Lipoprotein(a) as a Risk Factor for Recurrent Acute Myocardial Infarction and Mortality: Insights from Routine Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
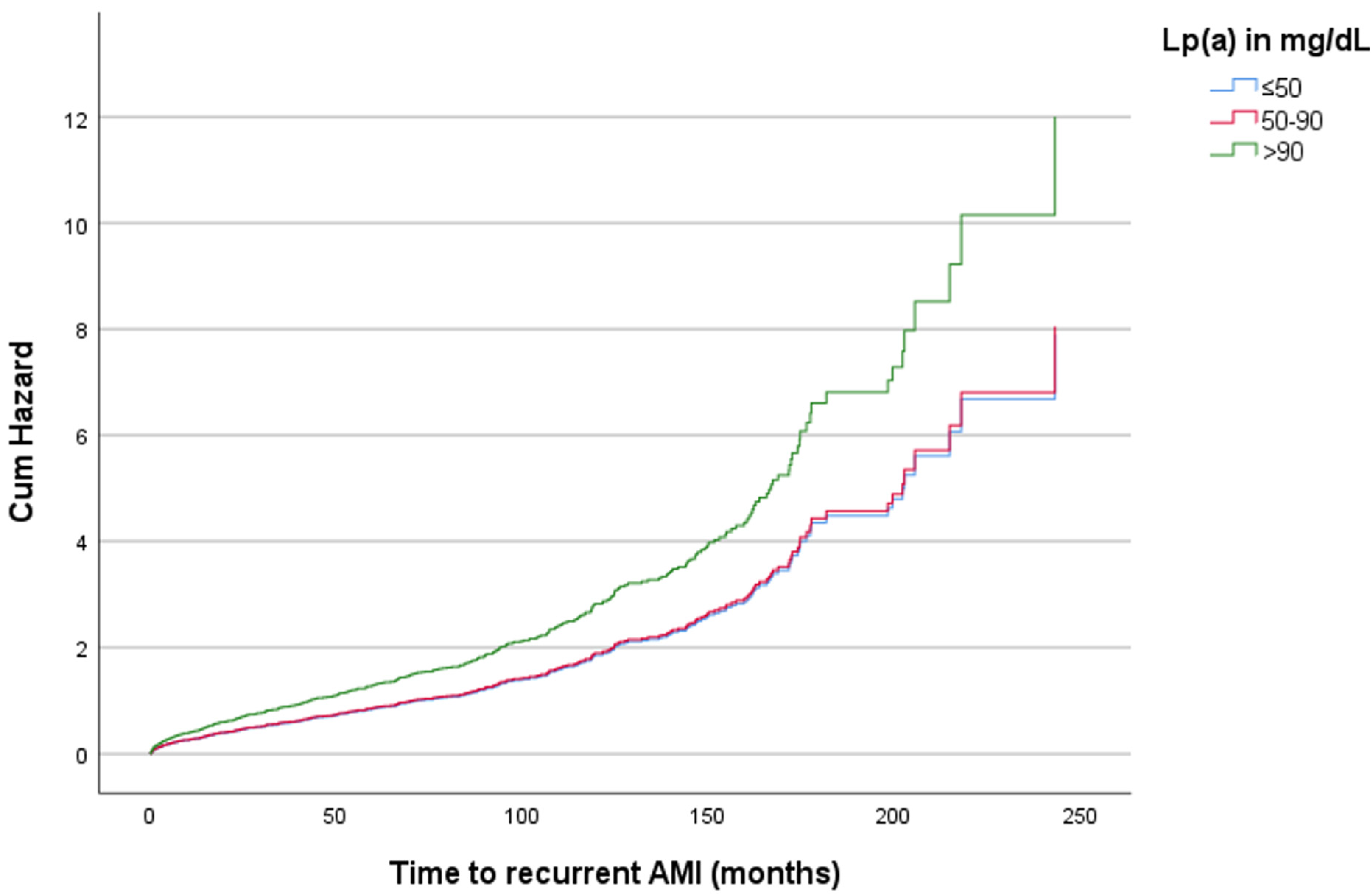
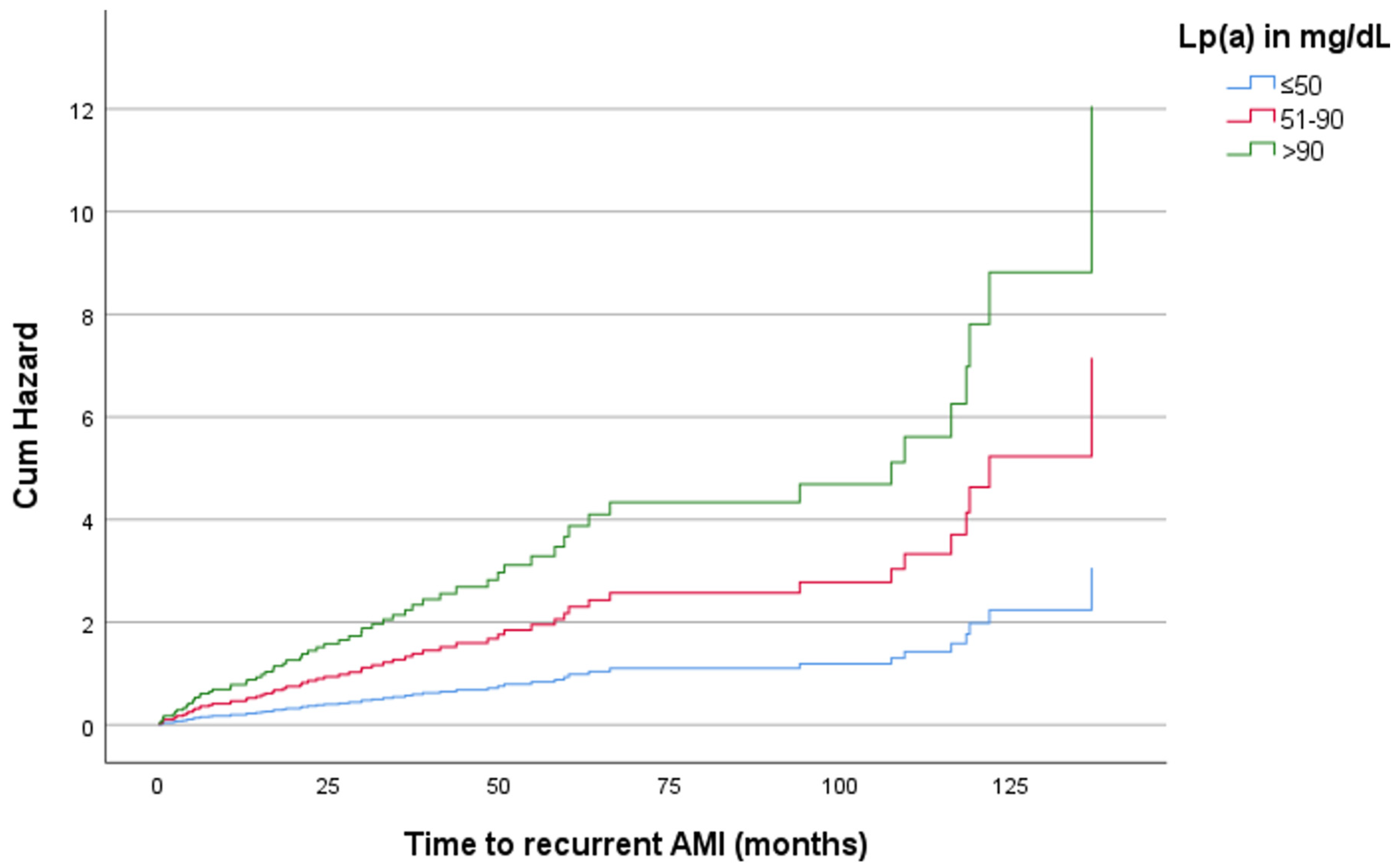
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| AMI | acute myocardial infarction |
| ASCVD | atherosclerotic cardiovascular disease |
| CV | cardiovascular |
| HDL-C | high-density lipoprotein cholesterol |
| ICD-10 | Tenth Revision of the International Classification of Diseases diagnosis codes |
| IQR | interquartile range |
| LDL-C | low-density lipoprotein cholesterol |
| Lp(a) | lipoprotein(a) |
| NSTEMI | myocardial infarction without ST-segment elevation |
| SD | standard deviation |
| STEMI | myocardial infarction with ST-segment elevation |
References
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in Atherosclerotic Cardiovascular Disease and Aortic Stenosis: A European Atherosclerosis Society Consensus Statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated Lipoprotein(a) and Risk of Aortic Valve Stenosis in the General Population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; et al. Lipoprotein(a) Concentration and the Risk of Coronary Heart Disease, Stroke, and Nonvascular Mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically Elevated Lipoprotein(a) and Increased Risk of Myocardial Infarction. JAMA 2009, 301, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Benn, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Extreme Lipoprotein(a) Levels and Risk of Myocardial Infarction in the General Population: The Copenhagen City Heart Study. Circulation 2008, 117, 176–184. [Google Scholar] [CrossRef]
- Patel, A.P.; Wang, M.; Pirruccello, J.P.; Ellinor, P.T.; Ng, K.; Kathiresan, S.; Khera, A.V. Lp(a) (Lipoprotein[a]) Concentrations and Incident Atherosclerotic Cardiovascular Disease: New Insights From a Large National Biobank. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 465–474. [Google Scholar] [CrossRef]
- Wilson, D.P.; Jacobson, T.A.; Jones, P.H.; Koschinsky, M.L.; McNeal, C.J.; Nordestgaard, B.G.; Orringer, C.E. Use of Lipoprotein(a) in Clinical Practice: A Biomarker Whose Time Has Come. A Scientific Statement from the National Lipid Association. J. Clin. Lipidol. 2019, 13, 374–392. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.; Cho, L.; Nicholls, S.J.; Kastelein, J.; Leitersdorf, E.; Landmesser, U.; Blaha, M.; Lincoff, A.M.; Morishita, R.; et al. Lipoprotein(a) Levels in a Global Population with Established Atherosclerotic Cardiovascular Disease. Open Heart 2022, 9, e002060. [Google Scholar] [CrossRef]
- Raitakari, O.; Kartiosuo, N.; Pahkala, K.; Hutri-Kähönen, N.; Bazzano, L.A.; Chen, W.; Urbina, E.M.; David R Jacobs, J.; Sinaiko, A.; Steinberger, J.; et al. Lipoprotein (a) in Youth and Prediction of Major Cardiovascular Outcomes in Adulthood. Circulation 2022, 147, 23. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Simony, S.B.; Mortensen, M.B.; Langsted, A.; Afzal, S.; Kamstrup, P.R.; Nordestgaard, B.G. Sex Differences of Lipoprotein(a) Levels and Associated Risk of Morbidity and Mortality by Age: The Copenhagen General Population Study. Atherosclerosis 2022, 355, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Waldeyer, C.; Makarova, N.; Zeller, T.; Schnabel, R.B.; Brunner, F.J.; Jørgensen, T.; Linneberg, A.; Niiranen, T.; Salomaa, V.; Jousilahti, P.; et al. Lipoprotein(a) and the Risk of Cardiovascular Disease in the European Population: Results from the BiomarCaRE Consortium. Eur. Heart J. 2017, 38, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Kalra, R.; Callas, P.W.; Alexander, K.S.; Zakai, N.A.; Wadley, V.; Arora, G.; Kissela, B.M.; Judd, S.E.; Cushman, M. Lipoprotein(a) and Risk of Ischemic Stroke in the REGARDS Study. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Gurdasani, D.; Sjouke, B.; Tsimikas, S.; Hovingh, G.K.; Luben, R.N.; Wainwright, N.W.J.; Pomilla, C.; Wareham, N.J.; Khaw, K.-T.; Boekholdt, S.M.; et al. Lipoprotein(a) and Risk of Coronary, Cerebrovascular, and Peripheral Artery Disease: The EPIC-Norfolk Prospective Population Study. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3058–3065. [Google Scholar] [CrossRef]
- Paré, G.; Çaku, A.; McQueen, M.; Anand, S.S.; Enas, E.; Clarke, R.; Boffa, M.B.; Koschinsky, M.; Wang, X.; Yusuf, S.; et al. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. Circulation 2019, 139, 1472–1482. [Google Scholar] [CrossRef]
- Šuran, D.; Blažun Vošner, H.; Završnik, J.; Kokol, P.; Sinkovič, A.; Kanič, V.; Kokol, M.; Naji, F.; Završnik, T. Lipoprotein(a) in Cardiovascular Diseases: Insight From a Bibliometric Study. Front. Public Health 2022, 10, 923797. [Google Scholar] [CrossRef]
- Virani, S.S.; Brautbar, A.; Davis, B.C.; Nambi, V.; Hoogeveen, R.C.; Sharrett, A.R.; Coresh, J.; Mosley, T.H.; Morrisett, J.D.; Catellier, D.J.; et al. Associations between Lipoprotein(a) Levels and Cardiovascular Outcomes in Black and White Subjects: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2012, 125, 241–249. [Google Scholar] [CrossRef]
- Welsh, P.; Al Zabiby, A.; Byrne, H.; Benbow, H.R.; Itani, T.; Farries, G.; Costa-Scharplatz, M.; Ferber, P.; Martin, L.; Brown, R.; et al. Elevated Lipoprotein(a) Increases Risk of Subsequent Major Adverse Cardiovascular Events (MACE) and Coronary Revascularisation in Incident ASCVD Patients: A Cohort Study from the UK Biobank. Atherosclerosis 2024, 389, 117437. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Morrow, D.A.; Tsimikas, S.; Sloan, S.; Ren, A.F.; Hoffman, E.B.; Desai, N.R.; Solomon, S.D.; Domanski, M.; Arai, K.; et al. Lipoprotein(a) for Risk Assessment in Patients with Established Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 63, 520–527. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Ballantyne, C.M.; Barter, P.J.; Kallend, D.; Leiter, L.A.; Leitersdorf, E.; McMurray, J.J.V.; Nicholls, S.J.; Olsson, A.G.; Shah, P.K.; et al. Association of Lipoprotein(a) With Risk of Recurrent Ischemic Events Following Acute Coronary Syndrome: Analysis of the Dal-Outcomes Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.C.; Clarke, R.; Watkins, H. Lp(a) (Lipoprotein[a]), an Exemplar for Precision Medicine. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 475–477. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Saeed, A.; Sun, W.; Agarwala, A.; Virani, S.S.; Nambi, V.; Coresh, J.; Selvin, E.; Boerwinkle, E.; Jones, P.H.; Ballantyne, C.M.; et al. Lipoprotein(a) Levels and Risk of Cardiovascular Disease Events in Individuals with Diabetes Mellitus or Prediabetes: The Atherosclerosis Risk in Communities Study. Atherosclerosis 2019, 282, 52–56. [Google Scholar] [CrossRef]
- Šuran, D.; Završnik, T.; Kokol, P.; Kokol, M.; Sinkovič, A.; Naji, F.; Završnik, J.; Blažun Vošner, H.; Kanič, V. Lipoprotein(a) As a Risk Factor in a Cohort of Hospitalised Cardiovascular Patients: A Retrospective Clinical Routine Data Analysis. J. Clin. Med. 2023, 12, 3220. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Nordestgaard, B.G.; Kamstrup, P.R. Low Lipoprotein(a) Levels and Risk of Disease in a Large, Contemporary, General Population Study. Eur. Heart J. 2021, 42, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Galasso, G.; De Angelis, E.; Silverio, A.; Di Maio, M.; Cancro, F.P.; Esposito, L.; Bellino, M.; Scudiero, F.; Damato, A.; Parodi, G.; et al. Predictors of Recurrent Ischemic Events in Patients With ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2021, 159, 44–51. [Google Scholar] [CrossRef]
- Kinlay, S.; Dobson, A.J.; Heller, R.F.; McELDUFF, P.; Alexander, H.; Dickeson, J. Risk of Primary and Recurrent Acute Myocardial Infarction From Lipoprotein(a) in Men and Women1. J. Am. Coll. Cardiol. 1996, 28, 870–875. [Google Scholar] [CrossRef]
- Miñana, G.; Gil-Cayuela, C.; Bodi, V.; de la Espriella, R.; Valero, E.; Mollar, A.; Marco, M.; García-Ballester, T.; Zorio, B.; Fernández-Cisnal, A.; et al. Lipoprotein(a) and Long-Term Recurrent Infarction after an Episode of ST-Segment Elevation Acute Myocardial Infarction. Coron. Artery Dis. 2020, 31, 378. [Google Scholar] [CrossRef]
- Wong, N.D.; Fan, W.; Hu, X.; Ballantyne, C.; Hoodgeveen, R.C.; Tsai, M.Y.; Browne, A.; Budoff, M.J. Lipoprotein(a) and Long-Term Cardiovascular Risk in a Multi-Ethnic Pooled Prospective Cohort. J. Am. Coll. Cardiol. 2024, 83, 1511–1525. [Google Scholar] [CrossRef]
- Bigazzi, F.; Minichilli, F.; Sbrana, F.; Pino, B.D.; Corsini, A.; Watts, G.F.; Sirtori, C.R.; Ruscica, M.; Sampietro, T. Gender Difference in Lipoprotein(a) Concentration as a Predictor of Coronary Revascularization in Patients with Known Coronary Artery Disease. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2021, 1866, 158869. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-H.; Ahn, J.-M.; Kang, D.-Y.; Lee, P.H.; Kang, S.-J.; Park, D.-W.; Lee, S.-W.; Kim, Y.-H.; Han, K.H.; Lee, C.W.; et al. Association of Lipoprotein(a) With Recurrent Ischemic Events Following Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2021, 14, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Derby, C.A.; Crawford, S.L.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid Changes during the Menopause Transition in Relation to Age and Weight: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 169, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Suk Danik, J.; Rifai, N.; Buring, J.E.; Ridker, P.M. Lipoprotein(a), Hormone Replacement Therapy, and Risk of Future Cardiovascular Events. J. Am. Coll. Cardiol. 2008, 52, 124–131. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Al-Rawas, A.M.; Al-Maqbali, M.; Al-Saleh, M.; Enriquez, M.B.; Al-Siyabi, S.; Al-Hashmi, K.; Al-Lawati, I.; Bulthuis, M.L.C.; et al. Systemic Oxidative Stress Is Increased in Postmenopausal Women and Independently Associates with Homocysteine Levels. Int. J. Mol. Sci. 2020, 21, 314. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Menopause as Risk Factor for Oxidative Stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef]
- Kork, F.; Jankowski, V.; Just, A.R.; Pfeilschifter, J.; Tepel, M.; Zidek, W.; Jankowski, J. Oxidized Low-Density Lipoprotein in Postmenopausal Women. J. Hypertens. 2014, 32, 1444–1449; discussion 1449. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, X.; Wang, R.; Zeng, J.; Zhang, K.; Yang, J.; Li, S.; Lin, X.; Jiang, Z.; Wang, G.; et al. Oxidized Lipoprotein(a) Increases Endothelial Cell Monolayer Permeability via ROS Generation. Lipids 2013, 48, 579–586. [Google Scholar] [CrossRef]
- Koschinsky, M.L.; Boffa, M.B. Oxidized Phospholipid Modification of Lipoprotein(a): Epidemiology, Biochemistry and Pathophysiology. Atherosclerosis 2022, 349, 92–100. [Google Scholar] [CrossRef]
- Feng, Z.; Li, H.-L.; Bei, W.-J.; Guo, X.-S.; Wang, K.; Yi, S.-X.; Luo, D.-M.; Li, X.; Chen, S.-Q.; Ran, P.; et al. Association of Lipoprotein(a) with Long-Term Mortality Following Coronary Angiography or Percutaneous Coronary Intervention. Clin. Cardiol. 2017, 40, 674–678. [Google Scholar] [CrossRef]
- Konishi, H.; Miyauchi, K.; Shitara, J.; Endo, H.; Wada, H.; Doi, S.; Naito, R.; Tsuboi, S.; Ogita, M.; Dohi, T.; et al. Impact of Lipoprotein(a) on Long-Term Outcomes in Patients with Diabetes Mellitus Who Underwent Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 118, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Ariyo, A.A.; Thach, C.; Tracy, R. Lp(a) Lipoprotein, Vascular Disease, and Mortality in the Elderly. N. Engl. J. Med. 2003, 349, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Cicero, A.F.G.; D’Addato, S.; D’Agostini, L.; Rosticci, M.; Giovannini, M.; Bertagnin, E.; Borghi, C.; Brisighella Heart Study Group. Serum Lipoprotein(a) Level as Long-Term Predictor of Cardiovascular Mortality in a Large Sample of Subjects in Primary Cardiovascular Prevention: Data from the Brisighella Heart Study. Eur. J. Intern. Med. 2017, 37, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Kamstrup, P.R.; Nordestgaard, B.G. High Lipoprotein(a) and High Risk of Mortality. Eur. Heart J. 2019, 40, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Kleber, M.E.; Tragante, V.; McCubrey, R.O.; Schmidt, A.F.; Direk, K.; Laufs, U.; Werner, C.; Koenig, W.; Rothenbacher, D.; et al. Relations between Lipoprotein(a) Concentrations, LPA Genetic Variants, and the Risk of Mortality in Patients with Established Coronary Heart Disease: A Molecular and Genetic Association Study. Lancet Diabetes Endocrinol. 2017, 5, 534–543. [Google Scholar] [CrossRef]
- Sawabe, M.; Tanaka, N.; Mieno, M.N.; Ishikawa, S.; Kayaba, K.; Nakahara, K.; Matsushita, S.; JMS Cohort Study Group. Low Lipoprotein(a) Concentration Is Associated with Cancer and All-Cause Deaths: A Population-Based Cohort Study (the JMS Cohort Study). PLoS ONE 2012, 7, e31954. [Google Scholar] [CrossRef]
- Langsted, A.; Kamstrup, P.R.; Nordestgaard, B.G. High Lipoprotein(a) and Low Risk of Major Bleeding in Brain and Airways in the General Population: A Mendelian Randomization Study. Clin. Chem. 2017, 63, 1714–1723. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Albers, J.J. Lipoprotein (a) Measurements for Clinical Application. J. Lipid Res. 2016, 57, 526–537. [Google Scholar] [CrossRef]
| Clinical Variable | Value |
|---|---|
| Age (years, mean ± SD) | 64.7 ± 12.2 |
| Women (%) | 31.5 |
| Diabetes mellitus (%) | 24.8 |
| Arterial hypertension (%) | 60.0 |
| STEMI/NSTEMI (%) | 36.7/63.3 |
| Laboratory data | Mean ± SD |
| Hemoglobin (g/L) | 135.0 ± 17.5 |
| Thrombocytes (109/L) | 226.1 ± 74.5 |
| HDL-C (mmol/L) | 1.1 ± 0.3 |
| Triglycerides (mmol/L) | 1.9 ± 1.2 |
| LDL-C (mmol/L) | 3.1 ± 1.1 |
| Creatinine (mcmol/L) | 91.9 ± 53.3 |
| Uric acid (mcmol/L) | 327.1 ± 107.3 |
| Medication | Percent (%) |
| Statins (no statin/moderate/high intensity) | 18.0/58.5/23.5 |
| Ezetimibe | 1.2 |
| Fenofibrate | 2.2 |
| Insulin | 9.1 |
| Oral antidiabetic drugs | 10.8 |
| Beta-blockers | 79.9 |
| Angiotensin-converting enzyme inhibitors | 75.5 |
| Angiotensin II receptor blockers | 12.4 |
| Mineralocorticoid receptor antagonists | 8.1 |
| Calcium channel antagonists | 12.9 |
| Alpha-adrenergic receptor antagonists | 2.8 |
| Lp(a) in mg/dL | Hazard Ratio | p-Value | 95% CI |
|---|---|---|---|
| Recurrent AMI | |||
| ≤50 | Reference | ||
| 51–90 | 1.01 | 0.921 | 0.768–1.340 |
| >90 | 1.51 | 0.013 | 1.093–2.094 |
| CV mortality | |||
| ≤50 | Reference | ||
| 51–90 | 1.13 | 0.300 | 0.899–1.412 |
| >90 | 1.14 | 0.348 | 0.869–1.487 |
| All-cause mortality | |||
| ≤50 | Reference | ||
| 51–90 | 1.09 | 0.310 | 0.923–1.285 |
| >90 | 1.20 | 0.090 | 0.972–1.477 |
| Sex | Age (Years) | Lp(a) (mg/dL) | Hazard Ratio | p-Value | 95% CI |
|---|---|---|---|---|---|
| Men | ≤65 | ≤50 | Reference | ||
| 51–90 | 1.26 | 0.354 | 0.773–2.054 | ||
| >90 | 1.06 | 0.839 | 0.597–1.887 | ||
| >65 | ≤50 | Reference | |||
| 51–90 | 0.45 | 0.046 | 0.201–0.986 | ||
| >90 | 1.18 | 0.681 | 0.529–2.646 | ||
| Women | ≤65 | ≤50 | Reference | ||
| 51–90 | 0.29 | 0.114 | 0.064–1.343 | ||
| >90 | 2.64 | 0.150 | 0.704–9.868 | ||
| >65 | ≤50 | Reference | |||
| 51–90 | 2.34 | 0.013 | 1.198–4.563 | ||
| >90 | 3.94 | <0.001 | 1.760–8.833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuran, D.; Kanič, V.; Kokol, P.; Završnik, T.; Verhnjak, F.; Žlahtič, B.; Sinkovič, A.; Naji, F.H. Lipoprotein(a) as a Risk Factor for Recurrent Acute Myocardial Infarction and Mortality: Insights from Routine Clinical Practice. Diagnostics 2024, 14, 2757. https://doi.org/10.3390/diagnostics14232757
Šuran D, Kanič V, Kokol P, Završnik T, Verhnjak F, Žlahtič B, Sinkovič A, Naji FH. Lipoprotein(a) as a Risk Factor for Recurrent Acute Myocardial Infarction and Mortality: Insights from Routine Clinical Practice. Diagnostics. 2024; 14(23):2757. https://doi.org/10.3390/diagnostics14232757
Chicago/Turabian StyleŠuran, David, Vojko Kanič, Peter Kokol, Tadej Završnik, Florjan Verhnjak, Bojan Žlahtič, Andreja Sinkovič, and Franjo Husam Naji. 2024. "Lipoprotein(a) as a Risk Factor for Recurrent Acute Myocardial Infarction and Mortality: Insights from Routine Clinical Practice" Diagnostics 14, no. 23: 2757. https://doi.org/10.3390/diagnostics14232757
APA StyleŠuran, D., Kanič, V., Kokol, P., Završnik, T., Verhnjak, F., Žlahtič, B., Sinkovič, A., & Naji, F. H. (2024). Lipoprotein(a) as a Risk Factor for Recurrent Acute Myocardial Infarction and Mortality: Insights from Routine Clinical Practice. Diagnostics, 14(23), 2757. https://doi.org/10.3390/diagnostics14232757







