebocyte Differentiation. Dermatology 1998, 196, 43–46. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The role of inflammation in the pathology of acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27–35. [Google Scholar]
- Carmina, E.; Godwin, A.J.; Stanczyk, F.Z.; Lippman, J.S.; Lobo, R.A. The association of serum androsterone glucuronide with inflammatory lesions in women with adult acne1. J. Endocrinol. Investig. 2002, 25, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Slayden, S.M.; Moran, C.; Sams, W.M.; Boots, L.R.; Azziz, R. Hyperandrogenemia in patients presenting with acne. Fertil. Steril. 2001, 75, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.G.; Fonseca, F.L.A.; Machado, C.D.A.S. Androgenic Hormone Profile of Adult Women with Acne. Dermatology 2013, 226, 167–171. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Kircik, L.H.; Stein Gold, L.; Thiboutot, D. Androgens, Androgen Receptors, and the Skin: From the Laboratory to the Clinic With Emphasis on Clinical and Therapeutic Implications. J. Drugs Dermatol. JDD 2020, 19, 30–35. [Google Scholar]
- Thiboutot, D.; Gilliland, K.; Light, J.; Lookingbill, D. Androgen Metabolism in Sebaceous Glands From Subjects With and Without Acne. Arch. Dermatol. 1999, 135, 1041–1045. [Google Scholar] [CrossRef]
- Dréno, B.; Poli, F.; Pawin, H.; Beylot, C.; Faure, M.; Chivot, M.; Auffret, N.; Moyse, D.; Ballanger, F.; Revuz, J. Development and evaluation of a Global Acne Severity Scale (GEA Scale) suitable for France and Europe: Global Acne Assessment Scale. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 43–48. [Google Scholar] [CrossRef]
- Auffret, N.; Claudel, J.-P.; Leccia, M.-T.; Poli, F.; Farhi, D.; Dréno, B. AFAST—Adult Female Acne Scoring Tool: An easy-to-use tool for scoring acne in adult females. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 824–828. [Google Scholar] [CrossRef]
- Burke, B.M.; Cunliffe, W.J. The assessment of acne vulgaris—The Leeds technique. Br. J. Dermatol. 1984, 111, 83–92. [Google Scholar] [CrossRef]
| PCOS Phenotypes | |||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Features | |||||
| Hyperandrogenia/hirsutism | X | X | X | ||
| Ovulatory dysfunction | X | X | X | ||
| Echographic polycystic ovaries/AMH | X | X | X | ||
| Diagnostic Criteria | |||||
| NIH 1990 | X | X | |||
| AE-PCOS Society | X | X | X | ||
| Rotterdam 2003 | X | X | X | X | |
| 2018/2023 guidelines | X | X | X | X | |
| Major Criteria | Minor Criteria |
|---|---|
| (1) More than 4 yellow dots in 4 images in the frontal area. (2) Lower average hair thickness in the frontal area compared with the occipital area. (3) >10% of thin hairs (<0.03 mm) in the frontal area. | (1) Increased frontal to occipital ratio of single-hair pilosebaceous units. (2) Vellus hairs. (3) Perifollicular discoloration. |
| Grade | Value | Definition |
|---|---|---|
| Clear | 0 | Normal, clear skin with no evidence of acne vulgaris |
| Almost clear | 1 | Rare non-inflammatory lesions present, with rare non-inflamed papules (papules must be resolving and may be hyperpigmented, though not pink-red) |
| Mild | 2 | Some non-inflammatory lesions are present, with few inflammatory lesions (papules, pustules only, no nodulocystic lesions) |
| Moderate | 3 | Non-inflammatory lesions predominate, with multiple inflammatory lesions evident; several to many comedones and papules/pustules; there may or may not be one small nodulocystic lesion |
| Severe | 4 | Inflammatory lesions are more apparent; many comedones and papules/pustules; there may or may not be a few nodulocystic lesions |
| Very severe | 5 | Highly inflammatory lesions predominate; variable number of comedones; many papules/pustules, and many nodulocystic lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taieb, A.; Feryel, A. Deciphering the Role of Androgen in the Dermatologic Manifestations of Polycystic Ovary Syndrome Patients: A State-of-the-Art Review. Diagnostics 2024, 14, 2578. https://doi.org/10.3390/diagnostics14222578
Taieb A, Feryel A. Deciphering the Role of Androgen in the Dermatologic Manifestations of Polycystic Ovary Syndrome Patients: A State-of-the-Art Review. Diagnostics. 2024; 14(22):2578. https://doi.org/10.3390/diagnostics14222578
Chicago/Turabian StyleTaieb, Ach, and Amri Feryel. 2024. "Deciphering the Role of Androgen in the Dermatologic Manifestations of Polycystic Ovary Syndrome Patients: A State-of-the-Art Review" Diagnostics 14, no. 22: 2578. https://doi.org/10.3390/diagnostics14222578
APA StyleTaieb, A., & Feryel, A. (2024). Deciphering the Role of Androgen in the Dermatologic Manifestations of Polycystic Ovary Syndrome Patients: A State-of-the-Art Review. Diagnostics, 14(22), 2578. https://doi.org/10.3390/diagnostics14222578







