Supporting Malaria Diagnosis Using Deep Learning and Data Augmentation
Abstract
1. Introduction
2. Related Work
2.1. Detection of Malaria Parasites in Thin Blood Smear Images
2.2. Detection of Malaria Parasites in Thick Blood Smear Images
3. Theoretical Framework
3.1. Malaria
3.1.1. Clinical Manifestations of Malaria
3.1.2. Malaria Diagnosis
3.2. Introduction to DL
Convolutional Neural Networks
3.3. YOLO Algorithm
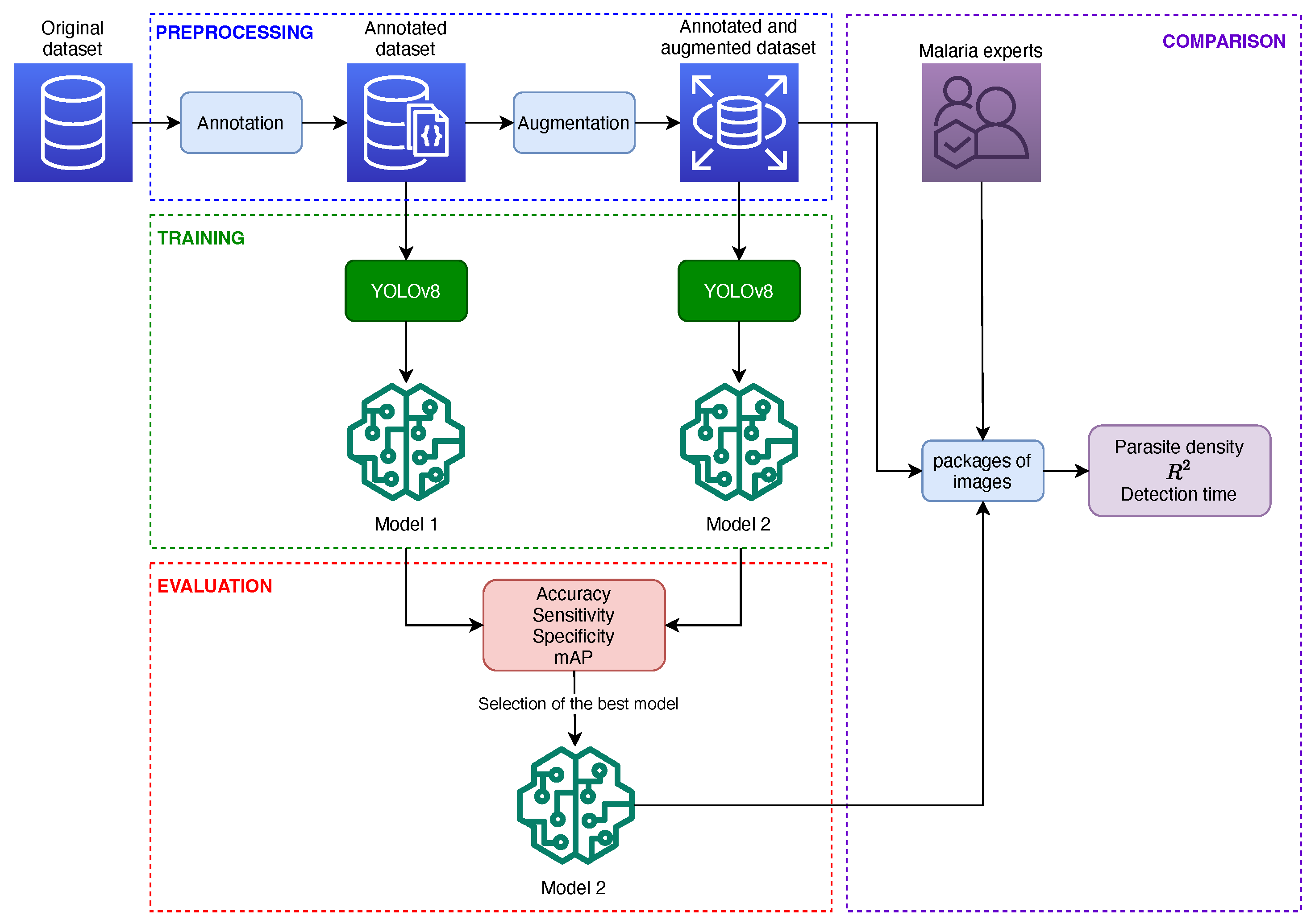
4. Materials and Methods
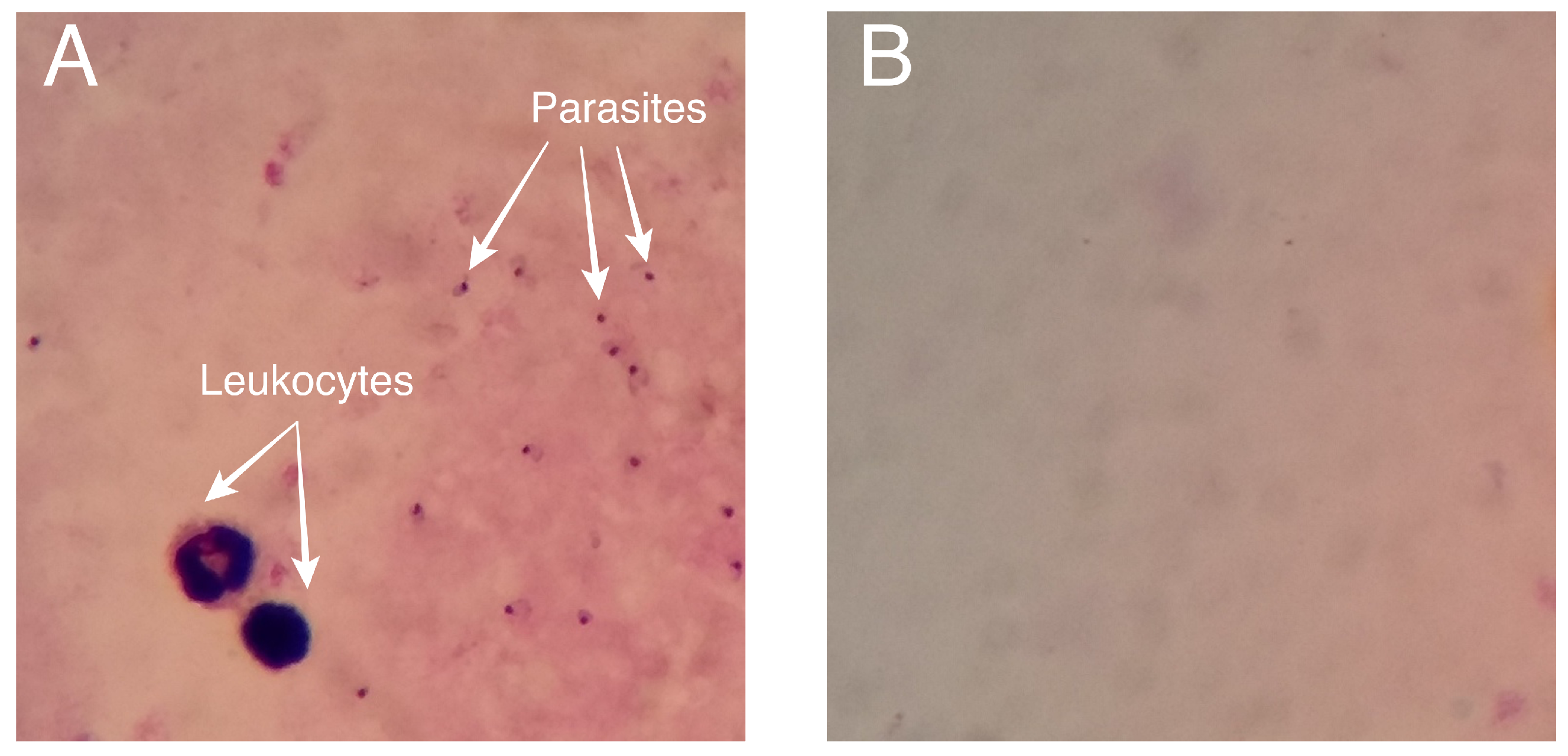
4.1. Dataset
4.2. Preprocessing
4.2.1. Annotation
4.2.2. Data Augmentation
4.3. Model Training
4.4. Model Evaluation
4.4.1. Evaluation of Predictive Capacity
4.4.2. Evaluation of Reading and Reporting Time
- for
- for
- for
5. Results
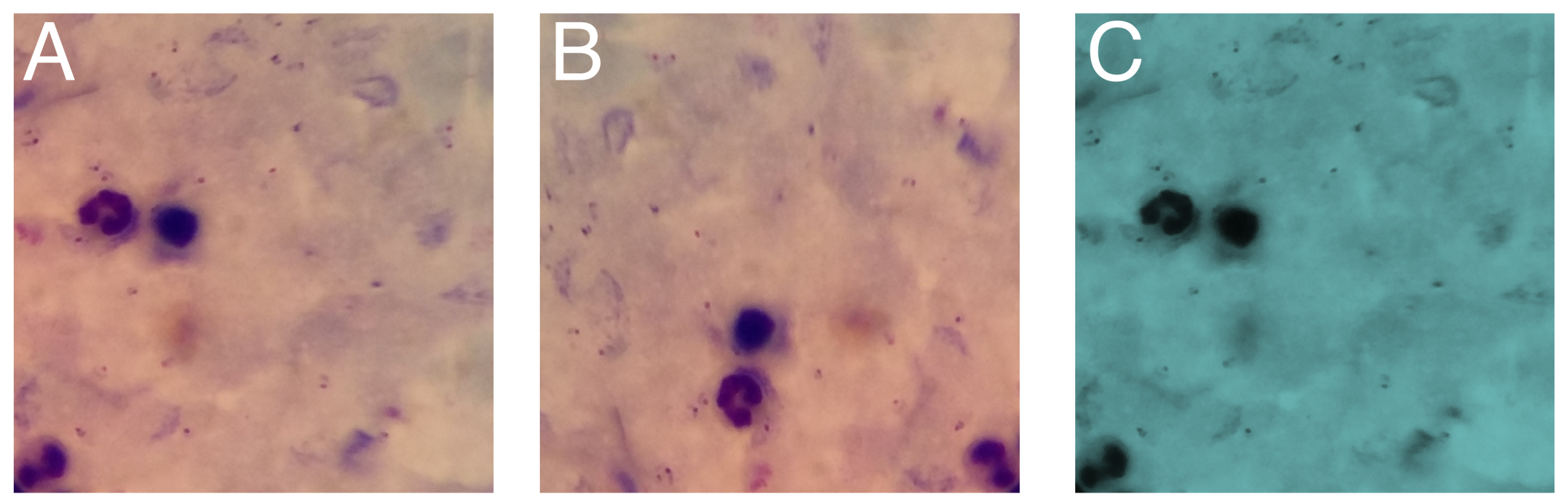
5.1. Data Augmentation
5.2. Performance of the Models in Detecting Parasites and Leukocytes
5.3. Parasite Density Calculation
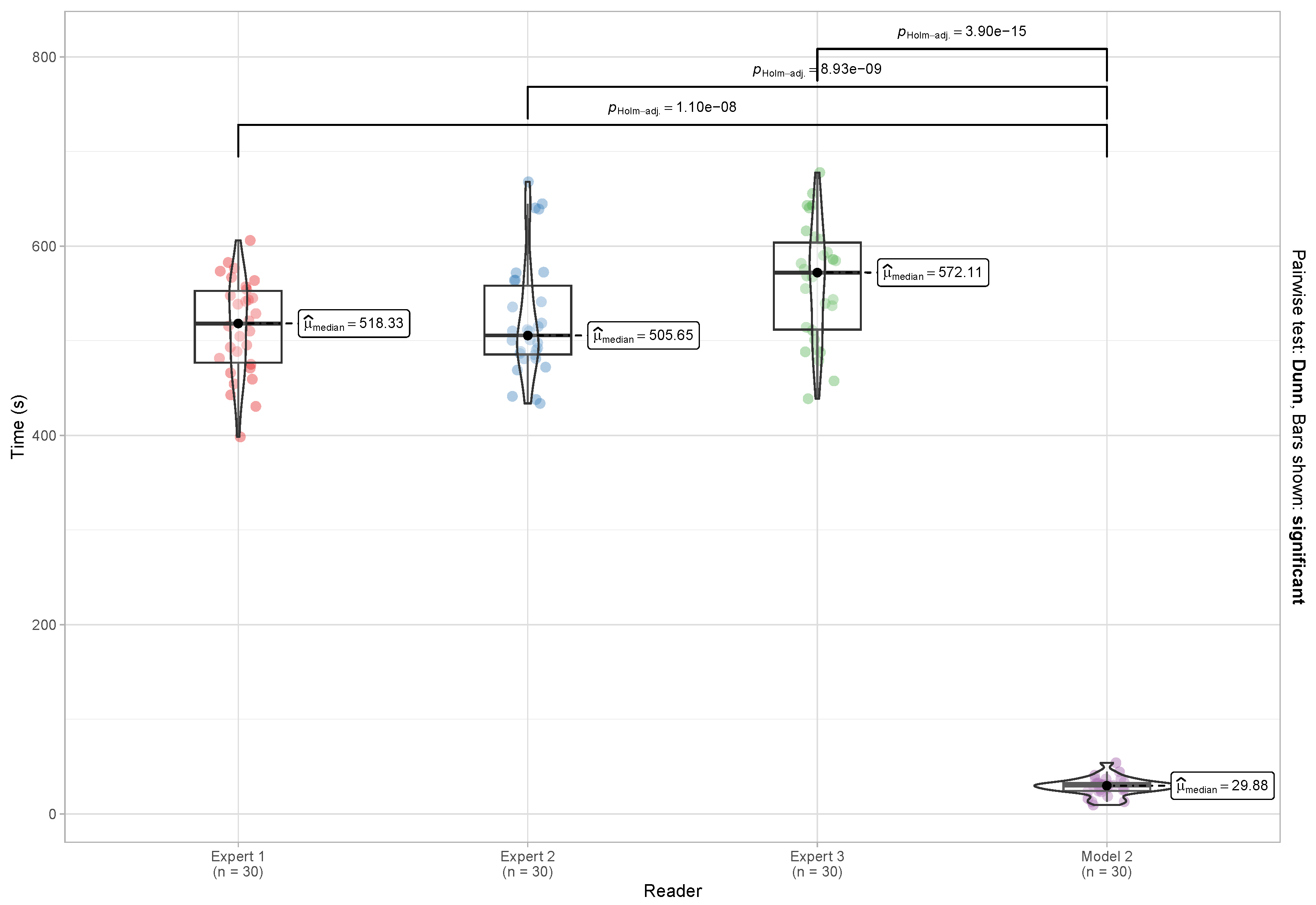
5.4. Reading and Reporting Time
6. Discussion
6.1. General Discussion
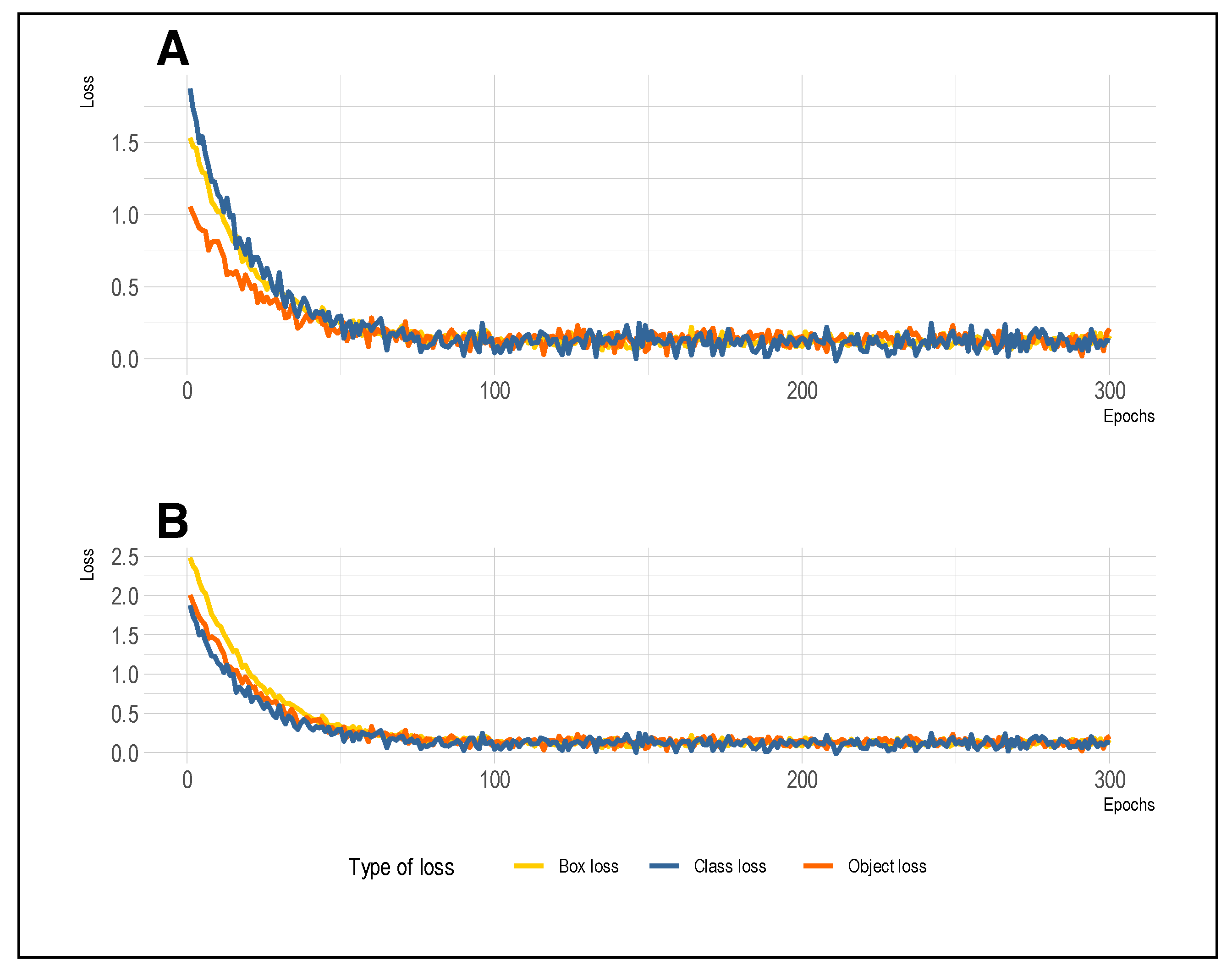
6.2. Training and Validation Loss
6.3. Quantitative Comparison with Previous Works
6.4. Qualitative Comparison with Previous Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuteja, R. Malaria—An overview. FEBS J. 2007, 274, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Bronzan, R.N.; McMorrow, M.L.; Kachur, S.P. Diagnosis of malaria: Challenges for clinicians in endemic and non-endemic regions. Mol. Diagn. Ther. 2008, 12, 299–306. [Google Scholar] [CrossRef]
- World Health Organization. Malaria Microscopy: Quality Assurance Manual Version 2; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Tangpukdee, N.; Duangdee, C.; Wilairatana, P.; Krudsood, S. Malaria diagnosis: A brief review. Korean J. Parasitol. 2009, 47, 93–102. [Google Scholar] [CrossRef]
- Janse, C.J.; Van Vianen, P.H. Flow Cytometry in Malaria Detection. Methods Cell Biol. 1994, 42, 295–318. [Google Scholar] [CrossRef]
- Linder, N.; Turkki, R.; Walliander, M.; Mårtensson, A.; Diwan, V.; Rahtu, E.; Pietikäinen, M.; Lundin, M.; Lundin, J. A malaria diagnostic tool based on computer vision screening and visualization of Plasmodium falciparum candidate areas in digitized blood smears. PLoS ONE 2014, 9, e104855. [Google Scholar] [CrossRef]
- Razzak, M.I. Malarial Parasite Classification using Recurrent Neural Network. Int. J. Image Process. 2015, 9, 69–79. [Google Scholar]
- Khan, M.I.; Acharya, B.; Singh, B.K.; Soni, J. Content Based Image Retrieval Approaches for Detection of Malarial Parasite in Blood Images. Int. J. Biom. Bioinform. 2011, 5, 97–110. [Google Scholar]
- Adi, K.; Pujiyanto, S.; Gernowo, R.; Pamungkas, A.; Putranto, A.B. Identifying the developmental phase of plasmodium falciparum in malaria-infected red blood cells using adaptive color segmentation and back propagation neural network. Int. J. Appl. Eng. Res. 2016, 11, 8754–8759. [Google Scholar]
- Yunda, L.; Alarcón, A.; Millán, J. Automated Image Analysis Method for p-vivax Malaria Parasite Detection in Thick Film Blood Images. Sist. Telemática 2012, 10, 9. [Google Scholar] [CrossRef]
- Elter, M.; Haßlmeyer, E.; Zerfaß, T. Detection of malaria parasites in thick blood films. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 5140–5144. [Google Scholar] [CrossRef]
- Rosado, L.; Da Costa, J.M.; Elias, D.; Cardoso, J.S. Automated Detection of Malaria Parasites on Thick Blood Smears via Mobile Devices. Procedia Comput. Sci. 2016, 90, 138–144. [Google Scholar] [CrossRef]
- World Health Organization. Malaria Parasite Counting: Malaria Microscopy Standard Operating Procedure; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Ikerionwu, C.; Ugwuishiwu, C.; Okpala, I.; James, I.; Okoronkwo, M.; Nnadi, C.; Orji, U.; Ebem, D.; Ike, A. Application of machine and deep learning algorithms in optical microscopic detection of Plasmodium: A malaria diagnostic tool for the future. Photodiagnosis Photodyn. Ther. 2022, 40, 103198. [Google Scholar] [CrossRef]
- World Health Organization. Microscopy Examination of Thick and Thin Blood Films for Identification of Malaria Parasites; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Siłka, W.; Wieczorek, M.; Siłka, J.; Woźniak, M. Malaria Detection Using Advanced Deep Learning Architecture. Sensors 2023, 23, 1501. [Google Scholar] [CrossRef]
- Marques, G.; Ferreras, A.; de la Torre-Diez, I. An ensemble-based approach for automated medical diagnosis of malaria using EfficientNet. Multimed. Tools Appl. 2022, 81, 28061–28078. [Google Scholar] [CrossRef] [PubMed]
- Loh, D.R.; Yong, W.X.; Yapeter, J.; Subburaj, K.; Chandramohanadas, R. A deep learning approach to the screening of malaria infection: Automated and rapid cell counting, object detection and instance segmentation using Mask R-CNN. Comput. Med. Imaging Graph. 2021, 88, 101845. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Rajesh Kanna, B. Deep learning approach to detect malaria from microscopic images. Multimed. Tools Appl. 2020, 79, 15297–15317. [Google Scholar] [CrossRef]
- Alkhaldi, T.M.; Hashim, A.N. Automatic Detection of Malaria Using Convolutional Neural Network. Math. Stat. Enginnering Appl. 2022, 71, 939–947. [Google Scholar]
- Yang, F.; Poostchi, M.; Yu, H.; Zhou, Z.; Silamut, K.; Yu, J.; Maude, R.J.; Jaeger, S.; Antani, S. Deep Learning for Smartphone-Based Malaria Parasite Detection in Thick Blood Smears. IEEE J. Biomed. Health Inform. 2020, 24, 1427–1438. [Google Scholar] [CrossRef]
- De Souza Oliveira, A.; Guimarães Fernandes Costa, M.; das Graças Vale Barbosa, M.; Ferreira Fernandes Costa Filho, C. A new approach for malaria diagnosis in thick blood smear images. Biomed. Signal Process. Control 2022, 78, 103931. [Google Scholar] [CrossRef]
- Chibuta, S.; Acar, A.C. Real-time Malaria Parasite Screening in Thick Blood Smears for Low-Resource Setting. J. Digit. Imaging 2020, 33, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Manescu, P.; Shaw, M.J.; Elmi, M.; Neary-Zajiczek, L.; Claveau, R.; Pawar, V.; Kokkinos, I.; Oyinloye, G.; Bendkowski, C.; Oladejo, O.A.; et al. Expert-level automated malaria diagnosis on routine blood films with deep neural networks. Am. J. Hematol. 2020, 95, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Malaria; CDC: Atlanta, GA, USA, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Malaria Diagnosis; CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Varo, R.; Chaccour, C.; Bassat, Q. Update on malaria. Med. Clín. (Engl. Ed.) 2020, 155, 395–402. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Malaria; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Kwambai, T.K.; Dhabangi, A.; Idro, R.; Opoka, R.; Watson, V.; Kariuki, S.; Kuya, N.A.; Onyango, E.D.; Otieno, K.; Samuels, A.M.; et al. Malaria Chemoprevention in the Postdischarge Management of Severe Anemia. N. Engl. J. Med. 2020, 383, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.D.; Lederman, E.R.; Barcus, M.J.; Prudhomme O’Meara, W.A.; Jordon, R.G.; Duong, S.; Muth, S.; Sismadi, P.; Bangs, M.J.; Prescott, W.R.; et al. Production and validation of durable, high quality standardized malaria microscopy slides for teaching, testing and quality assurance during an era of declining diagnostic proficiency. Malar. J. 2006, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Malaria Diagnostic Tests; CDC: Atlanta, GA, USA, 2023. [Google Scholar]
- Gidey, B.; Nega, D.; Abera, A.; Abebe, A.; Mekasha, S.; Tasew, G.; Haile, M.; Dillu, D.; Mehari, D.; Assefa, A.; et al. Mentorship on malaria microscopy diagnostic service in Ethiopia: Baseline competency of microscopists and performance of health facilities. Malar. J. 2021, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malaria rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Grobusch, M.P.; Schlagenhauf, P. Self-diagnosis and self-treatment of malaria by the traveler. In Travel Medicine, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 169–178. [Google Scholar] [CrossRef]
- World Health Organization. Quality and Safety Practices for Malaria Rapid Testing Services; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Universal Access to Malaria Diagnostic Testing: An Operational Manual; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Mfuh, K.O.; Achonduh-Atijegbe, O.A.; Bekindaka, O.N.; Esemu, L.F.; Mbakop, C.D.; Gandhi, K.; Leke, R.G.; Taylor, D.W.; Nerurkar, V.R. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar. J. 2019, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.G.; Lu, H. Hierarchical genetic algorithm based neural network design. In Proceedings of the 1st IEEE Symposium on Combinations of Evolutionary Computation and Neural Networks, San Antonio, TX, USA, 11–13 May 2000; pp. 168–175. [Google Scholar] [CrossRef]
- Shetty, D.; Harshavardhan, C.A.; Varma, M.J.; Navi, S.; Ahmed, M.R. Diving Deep into Deep Learning: History, Evolution, Types and Applications. Int. J. Innov. Technol. Explor. Eng. 2020, 9, 2835–2846. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Albawi, S.; Mohammed, T.A.; Al-Zawi, S. Understanding of a convolutional neural network. In Proceedings of the 2017 International Conference on Engineering and Technology, ICET 2017, Antalya, Turkey, 21–23 August 2017; IEEE: Piscataway, NJ, USA, 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Srinivas, S.; Sarvadevabhatla, R.K.; Mopuri, K.R.; Prabhu, N.; Kruthiventi, S.S.; Babu, R.V. An Introduction to Deep Convolutional Neural Nets for Computer Vision. In Deep Learning for Medical Image Analysis; Academic Press: Cambridge, MA, USA, 2017; pp. 25–52. [Google Scholar] [CrossRef]
- Gu, J.; Wang, Z.; Kuen, J.; Ma, L.; Shahroudy, A.; Shuai, B.; Liu, T.; Wang, X.; Wang, G.; Cai, J.; et al. Recent advances in convolutional neural networks. Pattern Recognit. 2018, 77, 354–377. [Google Scholar] [CrossRef]
- Redmon, J.; Farhadi, A. YOLOv3: An Incremental Improvement. arXiv 2018, arXiv:1804.02767. [Google Scholar]
- Fan, J.; Huo, T.; Li, X. A review of one-stage detection algorithms in autonomous driving. In Proceedings of the 2020 4th CAA International Conference on Vehicular Control and Intelligence, CVCI 2020, Hangzhou, China, 18–20 December 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 210–214. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, T.; Zhu, Z.; Zhao, Y. Real-time face mask detection method based on yolov3. Electronics 2021, 10, 837. [Google Scholar] [CrossRef]
- Jiang, P.; Ergu, D.; Liu, F.; Cai, Y.; Ma, B. A Review of Yolo Algorithm Developments. Procedia Comput. Sci. 2021, 199, 1066–1073. [Google Scholar] [CrossRef]
- Terven, J.; Córdova-Esparza, D.M.; Romero-González, J.A. A Comprehensive Review of YOLO Architectures in Computer Vision: From YOLOv1 to YOLOv8 and YOLO-NAS. Mach. Learn. Knowl. Extr. 2023, 5, 1680–1716. [Google Scholar] [CrossRef]
- Quinn, J.A.; Nakasi, R.; Mugagga, P.K.B.; Byanyima, P.; Lubega, W.; Andama, A. Deep Convolutional Neural Networks for Microscopy-Based Point of Care Diagnostics. arXiv 2016, arXiv:1608.02989. [Google Scholar]
- Microsoft Corporation. Visual Object Tagging Tool: An Electron App for Building End to End Object Detection Models from Images and Videos (Version 2.2.0); Microsoft Corporation: Redmon, WA, USA, 2019; Available online: https://github.com/microsoft/VoTT (accessed on 15 February 2021).
- Dyk, D.A.; Meng, X.L. The art of data augmentation. J. Comput. Graph. Stat. 2001, 10, 1–50. [Google Scholar] [CrossRef]
- Alexandrova, S.; Tatlock, Z.; Cakmak, M. RoboFlow: A flow-based visual programming language for mobile manipulation tasks. In Proceedings of the IEEE International Conference on Robotics and Automation, Seattle, WA, USA, 26–30 May 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 5537–5544. [Google Scholar] [CrossRef]
- Khosla, C.; Saini, B.S. Enhancing Performance of Deep Learning Models with different Data Augmentation Techniques: A Survey. In Proceedings of the International Conference on Intelligent Engineering and Management, ICIEM 2020, London, UK, 17–19 June 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 79–85. [Google Scholar] [CrossRef]
- Pattanaik, P.A.; Mittal, M.; Khan, M.Z. Unsupervised Deep Learning CAD Scheme for the Detection of Malaria in Blood Smear Microscopic Images. IEEE Access 2020, 8, 94936–94946. [Google Scholar] [CrossRef]
- Abdurahman, F.; Fante, K.A.; Aliy, M. Malaria parasite detection in thick blood smear microscopic images using modified YOLOV3 and YOLOV4 models. BMC Bioinform. 2021, 22, 112. [Google Scholar] [CrossRef]
- Nakasi, R.; Mwebaze, E.; Zawedde, A.; Tusubira, J.; Akera, B.; Maiga, G. A new approach for microscopic diagnosis of malaria parasites in thick blood smears using pre-trained deep learning models. SN Appl. Sci. 2020, 2, 1255. [Google Scholar] [CrossRef]
- Nakasi, R.; Mwebaze, E.; Zawedde, A. Mobile-aware deep learning algorithms for malaria parasites and white blood cells localization in thick blood smears. Algorithms 2021, 14, 17. [Google Scholar] [CrossRef]
- Molina, A.; Rodellar, J.; Boldú, L.; Acevedo, A.; Alférez, S.; Merino, A. Automatic identification of malaria and other red blood cell inclusions using convolutional neural networks. Comput. Biol. Med. 2021, 136, 104680. [Google Scholar] [CrossRef]
- Bakator, M.; Radosav, D. Deep learning and medical diagnosis: A review of literature. Multimodal Technol. Interact. 2018, 2, 47. [Google Scholar] [CrossRef]


| Model | Dataset | Parasite | Leukocyte | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | mAP | Accuracy | Sensitivity | Specificity | mAP | ||
| Model 1 | Original | 0.91 | 0.91 | 0.90 | 0.90 | 0.90 | 0.89 | 0.89 | 0.91 |
| Model 2 | Augmented | 0.95 | 0.94 | 0.93 | 0.95 | 0.98 | 0.95 | 0.97 | 0.97 |
| Study | Accuracy | Sensitivity | Specificity | mAP |
|---|---|---|---|---|
| [23] | 0.93 | 0.93 | 0.94 | NR |
| [57] | 0.89 | NR | NR | NR |
| [58] | NR | 0.94 | NR | 0.96 |
| [59] | NR | 0.93 | NR | 0.94 |
| [60] | NR | 0.93 | NR | 0.66 |
| Our work | 0.95 | 0.94 | 0.93 | 0.95 |
| Qualitative Criteria | Study | ||||||
|---|---|---|---|---|---|---|---|
| [61] | [23] | [14] | [58] | [24] | [26] | Our Work | |
| Use of thick-drop imaging |  |  |  |  |  |  |  |
| Detection of multiple classes (parasites and leukocytes) |  |  |  |  |  |  |  |
| Parasite density calculation according to WHO |  |  |  |  |  |  |  |
| Comparison with clinical experts |  |  |  |  |  |  |  |
| Evaluation of detection time |  |  |  |  |  |  |  |
| Easy to use and adaptable |  |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyos, K.; Hoyos, W. Supporting Malaria Diagnosis Using Deep Learning and Data Augmentation. Diagnostics 2024, 14, 690. https://doi.org/10.3390/diagnostics14070690
Hoyos K, Hoyos W. Supporting Malaria Diagnosis Using Deep Learning and Data Augmentation. Diagnostics. 2024; 14(7):690. https://doi.org/10.3390/diagnostics14070690
Chicago/Turabian StyleHoyos, Kenia, and William Hoyos. 2024. "Supporting Malaria Diagnosis Using Deep Learning and Data Augmentation" Diagnostics 14, no. 7: 690. https://doi.org/10.3390/diagnostics14070690
APA StyleHoyos, K., & Hoyos, W. (2024). Supporting Malaria Diagnosis Using Deep Learning and Data Augmentation. Diagnostics, 14(7), 690. https://doi.org/10.3390/diagnostics14070690






