Autoimmune Thyroid Diseases in Chronic Spontaneous Urticaria: The Role of Hormones, Anti-Thyroid Antibodies, and Ultrasound
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Methods
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Long, H.; Hu, Y.; He, L. Chronic spontaneous urticaria. In The Rose and Mackay Textbook of Autoimmune Diseases, 1st ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 1301–1331. [Google Scholar]
- Czarnecka-Operacz, M.; Sadowska-Przytocka, A.; Jenerowicz, D.; Szeliga, A.; Adamski, Z.; Łącka, K. Thyroid function and thyroid autoantibodies in patients with chronic spontaneous urticaria. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2017, 34, 566–572. [Google Scholar] [CrossRef]
- Rottem, M. Chronic urticaria and autoimmune thyroid disease: Is there a link? Autoimmun. Rev. 2003, 2, 69–72. [Google Scholar] [CrossRef]
- Confino-Cohen, R.; Chodick, G.; Shalev, V.; Leshno, M.; Kimhi, O.; Goldberg, A. Chronic urticaria and autoimmunity: Associations found in a large population study. J. Allergy Clin. Immunol. 2012, 129, 1307–1313. [Google Scholar] [CrossRef]
- Kolkhir, P.; Muñoz, M.; Asero, R.; Ferrer, M.; Kocatürk, E.; Metz, M.; Xiang, Y.K.; Maurer, M. Autoimmune chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2022, 149, 1819–1831. [Google Scholar] [CrossRef]
- Cherrez Ojeda, I.; Vanegas, E.; Felix, M.; Mata, V.; Cherrez, S.; Simancas-Racines, D.; Greiding, L.; Cano, J.; Cherrez, A.; Calderon, J.C. Etiology of chronic urticaria: The Ecuadorian experience. World Allergy Organ. J. 2018, 11, 1. [Google Scholar] [CrossRef]
- Tienforti, D.; Di Giulio, F.; Spagnolo, L.; Castellini, C.; Totaro, M.; Muselli, M.; Francavilla, S.; Baroni, M.G.; Barbonetti, A. Chronic urticaria and thyroid autoimmunity: A meta-analysis of case–control studies. J. Endocrinol. Investig. 2022, 45, 1317–1326. [Google Scholar] [CrossRef]
- Noor, S.F.; Jannat, S.; Khan, S.I.; Azzad, M.A. Clinical Association of Anti-TPO in Patients with Chronic Urticaria. J. Rangpur Med. Coll. 2023, 8, 29–33. [Google Scholar] [CrossRef]
- Leznoff, A.; Josse, R.G.; Denburg, J.; Dolovich, J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch. Dermatol. 1983, 119, 636–640. [Google Scholar] [CrossRef]
- Asero, R.; Cugno, M. Biomarkers of chronic spontaneous urticaria and their clinical implications. Expert Rev. Clin. Immunol. 2021, 17, 247–254. [Google Scholar] [CrossRef]
- Kolkhir, P.; André, F.; Church, M.K.; Maurer, M.; Metz, M. Potential blood biomarkers in chronic spontaneous urticaria. Clin. Exp. Allergy 2017, 47, 19–36. [Google Scholar] [CrossRef]
- Sardina, D.S.; Valenti, G.; Papia, F.; Uasuf, C.G. Exploring machine learning techniques to predict the response to omalizumab in chronic spontaneous urticaria. Diagnostics 2021, 11, 2150. [Google Scholar] [CrossRef]
- Mozena, J.D.; Tinana, A.; Negri, J.; Steinke, J.W.; Borish, L. Lack of a role for cross-reacting anti-thyroid antibodies in chronic idiopathic urticaria. J. Investig. Dermatol. 2010, 130, 1860–1865. [Google Scholar] [CrossRef]
- Gonzalez-Diaz, S.N.; Sanchez-Borges, M.; Rangel-Gonzalez, D.M.; Guzman-Avilan, R.I.; Canseco-Villarreal, J.I.; Arias-Cruz, A. Chronic urticaria and thyroid pathology. World Allergy Organ. J. 2020, 13, 100101. [Google Scholar] [CrossRef]
- Asero, R.; Ferrucci, S.M.; Calzari, P.; Consonni, D.; Cugno, M. Thyroid autoimmunity in CSU: A potential marker of omalizumab response? Int. J. Mol. Sci. 2023, 24, 7491. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Berghi, N.O. Immunological mechanisms implicated in the pathogenesis of chronic urticaria and Hashimoto thyroiditis. Iran. J. Allergy Asthma Immunol. 2017, 16, 358–366. [Google Scholar]
- Chiu, H.Y.; Muo, C.H.; Sung, F.C. Associations of chronic urticaria with atopic and autoimmune comorbidities: A nationwide population-based study. Int. J. Dermatol. 2018, 57, 822–829. [Google Scholar] [CrossRef]
- Carlucci, P.; Spataro, F.; Cristallo, M.; Di Gioacchino, M.; Nettis, E.; Gangemi, S. Immune-molecular link between thyroid and skin autoimmune diseases: A narrative review. J. Clin. Med. 2024, 13, 5594. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.S.; Wu, C.S. The essential role of anti-thyroid antibodies in chronic idiopathic urticaria. Endocr. Res. 2013, 38, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Aversano, M.; Caiazzo, P.; Iorio, G.; Ponticiello, L.; Lagana, B.; Leccese, F. Improvement of chronic idiopathic urticaria with L-thyroxine: A new TSH role in immune response? Allergy 2005, 60, 489–493. [Google Scholar] [CrossRef]
- Verneuil, L.; Leconte, C.; Ballet, J.J.; Coffin, C.; Laroche, D.; Izard, J.P.; Reznik, Y.; Leroy, D. Association between chronic urticaria and thyroid autoimmunity: A prospective study involving 99 patients. Dermatology 2004, 208, 98–103. [Google Scholar] [CrossRef]
- Kolkhir, P.; Metz, M.; Altrichter, S.; Maurer, M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: A systematic review. Allergy 2017, 72, 1440–1460. [Google Scholar] [CrossRef] [PubMed]
- Kasperska-Zajac, A.; Grzanka, A.; Damasiewicz-Bodzek, A. IL-6 transsignaling in patients with chronic spontaneous urticaria. PLoS ONE 2015, 10, e0145751. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Borro, M.; Ricciardi, L.; Gangemi, S. Prevalence of autoimmune and autoinflammatory diseases in chronic urticaria: Pathogenetic, diagnostic and therapeutic implications. Biomedicines 2023, 11, 410. [Google Scholar] [CrossRef]
- Yang, X.; Gao, T.; Shi, R.; Zhou, X.; Qu, J.; Xu, J.; Shan, Z.; Teng, W. Effect of iodine excess on Th1, Th2, Th17, and Treg cell subpopulations in the thyroid of NOD. H-2 h4 mice. Biol. Trace Elem. Res. 2014, 159, 288–296. [Google Scholar] [CrossRef]
- Valencia, X.; Lipsky, P.E. CD4+ CD25+ FoxP3+ regulatory T cells in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 2007, 3, 619–626. [Google Scholar] [CrossRef]
- Barzilai, A.; Baum, A.; Ben-Shoshan, M.; Tzanani, I.; Hakroush, R.; Coster, D.; Solomon, M.; Greenberger, S. Epidemiological and clinical characteristics of adult and pediatric patients with chronic spontaneous urticaria. J. Clin. Med. 2023, 12, 7482. [Google Scholar] [CrossRef]
- Díaz-Angulo, S.; López-Hoyos, M.; Muñoz Cacho, P.; Fernández, M.; López-Escobar, M.; Rodríguez, F.; González-López, M.A. Prevalence of thyroid autoimmunity in spanish patients with chronic idiopathic urticaria: A case-control study involving 343 subjects. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 692. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sung, N.H.; Lee, A.Y. Effect of levothyroxine treatment on clinical symptoms in hypothyroid patients with chronic urticaria and thyroid autoimmunity. Ann. Dermatol. 2016, 28, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zou, D.; Cai, H.; Liu, Y. Ultrasonography in the diagnosis of Hashimoto’s thyroiditis. Front. Biosci. 2016, 21, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.T.; Cui, W. Association of serum biochemical indexes and thyroid nodule with disease activity in patients with chronic spontaneous urticaria. Eur. J. Inflamm. 2022, 20, 1721727X221141789. [Google Scholar] [CrossRef]
| Variables | CSU (n = 43) | Control Group (n = 50) | p-Value |
|---|---|---|---|
| Gender, n (%) | 0.57 | ||
| Male | 5 (11.6) | 9 (18) | |
| Female | 38 (88.4) | 41 (82) | |
| Age, mean (SD) | 47.2 (14.8) | 46.30 (15.12) | |
| Age category, n (%) | 0.78 | ||
| 18–39 | 15 (35.7) | 15 (30.0) | |
| 40–64 | 23 (54.8) | 31 (62.0%) | |
| >65 | 4 (9.5) | 4 (8.0%) | |
| Resident area, n (%) | |||
| Urban | 21 (48.8) | NA | |
| Rural | 22 (51.2) | ||
| Occupation, n (%) | |||
| Homemaker | 11 (25.6) | NA | |
| Retiree | 7 (16.3) | ||
| Economist | 7 (16.3) | ||
| Salesperson | 4 (9.3) | ||
| Other | 14 (32.6) | ||
| Marital Status, n (%) | |||
| Married | 27 (62.8) | NA | |
| Single | 16 (37.2) | ||
| Variables | CSU (n = 43) | Control Group (n = 50) | p-Value |
|---|---|---|---|
| Duration of Illness, n (%) | |||
| <5 years | 24 (55.8) | NA | |
| 5–10 years | 4 (9.3) | ||
| >10 years | 13 (30.2) | ||
| Current Episode, n (%) | |||
| Hives | 22 (51.2) | NA | |
| Without hives | 21 (48.8) | ||
| Frequency of Occurrence Per Year, n (%) | |||
| None | 2 (4.7) | NA | |
| Low (1–3 times) | 30 (69.8) | ||
| Moderate (4–6 times) | 6 (14.0) | ||
| High (>6 times) | 5 (11.6) | ||
| Duration of Changes, n (%) | |||
| <12 h | 28 (65.1) | NA | |
| 12–24 h | 9 (20.9) | ||
| 24–72 h | 6 (14.0) | ||
| When Symptoms Are Worse, n (%) | |||
| Day | 11 (25.6) | NA | |
| Night | 19 (44.2) | ||
| No Difference | 13 (30.2) | ||
| Symptoms During Specific Times, n (%) | |||
| During Travel | 18 (62.1) | NA | |
| During Vacation | 22 (75.9) | ||
| During Weekends | 26 (89.7) | ||
| Allergies, n (%) | p < 0.001 | ||
| Yes | 20 (46.5) | 8 (16) | |
| No | 23 (53.5) | 42 (84) | |
| Infections, n (%) | p < 0.001 | ||
| H. pylori | 12 (27.3) | 2 (4) | |
| Candida | 10 (22.7) | 3 (6) | |
| E. coli | 3 (6.8) | 1 (2) | |
| None | 19 (43.2) | 44 (88) | |
| Comorbidities, n (%) | p < 0.001 | ||
| Hypothyroidism | 17 (27.9) | 2 (4) | |
| Hypertension | 12 (19.7) | 1 (2) | |
| Asthma | 4 (6.6) | 0 (0) | |
| Diabetes mellitus | 4 (6.6) | 0 (0) | |
| Depression | 3 (4.9) | 0 (0) | |
| Other | 10 (16.4) | 1 (2) | |
| None | 11 (18) | 46 (92) | |
| Variables | CSU (n = 43) | Control Group (n = 50) | p-Value |
|---|---|---|---|
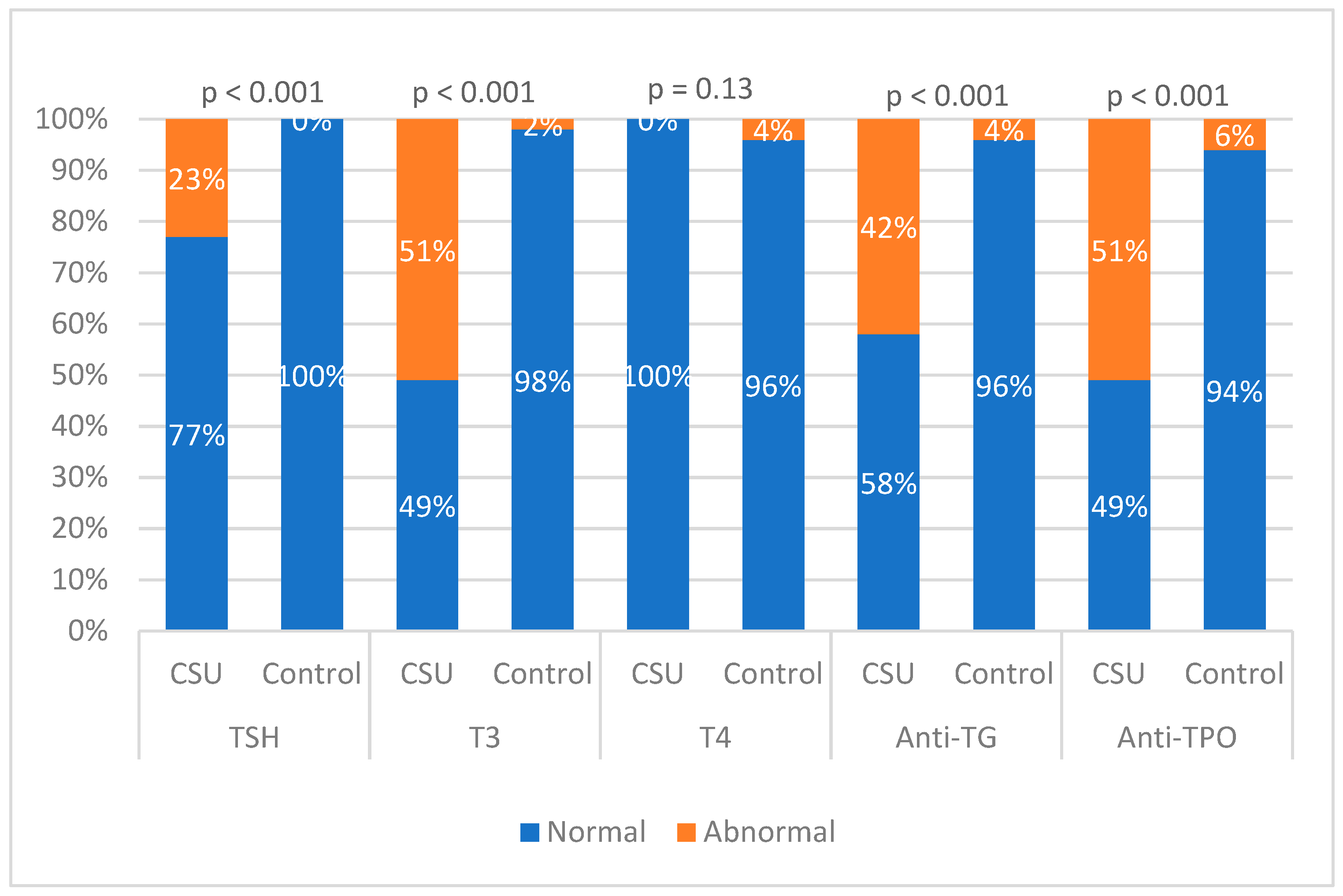
| TSH 0.4–4.0 µIU/mL | |||
| Normal | 31 (72.1) | 50 (100) | p < 0.001 |
| High | 10 (23.3) | 0 (0) | |
| Low | 2 (4.7) | 0 (0) | |
| Mean (SD) | 3.58 (3.76) | 2.2 (1.07) | |
| Median (rang) | 2.30 (0.05–16.70) | 2.25 (0.42–3.95) | |
| T3 0.8–2.0 ng/mL | |||
| Normal | 21 (48.8) | 47 (94) | p < 0.001 |
| High | 22 (51.2) | 1 (2) | |
| Low | 0 (0) | 2 (4) | |
| Mean (SD) | 2.82 (1.42) | 1.16 (0.52) | |
| Median (rang) | 2.2 (1.2–5.6) | 1.10 (0.16–2.62) | |
| T4 57.9–161 nmol/L | |||
| Normal | 41 (95.3) | 48 (96) | 0.13 |
| High | 0 (0) | 2 (4) | |
| Low | 2 (4.7) | 0 (0) | |
| Mean (SD) | 89.53 (25.99) | 134.07 (67.12) | |
| Median (rang) | 84.00 (50.00–161.00) | 123.63 (58.42–296.79) | |
| Anti TG ≤ 35 IU/mL | |||
| Normal | 25 (58.1) | 48 (96) | p < 0.001 |
| High | 18 (41.9) | 2 (4) | |
| Mean (SD) | 211.18 (541.38) | 20.97 (10.99) | |
| Median (rang) | 20.00 (0.00–2943.00) | 21.807 (0.541–44.115) | |
| Anti TPO ≤ 35 IU/mL | |||
| Normal | 21 (48.8) | 47 (94) | p < 0.001 |
| High | 22 (51.2) | 3 (6) | |
| Mean (SD) | 288.38 (378.47) | 19.279 (11.722) | |
| Median (rang) | 40.00 (0.00–1000.00) | 18.95 (1.06–47.28) | |
| Variables | CSU (n = 43) | Control Group (n = 50) | p-Value |
|---|---|---|---|
| Size, n (%) | 0.38 | ||
| Normal | 34 (79.07) | 43 (86) | |
| Enlarged | 5 (11.63) | 2 (4) | |
| Atrophic | 4 (9.3) | 5 (10) | |
| Volume of the right lobe (cm3) | |||
| Mean (SD) | 9543.23 (5018.56) | NA | |
| Mediana (rang) | 8370 (1440–24,000) | NA | |
| Volume of the left lobe (cm3) | |||
| Mean (SD) | 9131.47 (4576.35) | NA | |
| Mediana (rang) | 8064 (1584–21,600) | NA | |
| Istmus | |||
| Mean (SD) | 2.76 (1.2) | NA | |
| Mediana | 2.5 | NA | |
| Cyst, n (%) | 0.56 | ||
| Yes | 6 (13.9) | 4 (8) | |
| No | 37 (86.1) | 46 (92) | |
| Nodule, n (%) | p < 0.001 | ||
| Yes | 15 (34.88) | 3 (6) | |
| No | 28 (65.12) | 47 (94) | |
| Structure | p < 0.001 | ||
| Homogeneous | 12 (27.9) | 43 (86) | |
| Heterogeneous | 31 (72.1) | 7 (14) | |
| Autoimmune thyroid disease | p < 0.001 | ||
| Yes | 14 (32.55) | 2 (5) | |
| No | 30 (67.45) | 48 (95) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golušin, Z.; Maletin, N.; Denda, N.; Nišavić, M.; Radovanović, B.; Nikolić, O. Autoimmune Thyroid Diseases in Chronic Spontaneous Urticaria: The Role of Hormones, Anti-Thyroid Antibodies, and Ultrasound. Diagnostics 2025, 15, 608. https://doi.org/10.3390/diagnostics15050608
Golušin Z, Maletin N, Denda N, Nišavić M, Radovanović B, Nikolić O. Autoimmune Thyroid Diseases in Chronic Spontaneous Urticaria: The Role of Hormones, Anti-Thyroid Antibodies, and Ultrasound. Diagnostics. 2025; 15(5):608. https://doi.org/10.3390/diagnostics15050608
Chicago/Turabian StyleGolušin, Zoran, Nemanja Maletin, Nikola Denda, Miloš Nišavić, Bojan Radovanović, and Olivera Nikolić. 2025. "Autoimmune Thyroid Diseases in Chronic Spontaneous Urticaria: The Role of Hormones, Anti-Thyroid Antibodies, and Ultrasound" Diagnostics 15, no. 5: 608. https://doi.org/10.3390/diagnostics15050608
APA StyleGolušin, Z., Maletin, N., Denda, N., Nišavić, M., Radovanović, B., & Nikolić, O. (2025). Autoimmune Thyroid Diseases in Chronic Spontaneous Urticaria: The Role of Hormones, Anti-Thyroid Antibodies, and Ultrasound. Diagnostics, 15(5), 608. https://doi.org/10.3390/diagnostics15050608








