Abstract
Juvenile primary Sjögren syndrome (pSS) with renal involvement is extremely rare, reported approximately in 50 children, predominantly girls. Here, we present the first reported case of a male child with juvenile pSS with ocular surface disease (previously keratoconjunctivitis sicca), submandibular salivary gland involvement, and tubulointerstitial nephritis. First, two symptoms were clinically apparent at presentation. We illustrate here that kidney involvement in pSS should be actively looked for, as juvenile pSS may be associated with asymptomatic renal involvement. Immunophenotyping of peripheral blood cells using multicolor flow cytometry revealed at the time of diagnosis changes in both adaptive (T memory cells and B memory cells), and innate immunity (an increased activation of natural killer cells, as well as monocytes and neutrophils, and an increased representation of intermediate monocytes). Our case report points to the importance of kidney examination, early diagnosis and therapy in juvenile pSS, as well as highlights international collaboration to obtain more data for this rare disease.
1. Introduction
Swedish ophthalmologist Henrik Sjögren first published a description of Sjögren syndrome (SS) as a “keratoconjunctivitis sicca”. The incidence and prevalence in childhood remain unknown, and it is considered to be an underrecognised and underdiagnosed entity. Originally, SS was reported most often in menopausal women, and arthritis was a prominent feature of SS, accompanied by a raised erythrocyte sedimentation rate (ESR), anemia, and fever. Since this early SS description, the syndrome has been identified as a heterogeneous chronic multisystem autoimmune rheumatic entity. Juvenile primary SS (pSS) is characterized by inflammation of the exocrine glands. However, extraglandular or systemic features can also be part of the disorder. Compared to adults, eye and oral symptoms are rare in childhood. Clinical manifestations of SS might be heterogenous, including dry eyes (keratoconjunctivitis sicca, corneal ulcers, keratitis), dry mouth (leading to increased caries), major salivary gland swelling (parotid, submandibular), extraglandular manifestations (fatigue, arthritis, arthralgia/myalgia, Raynaud phenomenon), pulmonary (chronic cough, interstitial lung disease: nonspecific interstitial pneumonia, lymphocytic interstitial pneumonia, usual interstitial pneumonia, small airways disease), renal (tubulointerstitial nephritis, renal tubular acidosis—with distal being more common) or neurological (peripheral neuropathy, central nervous system disease: demyelinating disease, neuromyelitis optica) involvement. SS is described as pSS when there is no association with other autoimmune diseases and as secondary SS (sSS) when there is another autoimmune disease present (i.e., systemic lupus erythematosus, mixed connective tissue disease, primary biliary cirrhosis, autoimmune thyroiditis). There is a female predominance both in adulthood and childhood, with a female-to-male ratio of 5:1 to 7:1 in juvenile pSS. In children, the mean age at initial symptoms is 10 years, with diagnosis at a mean of 12 years [1,2,3,4]. The diagnosis is based both on clinical and laboratory criteria. However, the American College of Rheumatology (ACR)-European League Against Rheumatism (EULAR) classification criteria are available only for adults [5]. Pediatric diagnostic criteria were proposed more than 20 years ago by Bartunkova et al. [6], based on a series of 8 children (7 females and 1 male), but they have not been validated on other cohorts yet. Recently, Devauchelle-Pensec et al. published a French national diagnostic and care protocol, including recommendations for SS in children [7]. Therapy is based on organ involvement, taking into account heterogeneity and different severity of individual patients. Pharmacotherapy encompasses predominantly local therapy, hydroxychloroquine, glucocorticoids, methotrexate, and biological disease-modifying antirheumatic drugs (bDMARDs) [7,8,9]. However, the lack of good-quality studies does not allow clinical recommendations for the treatment of SS with childhood onset [8]. Thus, prospective randomized clinical trials are needed. Here, we report a rare case of a male child with pSS with renal involvement, describing the clinical course and diagnostics, including immunophenotyping and therapy. Published cases of pSS with renal involvement are also reviewed.
2. Case Description
In 2019, a 15.5-year-old male presented with a 1-week history of fever up to 38.5 °C with cough and rhinitis during the last two weeks. Sterile pyuria lasting two weeks was also present. An otorhinolaryngologist at a regional hospital prescribed antibiotics for maxillary sinusitis and lymphadenopathy. Non-specific kidney abnormalities (multiple regions of parenchymal inflammation bilaterally) were found on computer tomography (CT), and he was referred to our center with suspicion of systemic disease.
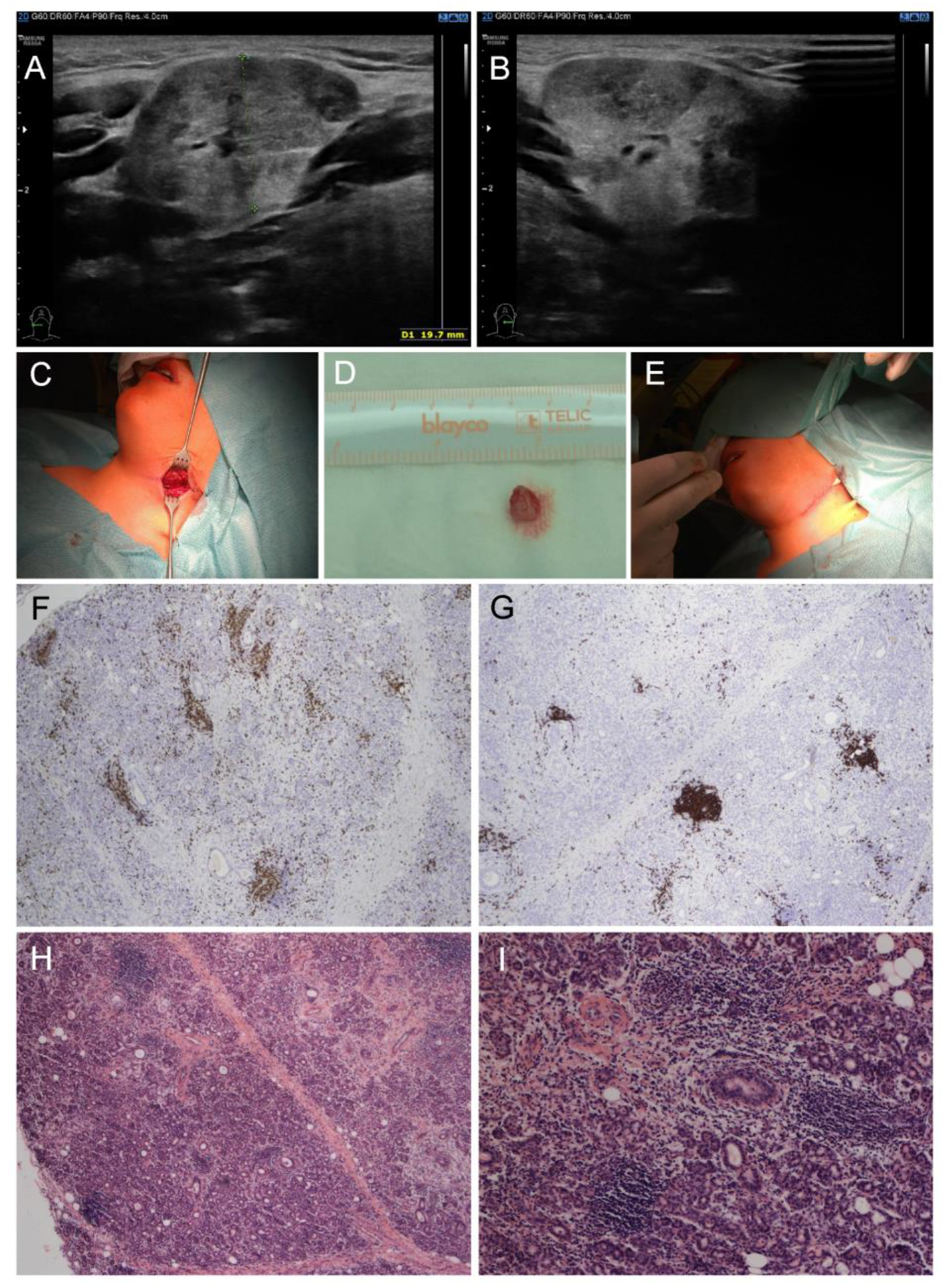
Physical examination revealed redness of both eyes, narrowed eyelids, prominent neck enlargement, large and painful solid submandibular salivary glands and submandibular lymphadenopathy. Our patient suffered from ocular dryness (sensation of sand in the eyes). Schirmer test was used to detect ocular dryness. The ophthalmologist recommended local therapy (prednisolone, vitamin A, hyaluronic acid and ectoin) due to ocular surface disease. Ultrasound examination proved enlarged submandibular glands with a rough structure and heterogenous echogenicity (Figure 1A,B). A working diagnosis of “sicca syndrome” with renal involvement was established due to sterile pyuria, elevated protein/creatinine and albumin/creatinine ratios, slightly decreased glomerular filtration rate (GFR), and kidney imaging abnormality. Further, mild erythrocyturia was detected repeatedly. Microalbuminuria was also present. On the other hand, Astrup (acidobasis status) was repeatedly with normal pH. Laboratory findings revealed elevated ESR (up to 110 mm/1st hour), C-reactive protein (CRP, 35 mg/L), leukocytosis (13.88 × 109/L) with neutrophilia, mild elevation in hepatic transaminases, hyperalbuminemia, polyclonal hypergammaglobulinemia, IgE elevation and hypovitaminosis D (25-hydroxyvitamin D level of 20.9 nmol/L). Antinuclear antibody (ANA), rheumatoid factor (RF), anti-extractable nuclear antigens (ENA), anti-Ro/SS-A and anti-La/SS-B autoantibodies were negative. Lupus anticoagulant was positive, while anti-cardiolipin IgG and anti-β2-glycoprotein 1 remained normal. Positron emission tomography (PET)-CT excluded malignancy. Further examination excluded IgG4-related, and other treatment/diseases (history of head and neck radiation treatment, active hepatitis C infection, acquired immunodeficiency syndrome, sarcoidosis, amyloidosis, graft versus host disease). Importantly, nephrology assessment revealed tubulointerstitial nephritis as the most probable diagnosis (sterile pyuria lasting four weeks, infection repeatedly excluded, while renal biopsy not carried out). Labial salivary gland biopsy revealed a focus score of 1, which met the 2016 ACR-EULAR SS classification criteria; however, a small amount of tissue did not allow the pathologist to exclude IgG4-related disease. Thus, a submandibular biopsy was performed and the findings supported the diagnosis of SS and excluded IgG4-related disease. Lymphocytic foci (i.e., foci with more than 50 lymphocytes) were found in the number of 5 per 4 mm2 of tissue in the submandibular salivary gland, resulting in a focus score of 3 (i.e., 2 or more lymphocytic foci per 4 mm2) (Figure 1C–I).
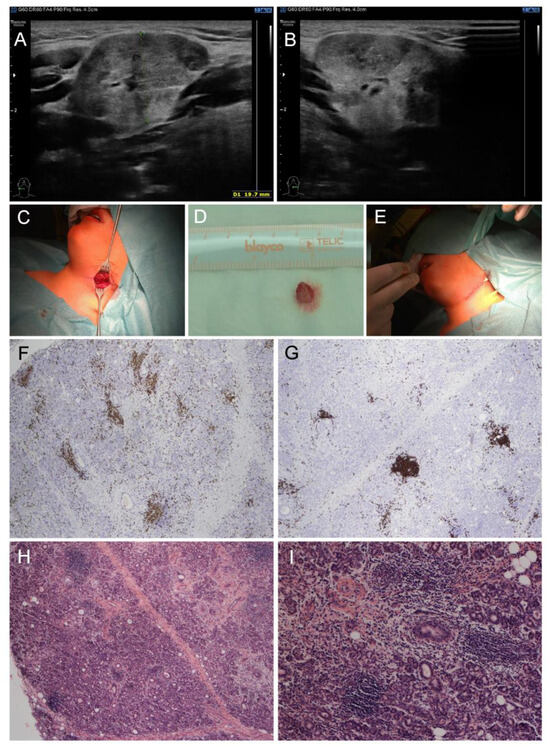
Figure 1.
Clinical and pathological examinations of a child male with pSS at the diagnosis. (A,B) Ultrasound: enlarged submandibular glands with a rough structure and heterogenous echogenicity. (C–E) Macroscopic findings: (C) submandibular salivary gland before probatory excision, (D) bioptic material from hypertrophic submandibular gland, (E) direct suture in submandibular region. (F–I) Histopathological findings in submandibular salivary gland: multifocal lymphocytic sialadenitis: (F) Immunohistochemistry of CD3 antibody labeling T lymphocytes, (G) immunohistochemistry of CD20 antibody labeling B lymphocytes, (H) hematoxylin–eosin staining of multifocal lymphocytic sialadenitis (40×), (I) hematoxylin–eosin staining of multifocal lymphocytic sialadenitis (100×).
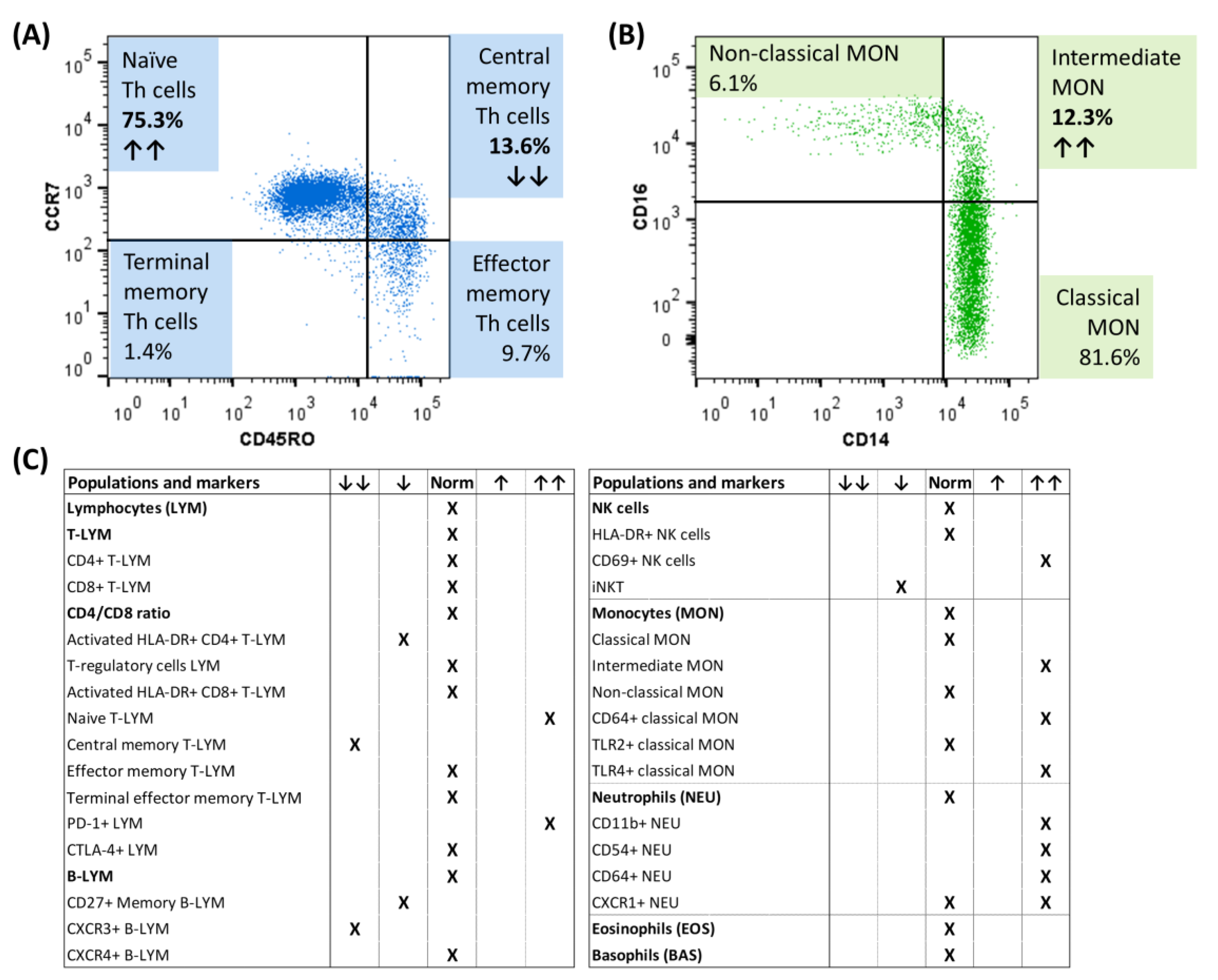
At the time of diagnosis, immunophenotyping of peripheral blood cells using multicolor flow cytometry revealed changes in T memory cells (decrease in central memory T cells, increase in PD1+ T cells) and B memory cells (decrease in CXCR3+ B cells) (Figure 2A). Regarding the innate immune cells, increased activation of natural killer (NK) cells (increased expression of the early activation marker CD69), as well as monocytes (increased number of CD64+ and TLR4+ monocytes) and neutrophils (increased expression of CD11b, CD54 and CD64) were detected, as well as an increased representation of intermediate monocytes (Figure 2B,C).
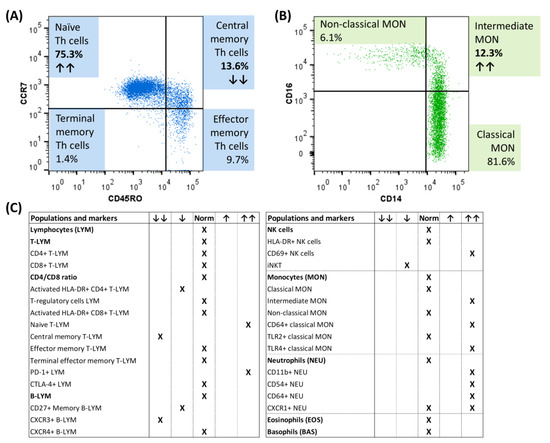
Figure 2.
Flow cytometric immunophenotyping of circulating immune cells of a child male with pSS at the diagnosis. (A) Distribution of memory T cell subsets according to CCR7 and CD45RO expression. (B) Representation of monocyte subsets. (C) Overview of the representation of immune populations/subpopulations and their activation in our patient with pSS at the time of diagnosis. ↑↑ indicates an increased relative percentage of the presented subpopulation/increased activation, while ↓↓ indicates a decrease from the healthy controls; CTLA-4+ = cytotoxic T lymphocyte-associated antigen positive, CXCR3+ = chemokine receptor CXCR3 positive, CXCR4+ = chemokine receptor CXCR4 positive, PD-1+ = programmed cell death protein positive, iNKT = invariant NK T cells, TLR2+ = toll-like receptor 2 positive, TLR4+ = toll-like receptor 4 positive.
A diagnosis of primary pSS was established by ACR-EULAR classification criteria for adult patients [5], as well as with the proposed diagnostic criteria for juvenile pSS [6]. The patient met six criteria proposed by Bartunkova et al. (oral and ocular clinical symptoms and four laboratory parameters: elevated ESR, polyclonal hyperimmunoglobulinemia, histological proof of lymphocytic infiltration of salivary glands, and objective documentation of ocular surface disease/dryness), and two ACR-EULAR criteria (focal lymphocytic sialadenitis and Schirmer). Due to nephrology indication (remained sterile pyuria, elevated albuminuria, and slow decrease in GFR) corticoid therapy was started (three 250 mg pulses of methylprednisolone followed by prednisone). Within 3 days after initiation of corticoid treatment, GFR and albuminuria normalized and sterile pyuria disappeared. Glycosuria (5.6 mmol/L) with hyperglycemia appeared as side effects. Weight gain and hypertension had developed, and thus antihypertensives (amlodipine and losartan) were prescribed. Due to his age, he was discussed with an adult rheumatologist, and therapy was amended with hydroxychloroquine. After three weeks, the patient left the hospital in good status. The following care was altered due to the COVID-19 pandemic with successful telemedicine and reinforced collaboration with regional pediatricians. The patient’s status further improved and only borderline anemia persisted. He managed to lose the weight via correction of eating habits and overall lifestyle. Four years after diagnosis, the patient is in good status, without medication, undergoing the transition process to the adult rheumatology. He attends a school.
3. Discussion
Here, we report the first case of a male child with juvenile pSS with ocular surface disease, submandibular salivary gland involvement, and tubulointerstitial nephritis. Renal involvement of pSS is a rare disease manifestation in childhood with 50 cases described in the literature (Table 1) [6,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. The largest published series by Basiaga et al. [29] encompassing 300 SS patients found 2 males among 20 patients with pSS and renal involvement (Table 1). Interestingly, renal involvement was found more frequently in children compared to adults (19.2% vs. 3.9%) (p = 0.005) in one study on SS including both pSS and sSS [25]. The same study described renal involvement to be present only in children without parotitis (p = 0.004) [25].

Table 1.
Published cases with juvenile pSS with renal involvement.
Kidney involvement should be actively looked for. Surprisingly, even one case with juvenile pSS detected by urine screening is described in the literature [13].
In adults, tubulointerstitial nephritis in SS is frequently accompanied by tubular syndromes—renal tubular acidosis, aminoaciduria, glycosuria with normal glycemia, Fanconi syndrome, and pseudo-Bartter syndrome. There were no above-mentioned syndromes detected in our patient.
Interestingly, no autoantibodies (ANA, ENA, anti-Ro/SSA, anti-La/SSB, RF) were detected in our pSS patient. In the pediatric pSS population, Virdee et al. reviewed a variability of positive serological markers, with anti-Ro/SSA, and anti-La/SSB antibodies between 36.4–84.6% and 27.3–65.4%, respectively. They even found a lower frequency of anti-Ro/anti-La in the male population. ANA and RF positivity varied in pediatric pSS populations between 63.6–96.2% and 27.3–75%, respectively [1].
Our patient suffered from ocular surface disease (ocular dryness). Ocular surface disease (previously keratoconjunctivitis sicca) and submandibular salivary gland enlargement are clinically apparent symptoms. Children diagnosed with pSS reported less dryness compared to adults [1]. In a study by Bartunkova et al., sicca syndrome was never seen during childhood, but it developed later in 3 out of 8 pSS patients [6].
In our patient, at the time of diagnosis, we also found changes in the immunophenotypes of circulating immune cells, particularly a decrease in central memory T cells, a decrease in CXCR3+ B cells, an increased proportion of intermediate monocytes, and increased activation of NK cells, monocytes, and neutrophils. To date, only a few studies have reported immune cell immunophenotypes in SS, mainly focusing on adults. Immunophenotyping of peripheral blood in adult pSS patients with low disease activity or in clinical remission revealed two subgroups of patients, characterized by distinct immune cell profiles [30]. Interestingly, patients with pSS and systemic lupus erythematosus had a similar immunologic architecture [30]. According to our knowledge, there is only a pilot study in 10 children with juvenile SS presented as a conference paper [31]. They detected elevated naïve and reduced frequencies of memory B cells and a dysregulation of the responder CD8+ T cell subpopulation compared to healthy controls [31]. The role of monocytes in SS has been supported by RNA-sequencing, where the transcriptomic profile of pSS patients has been shown to be enriched in intermediate and non-classical monocyte profiles [32,33]. Activated neutrophils have also been reported in SS patients [34,35], and it has been shown that they may contribute to inflammatory manifestations in pSS patients through induced extracellular trap formation (NETosis) [35]. Our data further support the key role of innate immune cells in the pathogenesis of pSS.
Therapy recommendations for adults have been developed by EULAR [9]. Recently, the French national diagnostic and care protocol includes therapeutic recommendations for SS in children [7]. Steroid and hydroxychloroquine therapies were found to be effective in treating renal disease [8].
SS could be associated with a higher risk of lymphoma [18]. The patient underwent PET-CT to exclude malignancy and is followed up with.
4. Conclusions
To the best of our knowledge, this is the first pediatric male case with tubulointerstitial nephritis, ocular surface disease (previously keratoconjunctivitis sicca), and submandibular salivary gland involvement. Our report highlights the importance of kidney examination, as well as early diagnosis and therapy. International collaboration is necessary to collect more data on juvenile pSS.
Author Contributions
Conceptualization, methodology, investigation, resources, collection and interpretation of clinical data, and writing—original draft preparation K.B.; clinical and laboratory data acquisition: K.B., H.F., P.H., P.M., R.P., J.M., J.C., P.C., Z.M. and E.K.; writing—review and editing, K.B., H.F., P.H., J.C., P.M., R.P., J.M., P.C., Z.M. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Czech Ministry of Health [grant DRO (FNOl, 00098892)] and by Palacky University (grant IGA_LF_2023_037).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of University Hospital Olomouc (reference number 160/23).
Informed Consent Statement
Informed consent was obtained from the guardian of the boy involved in the study. Written informed consent for publication from the patient’s guardians has been obtained.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Virdee, S.; Greenan-Barrett, J.; Ciurtin, C. A systematic review of primary Sjögren’s syndrome in male and pediatric populations. Clin. Rheumatol. 2017, 36, 2225–2236. [Google Scholar] [CrossRef]
- Hammenfors, D.S.; Valim, V.; Bica, B.E.; Pasoto, S.G.; Lilleby, V.; Nieto-González, J.C.; Silva, C.A.; Mossel, E.; Pereira, R.M.; Coelho, A.; et al. Juvenile Sjogren’s syndrome: Clinical characteristics with focus on salivary gland ultrasonography. Arthritis Care Res. 2020, 72, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.; Ciurtin, C. Sjögren Syndrome and Immunoglobulin-G4 Disease. In Textbook of Pediatric Rheumatology, 8th ed.; Petty, R.E., Laxer, R.M., Lindsley, C.B., Wedderburn, L.R., Mellins, E.D., Fuhlbrigge, R.C., Eds.; Elsevier Books: Philadelphia, PA, USA, 2021; pp. 417–426. [Google Scholar]
- Ciurtin, C.; Cho, Y.; Al-Obaidi, M.; Jury, E.; Price, E. Barriers to translational research in Sjögren’s syndrome with childhood onset: Challenges of recognising and diagnosing an orphan rheumatic disease. Lancet Rheumatol. 2021, 3, e138–e148. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 ACR-EULAR Classification Criteria for primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bartunkova, J.; Sediva, A.; Vencovsky, J.; Tesar, V. Primary Sjögren’s Syndrome in children and adolescents: Proposal for diagnostic criteria. Clin. Exp. Rheumatol. 1999, 17, 381–386. [Google Scholar] [PubMed]
- Devauchelle-Pensec, V.; Mariette, X.; Benyoussef, A.A.; Boisrame, S.; Cochener, B.; Cornec, D.; Nocturne, G.; Gottenberg, J.E.; Hachulla, E.; Labalette, P.; et al. French national diagnostic and care protocol for Sjögren’s disease. Rev. Med. Interne 2023, 44, 423–457. [Google Scholar] [CrossRef] [PubMed]
- Doolan, G.; Faizal, N.M.; Foley, C.; Al-Obaidi, M.; Jury, E.C.; Price, E.; Ramanan, A.V.; Lieberman, S.M.; Ciurtin, C. Treatment strategies for Sjögren’s syndrome with childhood onset: A systematic review of the literature. Rheumatology 2022, 61, 892–912. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjögren´s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef]
- Shioji, R.; Furuyama, T.; Onodera, S.; Saito, H.; Ito, H.; Sasaki, Y. Sjögren’s syndrome and renal tubular acidosis. Am. J. Med. 1970, 48, 456–463. [Google Scholar] [CrossRef]
- Chang, Y.C.; Huang, C.C.; Chiou, Y.Y.; Yu, C.Y. Renal tubular acidosis complicated with hypokalemic periodic paralysis. Pediatr. Neurol. 1995, 13, 52–54. [Google Scholar] [CrossRef]
- Kobayashi, I.; Furuta, H.; Tame, A.; Kawamura, N.; Kojima, K.; Endoh, M.; Okano, M.; Sakiyama, Y. Complications of childhood Sjogren syndrome. Eur. J. Pediatr. 1996, 155, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Suzuki, J.; Kume, K.; Suzuki, S.; Isome, M.; Kato, K.; Suzuki, H. Sjogren’ syndrome with membranous glomerulonephritis detected by urine screening of school children. Acta Paediatr. Jpn. 1996, 38, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Tomiita, M.; Saito, K.; Kohno, Y.; Shimojo, N.; Fujikawa, S.; Niimi, H. The clinical features of Sjögren’s syndrome in Japanese children. Acta Paediatr. Jpn. 1997, 39, 268–272. [Google Scholar] [CrossRef]
- Zawadzki, J. Permeability defect with bicarbonate leak as a mechanism of immune-related distal renal tubular acidosis. Am. J. Kidney Dis. 1998, 31, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, V.; Strike, H.; James-Ellison, M.; Tizard, E.J.; Ramanan, A.V. Renal tubular acidosis, arthritis and autoantibodies: Primary Sjogren’s syndrome in childhood. Rheumatology 2006, 45, 238–240. [Google Scholar] [CrossRef]
- Pessler, F.; Emery, H.; Dai, L.; Wu, Y.M.; Monash, B.; Cron, R.Q.; Pradhan, M. The spectrum of renal tubular acidosis in paediatric Sjogren syndrome. Rheumatology 2006, 45, 85–91. [Google Scholar] [CrossRef]
- Johnson, S.; Hulton, S.A.; Brundler, M.A.; Moss, C.; Huissoon, A.; Taylor, C.M. End-stage renal failure in adolescence with Sjogren’s syndrome autoantibodies SSA and SSB. Pediatr. Nephrol. 2007, 22, 1793–1797. [Google Scholar] [CrossRef]
- Skalova, S.; Minxova, L.; Slezak, R. Hypokalaemic Paralysis Revealing Sjogren’s Syndrome in a 16-Year Old Girl. Ghana Med. J. 2008, 42, 124–128. [Google Scholar]
- Maripuri, S.; Grande, J.P.; Osborn, T.G.; Fervenza, F.C.; Matteson, E.L.; Donadio, J.V.; Hogan, M.C. Renal involvement in primary Sjögren’s syndrome: A clinicopathologic study. Clin. J. Am. Soc. Nephrol. 2009, 4, 1423–1431. [Google Scholar] [CrossRef]
- Jung, S.K.; Park, K.H.; Yim, H.E.; Yoo, K.H.; Hong, Y.S.; Lee, J.W.; Won, N.H. Primary Sjogren’s syndrome with mesangial proliferative glomerulonephritis and IgA deposits in a child. Pediatr. Nephrol. 2010, 25, 567–568. [Google Scholar] [CrossRef]
- Kagan, M.; Bervina, N.; Vorobyeva, O.; Wouters, C.; Levtchenko, E. Pauci-immune crescentic glomerulonephritis complicating Sjogren’s syndrome in a 12-year-old girl. Pediatr. Nephrol. 2011, 26, 991–992. [Google Scholar] [CrossRef]
- Igarashi, T.; Itoh, Y.; Shimizu, A.; Igarashi, T.; Yoshizaki, K.; Fukunaga, Y. A case of juvenile Sjogren’s syndrome with interstitial nephritis. J. Nippon Med. Sch. 2012, 79, 286–290. [Google Scholar] [CrossRef]
- Bogdanović, R.; Basta-Jovanović, G.; Putnik, J.; Stajić, N.; Paripović, A. Renal involvement in primary Sjogren syndrome of childhood: Case report and literature review. Mod. Rheumatol. 2013, 23, 182–189. [Google Scholar] [CrossRef]
- Yokogawa, N.; Lieberman, S.M.; Sherry, D.D.; Vivino, F.B. Features of childhood Sjögren’s syndrome in comparison to adult Sjögren´s syndrome: Considerations in establishing child-specific diagnostic criteria. Clin. Exp. Rheumatol. 2016, 24, 343–351. [Google Scholar]
- Matsui, Y.; Takenouchi, T.; Narabayashi, A.; Ohara, K.; Nakahara, T.; Takahashi, T. Childhood Sjogren syndrome presenting as acute brainstem encephalitis. Brain Dev. 2016, 38, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Q.; Zhu, Y.; Zhao, M.; Liu, J.; Zhang, Z.; Gong, X. Nephrological disorders and neurological involvement in pediatric primary Sjogren syndrome: A case report and review of literature. Pediatr. Rheumatol. 2020, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Takezaki, S.; Tozawa, Y.; Ueki, M.; Hayashi, A.; Yamazaki, T.; Sato, Y.; Okamoto, T.; Yamada, M.; Ariga, T. Coexistence of acute poststreptococcal glomerulonephritis and acute rheumatic fever in a Japanese girl with primary Sjögren’s syndrome. Mod. Rheumatol. Case Rep. 2020, 4, 262–266. [Google Scholar] [CrossRef]
- Basiaga, M.L.; Stern, S.M.; Mehta, J.J.; Edens, C.; Randell, R.L.; Pomorska, A.; Irga-Jaworska, N.; Ibarra, M.F.; Bracaglia, C.; Nicolai, R.; et al. Childhood Sjögren syndrome: Features of an international cohort and application of the 2016 ACR/EULAR classification criteria. Rheumatology 2021, 60, 3144–3155. [Google Scholar] [CrossRef]
- Martin-Gutierrez, L.; Peng, J.; Thompson, N.L.; Robinson, G.A.; Naja, M.; Peckham, H.; Wu, W.; J’bari, H.; Ahwireng, N.; Waddington, K.E.; et al. Stratification of Patients with Sjögren’s Syndrome and Patients with Systemic Lupus erythematosus According to Two Shared Immune Cell Signatures, with Potential Therapeutic Implications. Arthritis Rheumatol. 2021, 73, 1626–1637. [Google Scholar] [CrossRef]
- Martin-Gutierrez, L.; Peckham, H.; Radziszewska, A.; Peng, J.; Nettey, O.; Jury, E.; Ciurtin, C. Juvenile Sjögren’s syndrome is characterised by dysregulated of B and T memory cell frequencies: A pilot immunophenotyping analysis of this rare disease phenotype. Rheumatology 2023, 62 (Suppl. 2), OA11. [Google Scholar] [CrossRef]
- Lopes, A.P.; Bekker, C.P.J.; Hillen, M.R.; Blokland, S.L.M.; Hinrichs, A.C.; Pandit, A.; Kruize, A.A.; Radstake, T.R.D.J.; van Roon, J.A.G. The Transcriptomic Profile of Monocytes from Patients with Sjögren’s Syndrome Is Associated with Inflammatory Parameters and Is Mimicked by Circulating Mediators. Front. Immunol. 2021, 12, 701656. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, R.; Zhang, M.; Wang, B.; Liao, Z.; Shi, G.; Li, Y. Abnormal Changes of Monocyte Subsets in Patients with Sjögren’s Syndrome. Front. Immunol. 2022, 13, 864920. [Google Scholar] [CrossRef]
- Torsteinsdóttir, I.; Gudbjörnsson, B.; Håkansson, L. Enhanced neutrophil and eosinophil adhesion in patients with primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 1998, 16, 255–262. [Google Scholar]
- Peng, Y.; Wu, X.; Zhang, S.; Deng, C.; Zhao, L.; Wang, M.; Wu, Q.; Yang, H.; Zhou, J.; Peng, L.; et al. The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary Sjögren’s syndrome. Arthritis Res. Ther. 2022, 24, 170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).