COVID-19-Associated Rhino-Orbital Mucormycosis: Histological and Electron Microscopy Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical and Legal Considerations
2.2. Study Design and Population
2.3. Preparation of Samples for the Histopathological Examination
2.4. Preparation of Samples for Electron Microscopy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinbrink, J.M.; Miceli, M.H. Mucormycosis. Infect. Dis. Clin. N. Am. 2021, 35, 435–452. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef]
- Chopra, S.; Setiya, S.; Waknis, P.P.; Kale, L.; Tidke, S. Various Treatment Modalities in COVID-19 Associated Facial Mucormycosis and the Need for its Surgical Management: A Systematic Review. J. Maxillofac. Oral. Surg. 2023, 1–22. [Google Scholar] [CrossRef]
- Baral, P.K.; Aziz, M.A.; Islam, M.S. Comparative risk assessment of COVID-19 associated mucormycosis and aspergillosis: A systematic review. Health Sci. Rep. 2022, 5, e789. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.K. COVID-19 associated pulmonary mucormycosis: A systematic review of published cases with review of literature. J. Fam. Med. Prim. Care 2022, 11, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Najeeb, H.; Naeem, A.; Dapke, K.; Phadke, R.; Asghar, M.S.; Shah, S.M.I.; De Berardis, D.; Ullah, I. COVID-19 Associated Mucormycosis: A Systematic Review from Diagnostic Challenges to Management. Diseases 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102146. [Google Scholar] [CrossRef] [PubMed]
- Saidha, P.K.; Kapoor, S.; Das, P.; Gupta, A.; Kakkar, V.; Kumar, A.; Arya, V. Mucormycosis of Paranasal Sinuses of Odontogenic Origin Post COVID19 Infection: A Case Series. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 3437–3441. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahuja, P. Risk Factors for Procurence of Mucormycosis and its Manifestations Post Covid-19: A Single Arm Retrospective Unicentric Clinical Study. Indian. J. Otolaryngol. Head. Neck Surg. 2022, 74, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Kondapavuluri, S.K.; Anchala, V.K.R.; Bandlapalli, S.; Gorantla, R.; Danaboyina, A.R.; Kondapavuluri, B.K.; Mandalapu, S. Spectrum of MR imaging findings of sinonasal mucormycosis in post COVID-19 patients. Br. J. Radiol. 2021, 94, 20210648. [Google Scholar] [CrossRef]
- Dave, T.V.; Gopinathan Nair, A.; Hegde, R.; Vithalani, N.; Desai, S.; Adulkar, N.; Kamal, S.; Mittal, R.; Bradoo, R.A. Clinical Presentations, Management and Outcomes of Rhino-Orbital-Cerebral Mucormycosis (ROCM) Following COVID-19: A Multi-Centric Study. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 488–495. [Google Scholar] [CrossRef]
- Sen, M.; Honavar, S.G.; Bansal, R.; Sengupta, S.; Rao, R.; Kim, U.; Sharma, M.; Sachdev, M.; Grover, A.K.; Surve, A.; et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India—Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J. Ophthalmol. 2021, 69, 1670–1692. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.U.S.; Arakeri, G.; Madikeri, G.; Shah, A.; Oeppen, R.S.; Brennan, P.A. COVID-19 associated mucormycosis (CAM) in India: A formidable challenge. Br. J. Oral. Maxillofac. Surg. 2021, 59, 1095–1098. [Google Scholar] [CrossRef]
- Hernández, J.L.; Buckley, C.J. Mucormycosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mahalaxmi, I.; Jayaramayya, K.; Venkatesan, D.; Subramaniam, M.D.; Renu, K.; Vijayakumar, P.; Narayanasamy, A.; Gopalakrishnan, A.V.; Kumar, N.S.; Sivaprakash, P.; et al. Mucormycosis: An opportunistic pathogen during COVID-19. Environ. Res. 2021, 201, 111643. [Google Scholar] [CrossRef]
- Walsh, T.J.; Gamaletsou, M.N.; McGinnis, M.R.; Hayden, R.T.; Kontoyiannis, D.P. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin. Infect. Dis. 2012, 54, S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Richardson, M.; Vallabhaneni, S.; Jackson, B.R.; Chiller, T. Emerging issues, challenges, and changing epidemiology of fungal disease outbreaks. Lancet Infect. Dis. 2017, 17, e403–e411. [Google Scholar] [CrossRef] [PubMed]
- Serris, A.; Danion, F.; Lanternier, F. Disease Entities in Mucormycosis. J. Fungi 2019, 5, 23. [Google Scholar] [CrossRef]
- Morace, G.; Borghi, E. Invasive mold infections: Virulence and pathogenesis of mucorales. Int. J. Microbiol. 2012, 2012, 349278. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef]
- Davitt, E.; Davitt, C.; Mazer, M.B.; Areti, S.S.; Hotchkiss, R.S.; Remy, K.E. COVID-19 disease and immune dysregulation. Best Pract. Res. Clin. Haematol. 2022, 35, 101401. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Shirbhate, E.; Patel, P.; Veerasamy, R.; Sharma, P.C.; Rajak, H. Corticosteroids for treatment of COVID-19: Effect, evidence, expectation and extent. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Das, A.; Mandal, J.; Shivaprakash, M.R.; George, V.K.; Tarai, B.; Rao, P.; Panda, N.; Verma, S.C.; Sakhuja, V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med. Mycol. 2006, 44, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, G. COVID-19-associated mucormycosis in India: Why such an outbreak? J. Mycol. Med. 2023, 33, 101393. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Jouvion, G.; de Chaumont, F.; Garcia-Hermoso, D.; Szczepaniak, C.; Renaudat, C.; Olivo-Marin, J.-C.; Chrétien, F.; Dromer, F.; Bretagne, S. Absence of Fungal Spore Internalization by Bronchial Epithelium in Mouse Models Evidenced by a New Bioimaging Approach and Transmission Electronic Microscopy. Am. J. Pathol. 2015, 185, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Eldsouky, S.M.; Shahat, A.K.; Al-Tabbakh, A.M.; El Rahman, S.M.A.; Marei, Y.M.; Mohammed, L.A.; El-Shimi, O.S.; Abdelmotaleb, D.S.; Marei, Y.M.; Elsayed, M.S.A.E. Clinical and mycological investigations of post-COVID-19 acute invasive fungal sinusitis. Laryngoscope Investig. Otolaryngol. 2022, 7, 1780–1789. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Kontoyiannis, D.P. Glucocorticoids and invasive fungal infections. Lancet 2003, 362, 1828–1838. [Google Scholar] [CrossRef]
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease–Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef]
- Nagalli, S.; Kikkeri, N.S. Mucormycosis in COVID-19: A systematic review of literature. Infez. Med. 2021, 29, 504–512. [Google Scholar] [CrossRef]
- Neagos, A.; Dumitru, M.; Vrinceanu, D.; Costache, A.; Marinescu, A.N.; Cergan, R. Ultrasonography used in the diagnosis of chronic rhinosinusitis: From experimental imaging to clinical practice. Exp. Ther. Med. 2021, 21, 611. [Google Scholar] [CrossRef]
- deShazo, R.D.; Chapin, K.; Swain, R.E. Fungal sinusitis. N. Engl. J. Med. 1997, 337, 254–259. [Google Scholar] [CrossRef]
- Burkett, A.; Saitornuang, S.; Leal, S.M. Zygomycetes. PathologyOutlines.com Website. Available online: https://www.pathologyoutlines.com/topic/microbiologyzygomycetes.html (accessed on 1 December 2023).
- Arndt, S.; Aschendorff, A.; Echternach, M.; Daemmrich, T.D.; Maier, W. Rhino-orbital-cerebral mucormycosis and aspergillosis: Differential diagnosis and treatment. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 71–76. [Google Scholar] [CrossRef]
- Guarner, J.; Brandt, M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011, 24, 247–280. [Google Scholar] [CrossRef]
- Zayet, S.; Zaghdoudi, A.; Ammari, L.; Kilani, B.; Tiouiri Benaissa, H. Cerebro-rhino-orbital mucormycosis and aspergillosis coinfection in a patient with diabetes mellitus: A case report. IDCases 2020, 23, e01022. [Google Scholar] [CrossRef]
- Rit, K.; Saha, R.; Dey, R.; Barik, G. Rhino-oculo-cerebral aspergillus and mucor co-infections in an immunocompromised patient with type 2 diabetes mellitus. Med. J. Dr. D.Y. Patil Univ. 2014, 7, 486. [Google Scholar] [CrossRef]
- Maiorano, E.; Favia, G.; Capodiferro, S.; Montagna, M.T.; Lo Muzio, L. Combined mucormycosis and aspergillosis of the oro-sinonasal region in a patient affected by Castleman disease. Virchows Arch. 2005, 446, 28–33. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, N.A.; El-Fattah, A.M.A.; Khafagy, Y.W. Invasive Fungal Sinusitis in Post COVID-19 Patients: A New Clinical Entity. Laryngoscope 2021, 131, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.L.; Hall, G.S.; Procop, G.W. Histologic features of zygomycosis: Emphasis on perineural invasion and fungal morphology. Arch. Pathol. Lab. Med. 2001, 125, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Sree Lakshmi, I.; Kumari, B.S.; Jyothi, C.; Devojee, M.; Padma Malini, K.; Sunethri, P.; Bheemrao Somalwar, S.; Kavitha, T. Histopathological Study of Mucormycosis in Post COVID-19 Patients and Factors Affecting it in a Tertiary Care Hospital. Int. J. Surg. Pathol. 2023, 31, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.H.; Brownstein, S.; Jackson, W.B.; Mintsioulis, G.; Gilberg, S.M.; al-Zeerah, B.F. Post-traumatic corneal mucormycosis caused by Absidia corymbifera. Ophthalmology 1997, 104, 1107–1111. [Google Scholar] [CrossRef]
- Romano, C.; Miracco, C.; Massai, L.; Piane, R.; Alessandrini, C.; Petrini, C.; Luzi, P. Case report. Fatal rhinocerebral zygomycosis due to Rhizopus oryzae. Mycoses 2002, 45, 45–49. [Google Scholar] [PubMed]
- Nagao, K.; Ota, T.; Tanikawa, A.; Takae, Y.; Mori, T.; Udagawa, S.-I.; Nishikawa, T. Genetic identification and detection of human pathogenic Rhizopus species, a major mucormycosis agent, by multiplex PCR based on internal transcribed spacer region of rRNA gene. J. Dermatol. Sci. 2005, 39, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Jeican, I.I.; Gheban, D.; Barbu-Tudoran, L.; Inișca, P.; Albu, C.; Ilieș, M.; Albu, S.; Vică, M.L.; Matei, H.V.; Tripon, S.; et al. Respiratory Nasal Mucosa in Chronic Rhinosinusitis with Nasal Polyps versus COVID-19: Histopathology, Electron Microscopy Analysis and Assessing of Tissue Interleukin-33. J. Clin. Med. 2021, 10, 4110. [Google Scholar] [CrossRef] [PubMed]
- Jeican, I.I.; Barbu Tudoran, L.; Florea, A.; Flonta, M.; Trombitas, V.; Apostol, A.; Dumitru, M.; Aluaș, M.; Junie, L.M.; Albu, S. Chronic Rhinosinusitis: MALDI-TOF Mass Spectrometry Microbiological Diagnosis and Electron Microscopy Analysis; Experience of the 2nd Otorhinolaryngology Clinic of Cluj-Napoca, Romania. J. Clin. Med. 2020, 9, 3973. [Google Scholar] [CrossRef]







| No./Gender/Age | University Center | Duration between the Diagnosis of COVID-19 and Mucormycosis | Diabetes | History of Corticosteroid Intake | Clinical Examination | Radio-Imaging Examination | Histopathological (HP) and Electron Microscopy Result |
|---|---|---|---|---|---|---|---|
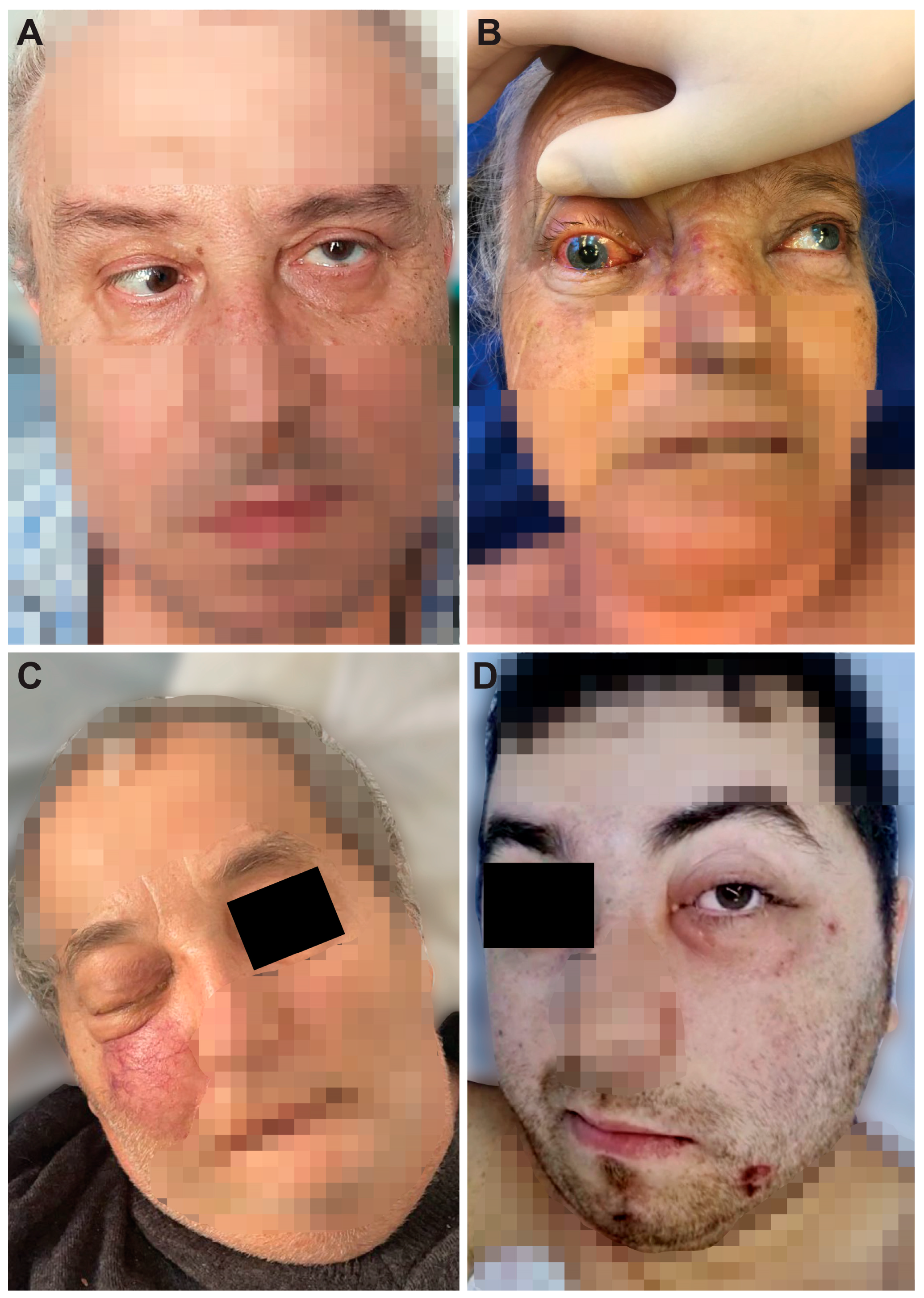
| 1, 57, M | Bucharest | one month | type 2, newly diagnosed | Yes | Immobility of the left eye, left incomplete blepharoptosis (Figure 1A). Extensive necrosis in the left nostril, adherent black crusts, purulent discharge in the left middle meatus. | MRI: Left proptosis, left maxillo-ethmoid rhinosinusitis (Figure 2A,B). | HP: Aseptate hyphae, polymorphonuclear, necrosis (Figure 4A), angioinvasion. SEM: Hyphae, rhizoids, immune cells. TEM: Aseptate hyphae. |
| 2, 66, F | Cluj-Napoca | six weeks | - | Yes | Swelling of the right zygomatic region, exophthalmos with immobility of the right eye, complete right blepharoptosis (Figure 1B). Extensive mucosal necrosis in the right nostril (Figure 3A–D). | MRI and CT performed at 2-week intervals. MRI: Right paramedian frontal cerebral intraparenchymal abscess (Figure 2C), altered sphericity of the right eyeball, bilateral pansinusitis with inhomogeneous content (Figure 2D,E), zygomatic abscess (Figure 2D, arrow). CT: Bone erosions in the right nasal cavity, zygomatic abscess (Figure 2F). Extensive lesions compared to previously performed MRI. | HP: Aseptate hyphae, angioinvasion, vascular necrosis, hemorrhage (Figure 4B), polymorphonuclear. SEM: Hyphae (Figure 5A), immune cells. TEM: Aseptate hyphae (Figure 6A,B), bacteria. |
| 3, 64, M | Cluj-Napoca | seven weeks | type 2, compensated | No | Right exophthalmos, immobility of the right eye, right oculomotor paralysis, mucopurulent secretions in the right nostril. | MRI and CT: Right pansinusitis with inhomogeneous content, right intraorbital abscess. | HP: Aseptate hyphae, angioinvasion, polymorphonuclear. SEM: Hyphae, immune cells (Figure 5B–D). TEM: Aseptate hyphae, bacteria. |
| 4, 73, M | Cluj-Napoca | one month | type 2, decompensated | Yes | Mucopurulent secretions in the left nostril. | CT: Left maxillo-ethmoid rhinosinusitis with inhomogeneous content. | HP: Wide aseptate hyphae, angioinvasion (Figure 4C–E), polymorphonuclear. SEM: Hyphae, immune cells, bacterial biofilm. TEM: Aseptate hyphae. |
| 5, 69, F | Timisoara | two months | type 2, decompensated | Yes | Denudation and infiltration of the hard palate (Figure 3E), oronasal fistula (Figure 3F), mucosal necrosis in the right nasal cavity, perforation of the nasal septum (Figure 3G–J). | CT: Right pansinusitis with inhomogeneous content. | HP: Wide aseptate hyphae, angioinvasion, polymorphonuclear. SEM: Hyphae, immune cells, bacterial biofilm. TEM: Aseptate hyphae, bacteria (Figure 6D). |
| 6, 65, F | Timișoara | two months | - | No | Ulcer-necrotic lesions of the right nasal cavity. | CT: Right pansinusitis, bone erosions (Figure 2 G,H). | HP: Wide aseptate hyphae, angioinvasion, polymorphonuclear. SEM: Hyphae, rhizoids (Figure 5E), immune cells. TEM: Aseptate hyphae. |
| 7, 62, M | Timișoara | six weeks | type 2, compensated | Yes | Right exophthalmos, orbital cellulitis, right complete blepharoptosis (Figure 1C). Extensive ulceronecrotic lesions in the right nasal cavity. | CT: Right proptosis, altered sphericity of the right eyeball, right pansinusitis. | HP: Aseptate hyphae, angioinvasion, polymorphonuclear, septate hyphae (Figure 4F,G). SEM: Hyphae, immune cells, bacterial biofilm. TEM: Neutrophils, bacterial biofilm (Figure 6E–H). |
| 8, 30, M | Timișoara | one month | type 1, decompensated | Yes | Exophthalmos, left orbital cellulitis (Figure 1D). Erosion, bone erosion, left superior alveolar rim, gingival hemorrhage (Figure 3K,L). Abnormal tooth mobility. Muco-purulent nasal secretions. | MRI: Left proptosis, left maxillary rhinosinusitis. | HP: Aseptate hyphae, angioinvasion, invasion of perineural lymphatics, necrosis (Figure 4H–J). SEM: Hyphae, rhizoids, immune cells, bacterial biofilm (Figure 5F,G). TEM: Aseptate hyphae, bacteria. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeican, I.I.; Horhat, D.I.; Dumitru, M.; Florea, A.; Barbu-Tudoran, L.; Gheban, B.-A.; Anton, V.; Toader, C.; Aluaș, M.; Siserman, C.V.; et al. COVID-19-Associated Rhino-Orbital Mucormycosis: Histological and Electron Microscopy Characteristics. Diagnostics 2024, 14, 429. https://doi.org/10.3390/diagnostics14040429
Jeican II, Horhat DI, Dumitru M, Florea A, Barbu-Tudoran L, Gheban B-A, Anton V, Toader C, Aluaș M, Siserman CV, et al. COVID-19-Associated Rhino-Orbital Mucormycosis: Histological and Electron Microscopy Characteristics. Diagnostics. 2024; 14(4):429. https://doi.org/10.3390/diagnostics14040429
Chicago/Turabian StyleJeican, Ionuț Isaia, Delia Ioana Horhat, Mihai Dumitru, Adrian Florea, Lucian Barbu-Tudoran, Bogdan-Alexandru Gheban, Vlad Anton, Corneliu Toader, Maria Aluaș, Costel Vasile Siserman, and et al. 2024. "COVID-19-Associated Rhino-Orbital Mucormycosis: Histological and Electron Microscopy Characteristics" Diagnostics 14, no. 4: 429. https://doi.org/10.3390/diagnostics14040429
APA StyleJeican, I. I., Horhat, D. I., Dumitru, M., Florea, A., Barbu-Tudoran, L., Gheban, B.-A., Anton, V., Toader, C., Aluaș, M., Siserman, C. V., Balica, N., Vrînceanu, D., & Albu, S. (2024). COVID-19-Associated Rhino-Orbital Mucormycosis: Histological and Electron Microscopy Characteristics. Diagnostics, 14(4), 429. https://doi.org/10.3390/diagnostics14040429











