Instrumented Balance Error Scoring System in Children and Adolescents—A Cross Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
2.3. BESS
2.4. Posturography
2.5. Feedback
2.6. Statistical Analysis
3. Results
3.1. Study Population
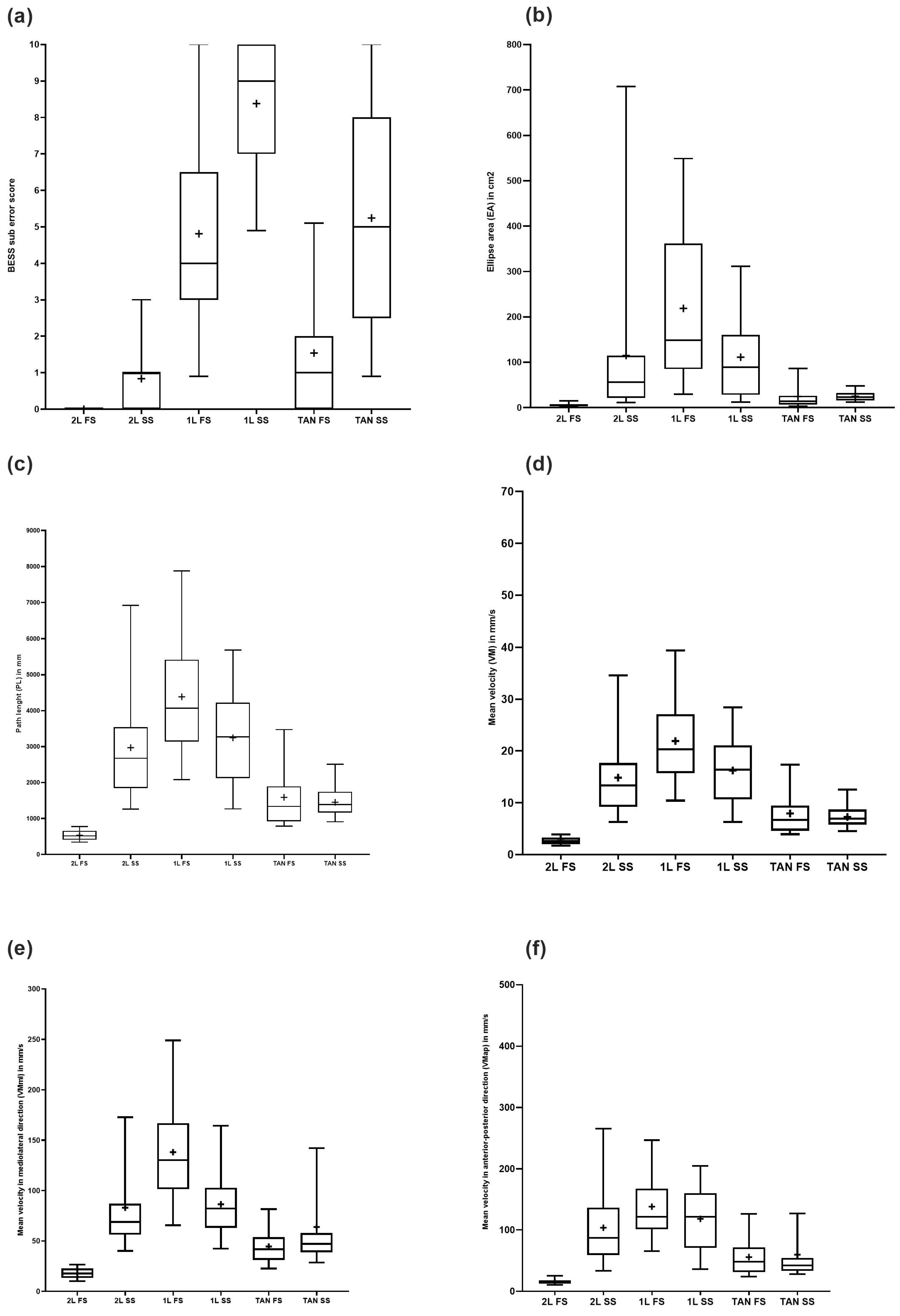
3.2. BESS
3.3. Posturography
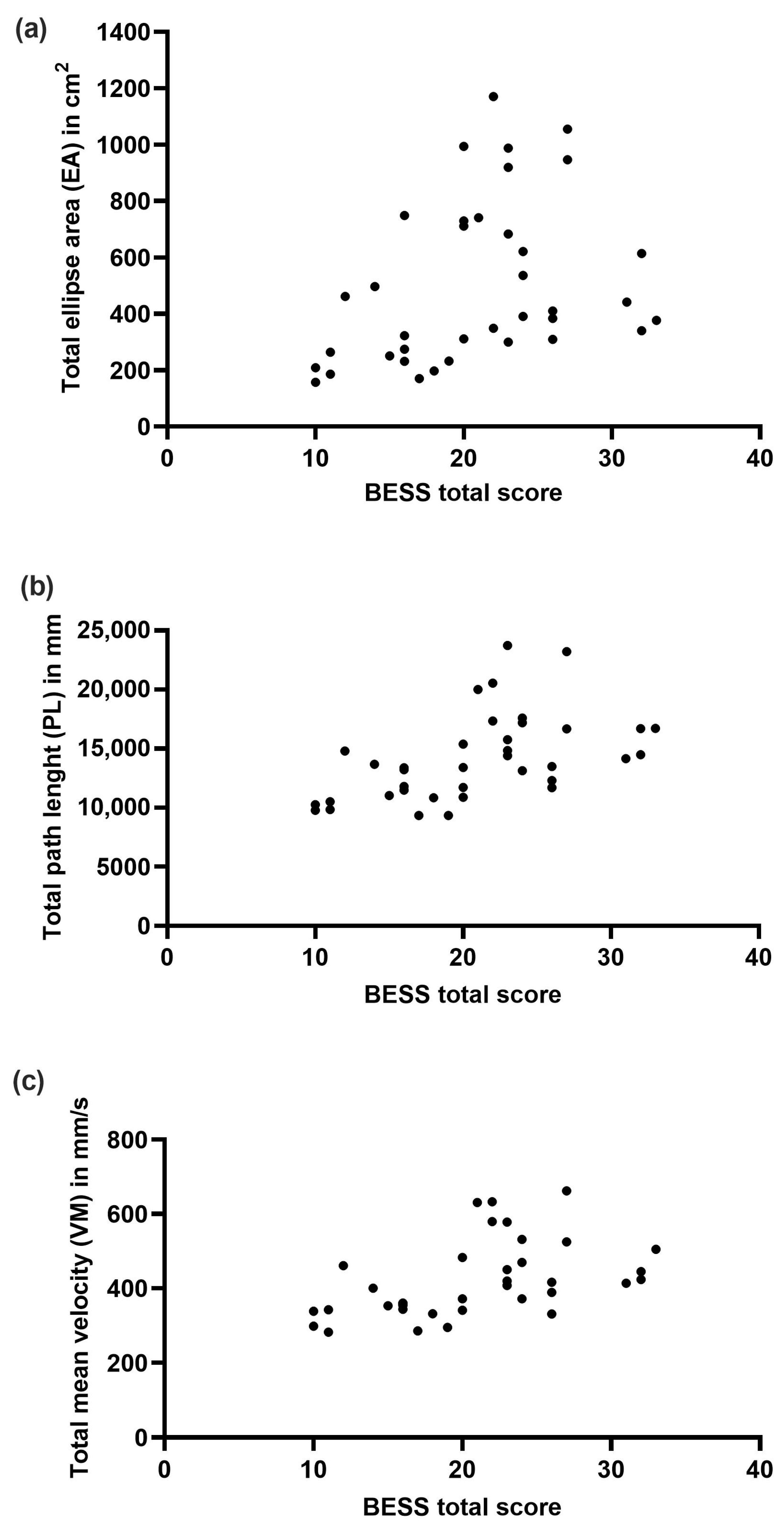
3.4. Correlation of BESS and Force Plate Measures
3.5. Test–Retest Reliability of Instrumented BESS
3.6. Feedback
4. Discussion
4.1. BESS
4.2. Posturography
4.3. Correlation of BESS and Force Plate Measures
4.4. Test–Retest Reliability
4.5. Feedback
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths–United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brazinova, A.; Rehorcikova, V.; Taylor, M.S.; Buckova, V.; Majdan, M.; Psota, M.; Peeters, W.; Feigin, V.; Theadom, A.; Holkovic, L.; et al. Epidemiology of Traumatic Brain Injury in Europe: A Living Systematic Review. J. Neurotrauma 2018, 38, 1411–1440. [Google Scholar] [CrossRef] [PubMed]
- Peeters, W.; van den Brande, R.; Polinder, S.; Brazinova, A.; Steyerberg, E.W.; Lingsma, H.F.; Maas, A.I. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015, 157, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 36 (Suppl. S43), 28–60. [Google Scholar] [CrossRef] [PubMed]
- Coulter, I.C.; Forsyth, R.J. Paediatric traumatic brain injury. Curr. Opin. Pediatr. 2019, 31, 769–774. [Google Scholar] [CrossRef]
- Gelineau-Morel, R.N.; Zinkus, T.P.; Le Pichon, J.B. Pediatric Head Trauma: A Review and Update. Pediatr. Rev. 2019, 40, 468–481. [Google Scholar] [CrossRef]
- Bernard, C.; McKinlay, A.; Krieser, D.; Testa, R.; Ponsford, A.J. Acute post-concussive symptoms in young children. Brain Inj. 2017, 31, 1414–1421. [Google Scholar] [CrossRef]
- Babcock, L.; Byczkowski, T.; Wade, S.L.; Ho, M.; Mookerjee, S.; Bazarian, J.J. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013, 167, 156–161. [Google Scholar] [CrossRef]
- Kapadia, M.; Scheid, A.; Fine, E.; Zoffness, R. Review of the Management of Pediatric Post-Concussion Syndrome-a Multi-Disciplinary, Individualized Approach. Curr. Rev. Musculoskelet. Med. 2019, 12, 57–66. [Google Scholar] [CrossRef]
- Lumba-Brown, A.; Yeates, K.O.; Sarmiento, K.; Breiding, M.J.; Haegerich, T.M.; Gioia, G.A.; Turner, M.; Benzel, E.C.; Suskauer, S.J.; Giza, C.C.; et al. Centers for Disease Control and Prevention Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury among Children. JAMA Pediatr. 2018, 172, e182853. [Google Scholar] [CrossRef]
- Degani, A.M.; Santos, M.M.; Leonard, C.T.; Rau, T.F.; Patel, S.A.; Mohapatra, S.; Danna-Dos-Santos, A. The effects of mild traumatic brain injury on postural control. Brain Inj. 2017, 31, 49–56. [Google Scholar] [CrossRef]
- Bonke, E.M.; Southard, J.; Buckley, T.A.; Reinsberger, C.; Koerte, I.K.; Howell, D.R. The effects of repetitive head impacts on postural control: A systematic review. J. Sci. Med. Sport 2021, 24, 247–257. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.L.; Nagai, T.; Webster, K.E.; Hewett, T.E. Musculoskeletal Injury Risk after Sport-Related Concussion: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2019, 47, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Lynall, R.C.; Buckley, T.A.; Herman, D.C. Neuromuscular Control Deficits and the Risk of Subsequent Injury after a Concussion: A Scoping Review. Sports Med. 2018, 48, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.A.; Howard, C.M.; Oldham, J.R.; Lynall, R.C.; Swanik, C.B.; Getchell, N. No Clinical Predictors of Postconcussion Musculoskeletal Injury in College Athletes. Med. Sci. Sports Exerc. 2020, 52, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Potter, M.N.; Kirkwood, M.W.; Wilson, P.E.; Provance, A.J.; Wilson, J.C. Clinical predictors of symptom resolution for children and adolescents with sport-related concussion. J. Neurosurg. Pediatr. 2019, 24, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Mayer, A.R.; Master, C.L.; Leddy, J.; Zemek, R.; Meier, T.B.; Owen Yeates, K.; Arbogast, K.B.; Mannix, R.; Meehan, W.P., 3rd. Prognosis for Persistent Post Concussion Symptoms using a Multifaceted Objective Gait and Balance Assessment Approach. Gait Posture 2020, 79, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.A.; Anderson, V.; Babl, F.E.; Gioia, G.A.; Giza, C.C.; Meehan, W.; Moser, R.S.; Purcell, L.; Schatz, P.; Schneider, K.J.; et al. What is the difference in concussion management in children as compared with adults? A systematic review. Br. J. Sports Med. 2017, 51, 949–957. [Google Scholar] [CrossRef]
- Comeau, D.; Pfeifer, N. Diagnosis of Concussion on the Sidelines. Semin. Pediatr. Neurol. 2019, 30, 26–34. [Google Scholar] [CrossRef]
- Broglio, S.P.; Cantu, R.C.; Gioia, G.A.; Guskiewicz, K.M.; Kutcher, J.; Palm, M.; Valovich McLeod, T.C. National Athletic Trainers’ Association position statement: Management of sport concussion. J. Athl. Train. 2014, 49, 245–265. [Google Scholar] [CrossRef]
- Bell, D.R.; Guskiewicz, K.M.; Clark, M.A.; Padua, D.A. Systematic review of the balance error scoring system. Sports Health 2011, 3, 287–295. [Google Scholar] [CrossRef]
- Echemendia, R.J.; Brett, B.L.; Broglio, S.; Davis, G.A.; Giza, C.C.; Guskiewicz, K.M.; Harmon, K.G.; Herring, S.; Howell, D.R.; Master, C.; et al. Sport concussion assessment tool–6 (SCAT6). Br. J. Sports Med. 2023, 57, 622–631. [Google Scholar] [CrossRef]
- Onate, J.A.; Beck, B.C.; Van Lunen, B.L. On-field testing environment and balance error scoring system performance during preseason screening of healthy collegiate baseball players. J. Athl. Train. 2007, 42, 446–451. [Google Scholar]
- Cushman, D.; Hendrick, J.; Teramoto, M.; Fogg, B.; Bradley, S.; Hansen, C. Reliability of the balance error scoring system in a population with protracted recovery from mild traumatic brain injury. Brain Inj. 2018, 32, 569–574. [Google Scholar] [CrossRef]
- Krafczyk, S.; Tietze, S.; Swoboda, W.; Valkovič, P.; Brandt, T. Artificial neural network: A new diagnostic posturographic tool for disorders of stance. Clin. Neurophysiol. 2006, 117, 1692–1698. [Google Scholar] [CrossRef]
- Ickenstein, G.W.; Ambach, H.; Kloditz, A.; Koch, H.; Isenmann, S.; Reichmann, H.; Ziemssen, T. Static posturography in aging and Parkinson’s disease. Front. Aging Neurosci. 2012, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Pozzilli, C. The clinical relevance of force platform measures in multiple sclerosis: A review. Mult. Scler. Int. 2013, 2013, 756564. [Google Scholar] [CrossRef] [PubMed]
- Quatman-Yates, C.C.; Bonnette, S.; Hugentobler, J.A.; Mede, B.; Kiefer, A.W.; Kurowski, B.G.; Riley, M.A. Postconcussion Postural Sway Variability Changes in Youth: The Benefit of Structural Variability Analyses. Pediatr. Phys. Ther. 2015, 27, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.O.; Levy, S.S.; Seay, S.W.; Goble, D.J. An alternative to the balance error scoring system: Using a low-cost balance board to improve the validity/reliability of sports-related concussion balance testing. Clin. J. Sport Med. 2014, 24, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, M.; Wilson, K.E.; Hunt, A.; Keightley, M.; Zabjek, K.; Taha, T.; Gagnon, I.; Reed, N. Postural Stability in Healthy Child and Youth Athletes: The Effect of Age, Sex, and Concussion-Related Factors on Performance. Sports Health 2018, 10, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.J.; Cassidy, J.D.; Holm, L.; Kraus, J.; Coronado, V.G. Methodological issues and research recommendations for mild traumatic brain injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 36 (Suppl. S43), 113–125. [Google Scholar] [CrossRef]
- Crouchman, M.; Rossiter, L.; Colaco, T.; Forsyth, R. A practical outcome scale for paediatric head injury. Arch. Dis. Child. 2001, 84, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Maurer, C.; Peterka, R.J. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J. Neurophysiol. 2005, 93, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.; O’Hare, N. A quality control procedure for force platforms. Physiol. Meas. 2000, 21, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Freitas, S.M. Revision of posturography based on force plate for balance evaluation. Rev. Bras. Fisioter. 2010, 14, 183–192. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall: London, UK, 2020. [Google Scholar]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Alsalaheen, B.A.; Haines, J.; Yorke, A.; Stockdale, K.; Broglio, S.P. Reliability and concurrent validity of instrumented balance error scoring system using a portable force plate system. Phys. Sportsmed. 2015, 43, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Cushman, D.; Anderson, N.; Chen, W.; Cheng, C.; Hon, S.D.; Hung, M. A Normative Dataset of the Balance Error Scoring System in Children Aged Between 5 and 14. Clin. J. Sport Med. 2016, 26, 497–501. [Google Scholar] [CrossRef]
- Khanna, N.K.; Baumgartner, K.; LaBella, C.R. Balance Error Scoring System Performance in Children and Adolescents with No History of Concussion. Sports Health 2015, 7, 341–345. [Google Scholar] [CrossRef]
- Valovich McLeod, T.C.; Barr, W.B.; McCrea, M.; Guskiewicz, K.M. Psychometric and measurement properties of concussion assessment tools in youth sports. J. Athl. Train. 2006, 41, 399–408. [Google Scholar]
- Verbecque, E.; da Costa, P.H.; Meyns, P.; Desloovere, K.; Vereeck, L.; Hallemans, A. Age-related changes in postural sway in preschoolers. Gait Posture 2016, 44, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ozinga, S.J.; Linder, S.M.; Koop, M.M.; Dey, T.; Figler, R.; Russman, A.N.; So, R.; Rosenthal, A.H.; Cruickshank, J.; Alberts, J.L. Normative Performance on the Balance Error Scoring System by Youth, High School, and Collegiate Athletes. J. Athl. Train. 2018, 53, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Hanson, E.; Sugimoto, D.; Stracciolini, A.; Meehan, W.P., 3rd. Assessment of the Postural Stability of Female and Male Athletes. Clin. J. Sport Med. 2017, 27, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Lin, W.H. The influence of gender and somatotype on single-leg upright standing postural stability in children. J. Appl. Biomech. 2007, 23, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Riach, C.L.; Starkes, J.L. Velocity of centre of pressure excursions as an indicator of postural control systems in children. Gait Posture 1994, 2, 167–172. [Google Scholar] [CrossRef]
- Rival, C.; Ceyte, H.; Olivier, I. Developmental changes of static standing balance in children. Neurosci. Lett. 2005, 376, 133–136. [Google Scholar] [CrossRef]
- Goble, D.J.; Brar, H.; Brown, E.C.; Marks, C.R.; Baweja, H.S. Normative data for the Balance Tracking System modified Clinical Test of Sensory Integration and Balance protocol. Med. Devices 2019, 12, 183–191. [Google Scholar] [CrossRef]
- Condon, C.; Cremin, K. Static balance norms in children. Physiother. Res. Int. 2014, 19, 1–7. [Google Scholar] [CrossRef]
- Cochrane, G.D.; Christy, J.B.; Almutairi, A.; Busettini, C.; van Heyningen, H.K.K.; Weise, K.K.; Swanson, M.W.; Gould, S.J. Vestibular, Oculomotor, and Balance Functions in Children with and without Concussion. J. Head Trauma Rehabil. 2021, 36, 264–273. [Google Scholar] [CrossRef]
- Verbecque, E.; Vereeck, L.; Hallemans, A. Postural sway in children: A literature review. Gait Posture 2016, 49, 402–410. [Google Scholar] [CrossRef]
- Ludwig, O.; Kelm, J.; Hammes, A.; Schmitt, E.; Fröhlich, M. Neuromuscular performance of balance and posture control in childhood and adolescence. Heliyon 2020, 6, e04541. [Google Scholar] [CrossRef]
- Blanchet, M.; Prince, F.; Messier, J. Development of postural stability limits: Anteroposterior and mediolateral postural adjustment mechanisms do not follow the same maturation process. Hum. Mov. Sci. 2019, 63, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef]
- Kirshenbaum, N.; Riach, C.L.; Starkes, J.L. Non-linear development of postural control and strategy use in young children: A longitudinal study. Exp. Brain Res. 2001, 140, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Tsiros, M.D.; Brinsley, J.; Mackintosh, S.; Thewlis, D. Relationships between adiposity and postural control in girls during balance tasks of varying difficulty. Obes. Res. Clin. Pract. 2019, 13, 358–364. [Google Scholar] [CrossRef]
- Pilz, F.; Vill, K.; Rawer, R.; Bonfert, M.; Tacke, M.; Heussinger, N.; Muller-Felber, W.; Blaschek, A. Mechanography in children: Pediatric references in postural control. J. Musculoskelet. Neuronal Interact. 2022, 22, 431–454. [Google Scholar] [PubMed]
- Nolan, L.; Grigorenko, A.; Thorstensson, A. Balance control: Sex and age differences in 9- to 16-year-olds. Dev. Med. Child. Neurol. 2005, 47, 449–454. [Google Scholar] [CrossRef]
- Rinaldi, N.M.; Polastri, P.F.; Barela, J.A. Age-related changes in postural control sensory reweighting. Neurosci. Lett. 2009, 467, 225–229. [Google Scholar] [CrossRef]
- Peterson, M.L.; Christou, E.; Rosengren, K.S. Children achieve adult-like sensory integration during stance at 12-years-old. Gait Posture 2006, 23, 455–463. [Google Scholar] [CrossRef]
- Valente, M. Maturational effects of the vestibular system: A study of rotary chair, computerized dynamic posturography, and vestibular evoked myogenic potentials with children. J. Am. Acad. Audiol. 2007, 18, 461–481. [Google Scholar] [CrossRef]
- Guzman, J.; Aktan, N. Comparison of the Wii Balance Board and the BESS tool measuring postural stability in collegiate athletes. Appl. Nurs. Res. 2016, 29, 1–4. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Mancini, M.; Fino, P.C.; Chesnutt, J.; Swanson, C.W.; Markwardt, S.; Chapman, J.C. Sensor-Based Balance Measures Outperform Modified Balance Error Scoring System in Identifying Acute Concussion. Ann. Biomed. Eng. 2017, 45, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Finnoff, J.T.; Peterson, V.J.; Hollman, J.H.; Smith, J. Intrarater and Interrater Reliability of the Balance Error Scoring System (BESS). PM&R 2009, 1, 50–54. [Google Scholar]
- Hansen, C.; Cushman, D.; Chen, W.; Bounsanga, J.; Hung, M. Reliability Testing of the Balance Error Scoring System in Children Between the Ages of 5 and 14. Clin. J. Sport Med. 2017, 27, 64–68. [Google Scholar] [CrossRef]
| BESS Subscores and Total Scores | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Cohort | Group Comparison Age | Group Comparison Sex | |||||||
| n = 37 | <12 y.o.a. | ≥12 y.o.a. | Female | Male | |||||
| n = 11 | n = 26 | n = 18 | n = 19 | ||||||
| Testing Position | Mean ± SD | 95% CI | (Range) | Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p |
| 2L FS | 0 ± 0 | [0; 0] | (0–0) | 0 | 0 | n.a. | 0 | 0 | n.a. |
| 1L FS | 4.80 ± 2.83 | [3.87; 5.75] | (0–10) | 6.36 ± 3.33 | 4.15 ± 2.36 | 0.087 | 4.50 ± 2.28 | 5.11 ± 3.30 | 0.660 |
| Tan FS | 1.54 ± 1.64 | [0.99; 2.09] | (0–6) | 2.18 ± 1.62 | 1.27 ± 1.59 | 0.065 | 1.33 ± 1.72 | 1.74 ± 1.60 | 0.298 |
| 2L SS | 0.84 ± 1.01 | [0.50; 1.18] | (0–3) | 1.09 ± 1.30 | 0.73 ± 0.87 | 0.635 | 0.72 ± 0.75 | 0.95 ± 1.22 | 0.988 |
| 1L SS | 8.38 ± 1.76 | [7.79; 8.96] | (4–10) | 9.18 ± 1.25 | 8.04 ± 1.84 | 0.075 | 8.17 ± 1.65 | 8.58 ± 1.87 | 0.327 |
| Tan SS | 5.24 ± 2.82 | [4.29; 6.20] | (0–10) | 4.55 ± 2.84 | 5.54 ± 2.87 | 0.342 | 5.28 ± 3.30 | 5.21 ± 2.46 | 0.940 |
| BESS Total score | 20.81 ± 6.28 | [18.72; 22.90] | (10–33) | 23.36 ± 6.01 | 19.73 ± 6.15 | 0.108 | 20.00 ± 5.88 | 21.58 ± 6.69 | 0.45 |
| Total Cohort | Group Comparison Age | Group Comparison Sex | |||||
|---|---|---|---|---|---|---|---|
| n = 37 | <12 y.o.a. | ≥12 y.o.a. | Female | Male | |||
| n = 11 | n = 26 | n = 18 | n = 19 | ||||
| Testing Position | Mean ± SD | Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p |
| Ellipse Area in cm2 | |||||||
| 2L FS | 5.62 ± 3.35 | 6.55 ± 3.77 | 5.23 ± 3.15 | 0.242 | 5.91 ± 4.23 | 5.35 ± 2.33 | 0.869 |
| 1L FS | 115.10 ± 171.0 | 237.33 ± 270.12 | 63.39 ± 59.84 | 0.023 | 52.39 ± 32.31 | 174.51 ± 223.29 | 0.221 |
| Tan FS | 24.7 ± 31.49 | 32.57 ± 24.83 | 21.38 ± 33.80 | 0.043 | 18.39 ± 18.49 | 30.68 ± 39.78 | 0.142 |
| 2L SS | 25.03 ± 11.50 | 24.01 ± 11.07 | 25.49 ± 11.86 | 0.658 | 25.67 ± 13.07 | 24.44 ± 10.11 | 0.893 |
| 1L SS | 218.78 ± 168.65 | 313.72 ± 149.51 | 178.61 ± 162.40 | 0.007 | 226.26 ± 180.86 | 211.69 ± 160.90 | 0.893 |
| Tan SS | 111.25 ± 99.30 | 79.07 ± 76.80 | 124.87 ± 105.79 | 0.150 | 126.90 ± 121.97 | 96.42 ± 72.06 | 0.775 |
| Total Score | 500.49 ± 286.38 | 693.25 ± 317.99 | 418.94 ± 233.21 | 0.014 | 455.51 ± 233.08 | 543.10 ± 329.82 | 0.425 |
| Path Length PL in mm | |||||||
| 2L FS | 534.11 ± 138.20 | 597.92 ± 141.46 | 507.11 ± 130.19 | 0.067 | 516.33 ± 145.70 | 550.96 ± 132.44 | 0.450 |
| 1L FS | 2972.24 ± 1586.65 | 3884.95 ± 2235.32 | 2586.00 ± 1051.13 | 0.100 | 2488.67 ± 810.37 | 3430.36 ± 1989.80 | 0.220 |
| Tan FS | 1589.48 ± 773.37 | 1997.36 ± 870.53 | 1416.90 ± 673.77 | 0.028 | 1418.72 ± 802.35 | 1751.23 ± 729.00 | 0.066 |
| 2L SS | 1454.29 ± 410.34 | 1485.20 ± 560.13 | 1441.21 ± 341.13 | 0.780 | 1413.76 ± 344.54 | 1392.69 ± 470.58 | 0.869 |
| 1L SS | 4386.91 ± 1859.00 | 5350.72 ± 1361.22 | 3979.15 ± 1911.45 | 0.002 | 3797.31 ± 972.92 | 4945.48 ± 2311.38 | 0.210 |
| Tan SS | 3246.75 ± 1319.72 | 3140.53 ± 1416.29 | 3291.69 ± 1303.30 | 0.765 | 3216.22 ± 1470.38 | 3275.68 ± 1199.85 | 0.890 |
| Total Score | 14,183.78 ± 3654.10 | 16,456.69 ± 3527.04 | 13,222.17 ± 3319.14 | 0.008 | 12,851.01 ± 2229.90 | 15,446.41 ± 4306.92 | 0.057 |
| Mean Velocity in mm/s | |||||||
| 2L FS | 2.65 ± 0.70 | 2.99 ± 0.70 | 2.51 ± 0.66 | 0.047 | 2.58 ± 0.73 | 2.72 ± 0.68 | 0.578 |
| 1L FS | 14.86 ± 7.39 | 19.42 ± 11.18 | 12.93 ± 5.26 | 0.100 | 12.44 ± 4.05 | 17.15 ± 9.95 | 0.220 |
| Tan FS | 7.95 ± 3.87 | 9.99 ± 4.35 | 7.08 ± 13.37 | 0.028 | 7.09 ± 4.01 | 8.76 ± 3.64 | 0.066 |
| 2L SS | 7.27 ± 2.05 | 7.43 ± 2.80 | 7.01 ± 1.71 | 0.781 | 7.07 ± 1.72 | 7.46 ± 2.35 | 0.869 |
| 1L SS | 21.93 ± 9.29 | 26.75 ± 6.81 | 18.89 ± 9.56 | 0.002 | 18.98 ± 4.86 | 24.72 ± 11.56 | 0.210 |
| Tan SS | 16.23 ± 6.60 | 15.70 ± 7.08 | 16.45 ± 6.52 | 0.755 | 16.08 ± 7.35 | 16.38 ± 6.00 | 0.893 |
| Total Score | 434.06 ± 126.00 | 494.88 ± 106.92 | 408.33 ± 126.38 | 0.011 | 406.33 ± 130.33 | 460.33 ± 119.21 | 0.066 |
| Total Cohort | Group Comparison Age | Group Comparison Sex | |||||
|---|---|---|---|---|---|---|---|
| n = 37 | < 12 y.o.a. | ≥ 12 y.o.a. | Female | Male | |||
| n = 11 | n = 26 | n = 18 | n = 19 | ||||
| Testing Position | Mean ± SD | Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p |
| Mean Velocity ML in mm/s | |||||||
| 2L FS | 18.05 ± 5.20 | 19.69 ± 4.44 | 17.35 ± 5.42 | 0.216 | 17.87 ± 5.61 | 18.22 ± 4.93 | 0.845 |
| 1L FS | 82.99 ± 44.60 | 114.18 ± 65.00 | 69.79 ± 23.76 | 0.014 | 66.24 ± 15.89 | 98.85 ± 56.52 | 0.070 |
| Tan FS | 44.49 ± 17.37 | 52.39 ± 16.73 | 41.14 ± 16.83 | 0.051 | 38.34 ± 15.86 | 50.31 ± 17.09 | 0.019 |
| 2L SS | 63.80 ± 92.85 | 49.65 ± 21.05 | 69.79 ± 110.05 | 0.349 | 77.84 ± 132.43 | 50.50 ± 17.16 | 0.999 |
| 1L SS | 138.29 ± 54.83 | 173.69 ± 41.69 | 123.31 ± 53.38 | 0.002 | 120.79 ± 30.89 | 154.87 ± 67.20 | 0.178 |
| Tan SS | 86.45 ± 33.30 | 85.28 ± 36.26 | 86.94 ± 32.71 | 0.892 | 85.24 ± 34.43 | 87.59 ± 33.09 | 0.834 |
| Mean Velocity AP in mm/s | |||||||
| 2L FS | 15.56 ± 4.37 | 18.33 ± 4.88 | 14.38 ± 3.63 | 0.031 | 14.77 ± 4.52 | 16.31 ± 4.20 | 0.159 |
| 1L FS | 83.21 ± 61.13 | 133.38 ± 81.74 | 90.99 ± 46.41 | 0.140 | 89.22 ± 36.06 | 117.22 ± 76.44 | 0.480 |
| Tan FS | 55.96 ± 31.52 | 74.07 ± 38.05 | 48.30 ± 25.42 | 0.017 | 51.64 ± 34.62 | 60.04 ± 28.61 | 0.118 |
| 2L SS | 59.86 ± 94.01 | 44.41 ± 14.85 | 66.40 ± 111.82 | 0.730 | 76.50 ± 134.06 | 44.07 ± 13.51 | 0.620 |
| 1L SS | 138.17 ± 67.51 | 162.95 ± 45.82 | 127.68 ± 73.06 | 0.012 | 118.40 ± 35.25 | 156.90 ± 84.74 | 0.233 |
| Tan SS | 118.23 ± 53.88 | 112.41 ± 56.42 | 120.69 ± 53.71 | 0.675 | 86.84 ± 61.90 | 118.81 ± 46.75 | 0.948 |
| BESS Score | Ellipse Area (cm2) | Mean Velocity (mm/s) | Path Length (mm) | |||
|---|---|---|---|---|---|---|
| Testing position | Spearman’s Rho (rs) | p | Spearman’s Rho (rs) | p | Spearman’s Rho (rs) | p |
| 2L FS | % | % | % | % | % | % |
| 1L FS | 0.61 | <0.001 | 0.59 | <0.001 | 0.59 | <0.001 |
| Tan FS | 0.77 | <0.001 | 0.74 | <0.001 | 0.74 | <0.001 |
| 2L SS | 0.43 | <0.001 | 0.45 | 0.005 | 0.45 | 0.005 |
| 1L SS | 0.30 | 0.070 | 0.25 | 0.141 | 0.25 | 0.141 |
| Tan SS | 0.76 | <0.001 | 0.81 | <0.001 | 0.81 | <0.001 |
| Total Score | 0.46 | 0.004 | 0.54 | <0.001 | 0.60 | <0.001 |
| BESS | First Round | Second Round | Spearman’s Rho | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | rs | p | |
| FS 2L | 0.0 ± 0.0 | 0.03 ± 0.167 | n.c. | |
| FS 1L | 4.67 ± 2.77 | 3.81 ± 2.94 | 0.799 | <0.001 |
| FS Tan | 1.56 ± 1.66 | 1.58 ± 1.96 | 0.798 | <0.001 |
| SS 2L | 0.78 ± 0.96 | 0.28 ± 0.62 | 0.249 | 0.143 |
| SS 1L | 8.33 ± 1.76 | 8.33 ± 1.94 | 0.489 | 0.002 |
| SS Tan | 5.33 ± 2.85 | 5.03 ± 3.03 | 0.678 | <0.001 |
| Total Score | 20.81 ± 6.28 | 18.81 ± 6.79 | 0.734 | <0.001 |
| EA | First Round | Second Round | Spearman’s Rho | |
| Mean ± SD | Mean ± SD | rs | p | |
| FS 2L | 5.56 ± 3.38 | 6.04 ± 5.23 | 0.508 | 0.001 |
| FS 1L | 116.22 ± 173.32 | 104.18 ± 178.10 | 0.581 | <0.001 |
| FS Tan | 20.68 ± 20.13 | 20.34 ± 23.05 | 0.392 | 0.016 |
| SS 2L | 25.28 ± 11.56 | 17.72 ± 5.56 | 0.178 | 0.299 |
| SS 1L | 221.97 ± 169.91 | 159.68 ± 142.41 | 0.435 | 0.008 |
| SS Tan | 114.04 ± 99.22 | 81.88 ± 84.98 | 0.415 | 0.012 |
| PL | First Round | Second Round | Spearman’s Rho | |
| Mean ± SD | Mean ± SD | rs | p | |
| FS 2L | 534.11 ± 138.20 | 620.65 ± 193.87 | 0.569 | <0.001 |
| FS 1L | 2972.24 ± 1586.65 | 2764.13 ± 1634.90 | 0.530 | <0.001 |
| FS Tan | 1589.48 ± 773.37 | 1510.59 ± 783.48 | 0.524 | 0.001 |
| SS 2L | 1454.29 ± 410.34 | 1151.09 ± 264.54 | 0.465 | 0.004 |
| SS 1L | 4386.91 ± 1859.00 | 3961.14 ± 1678.25 | 0.675 | <0.001 |
| SS Tan | 3246.75 ± 1319.72 | 2847.35 ± 1249.81 | 0.552 | <0.001 |
| v mean | First Round | Second Round | Spearman’s Rho | |
| Mean ± SD | Mean ± SD | rs | p | |
| FS 2L | 2.65 ± 0.71 | 3.10 ± 0.97 | 0.596 | <0.001 |
| FS 1L | 14.84 ± 8.04 | 13.82 ± 8.17 | 0.530 | <0.001 |
| FS Tan | 7.69 ± 3.59 | 7.55 ± 3.92 | 0.524 | 0.001 |
| SS 2L | 7.32 ± 2.06 | 5.75 ± 1.32 | 0.465 | 0.004 |
| SS 1L | 22.12 ± 9.36 | 19.08 ± 8.39 | 0.675 | <0.001 |
| SS Tan | 16.53 ± 6.43 | 14.24 ± 6.25 | 0.552 | <0.001 |
| Questions | Very Much | Somewhat | Undecided | Not Really | Not at All | Mean ± SD | Median | Mode |
|---|---|---|---|---|---|---|---|---|
| Do you feel well? | 17 | 16 | 2 | 1 | 0 | 4.36 ± 0.72 | 4 | 5 |
| Did you enjoy the testing on the force plate? | 10 | 19 | 6 | 1 | 0 | 4.06 ± 0.75 | 1 | 4 |
| Was the testing on the force plate well-explained? | 33 | 3 | 0 | 0 | 0 | 4.92 ± 0.28 | 0 | 5 |
| Was testing on the force plate easy for you? | 3 | 7 | 22 | 3 | 1 | 3.22 ± 0.83 | 3 | 3 |
| Doctor’s visit | Physical education | Sports | Computer game | Other | ||||
| What did the tests remind you most of? | 11 | 11 | 8 | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönberg, N.K.T.; Poppel, J.; Howell, D.; Wagner, J.; Höfinger, M.; Fabri, N.; Bonke, E.M.; Rojczyk, P.; Hösl, M.; Kiwull, L.; et al. Instrumented Balance Error Scoring System in Children and Adolescents—A Cross Sectional Study. Diagnostics 2024, 14, 513. https://doi.org/10.3390/diagnostics14050513
Schönberg NKT, Poppel J, Howell D, Wagner J, Höfinger M, Fabri N, Bonke EM, Rojczyk P, Hösl M, Kiwull L, et al. Instrumented Balance Error Scoring System in Children and Adolescents—A Cross Sectional Study. Diagnostics. 2024; 14(5):513. https://doi.org/10.3390/diagnostics14050513
Chicago/Turabian StyleSchönberg, Nils K. T., Julius Poppel, David Howell, Johanna Wagner, Michael Höfinger, Nicole Fabri, Elena M. Bonke, Philine Rojczyk, Matthias Hösl, Lorenz Kiwull, and et al. 2024. "Instrumented Balance Error Scoring System in Children and Adolescents—A Cross Sectional Study" Diagnostics 14, no. 5: 513. https://doi.org/10.3390/diagnostics14050513
APA StyleSchönberg, N. K. T., Poppel, J., Howell, D., Wagner, J., Höfinger, M., Fabri, N., Bonke, E. M., Rojczyk, P., Hösl, M., Kiwull, L., Schröder, S. A., Blaschek, A., Vill, K., Koerte, I. K., Huppert, D., Heinen, F., & Bonfert, M. V. (2024). Instrumented Balance Error Scoring System in Children and Adolescents—A Cross Sectional Study. Diagnostics, 14(5), 513. https://doi.org/10.3390/diagnostics14050513







