Multiparametric Cardiac Magnetic Resonance Assessment in Sickle Beta Thalassemia
Abstract
:1. Introduction
2. Materials and Methods
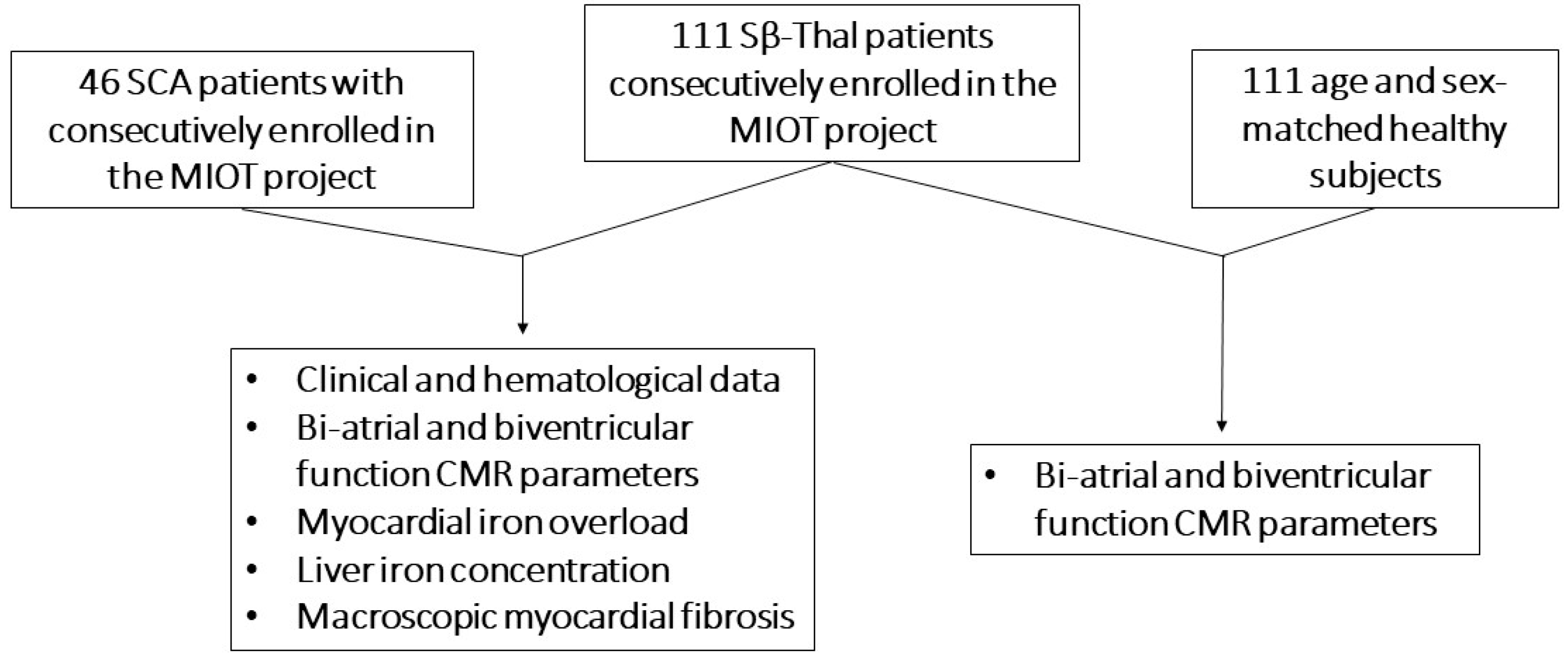
2.1. Study Population
2.2. Magnetic Resonance Imaging (MRI)
2.3. Diagnostic Criteria
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Clinical and MRI Findings in Sβ-Thal Patients
3.3. Comparison of Clinical and MRI Findings between Sβ-Thal and SCA Patients
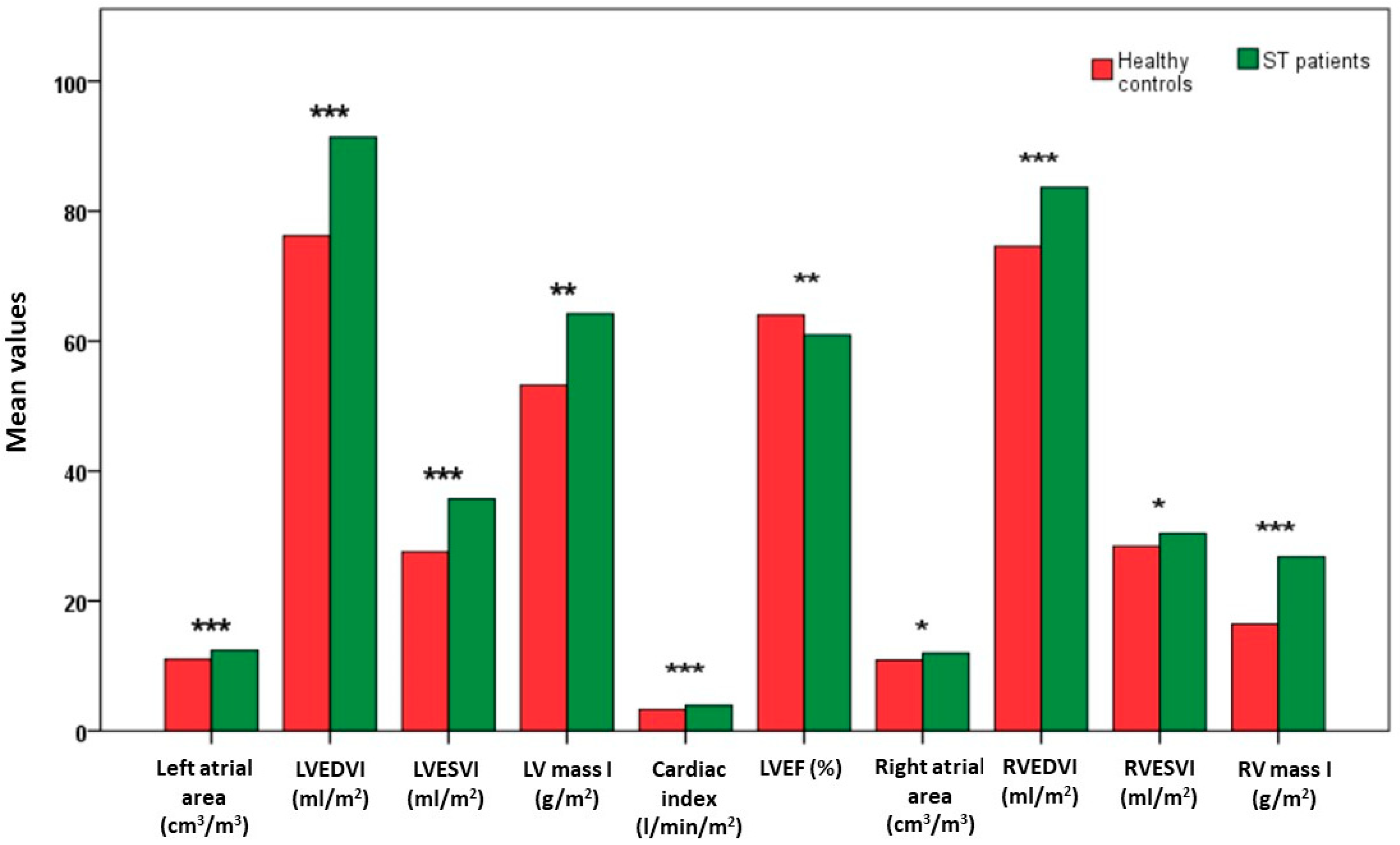
3.4. Comparison of Bi-Atrial and Biventricular Function MR Parameters between Sβ-Thal Patients and Healthy Controls
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lane, P.A. Sickle Cell Disease. Pediatr. Clin. N. Am. 1996, 43, 639–664. [Google Scholar] [CrossRef] [PubMed]
- el-Hazmi, M.A.; Warsy, A.S.; al-Swailem, A.R.; al-Faleh, F.Z.; al-Jabbar, F.A. Genetic Compounds—Hb S, Thalassaemias and Enzymopathies: Spectrum of Interactions. J. Trop. Pediatr. 1994, 40, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Adekile, A.D.; Akbulut, N.; Azab, A.F.; Al-Sharida, S.; Thomas, D. The Sickle β-Thalassemia Phenotype. J. Pediatr. Hematol. Oncol. 2017, 39, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Loukopoulos, D. Current Status of Thalassemia and the Sickle Cell Syndromes in Greece. Semin. Hematol. 1996, 33, 76–86. [Google Scholar] [PubMed]
- Mukherjee, M.B.; Nadkarni, A.H.; Gorakshakar, A.C.; Ghosh, K.; Mohanty, D.; Colah, R.B. Clinical, Hematologic and Molecular Variability of Sickle Cell-β Thalassemia in Western India. Indian. J. Hum. Genet. 2010, 16, 154–158. [Google Scholar] [CrossRef]
- Aessopos, A.; Farmakis, D.; Trompoukis, C.; Tsironi, M.; Moyssakis, I.; Tsaftarides, P.; Karagiorga, M. Cardiac Involvement in Sickle Beta-Thalassemia. Ann. Hematol. 2009, 88, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Murphy, C.; Eakins, E.; Rondinelli, M.B.; Daves, M.; Vecchiato, C.; Wolf, D.; Mc Mahon, C.; Smith, O.P. Insight into the Complex Pathophysiology of Sickle Cell Anaemia and Possible Treatment. Eur. J. Haematol. 2019, 102, 319–330. [Google Scholar] [CrossRef]
- Steinberg, M.H. Sickle Cell Anemia, the First Molecular Disease: Overview of Molecular Etiology, Pathophysiology, and Therapeutic Approaches. ScientificWorldJournal 2008, 8, 1295–1324. [Google Scholar] [CrossRef] [PubMed]
- Serjeant, G.R. The Natural History of Sickle Cell Disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011783. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Quota, A.; Messina, G.; Ricchi, P.; Bagnato, S.; Gerardi, C.; Lisi, R.; Cuccia, L.; Renne, S.; et al. Prognostic Value of Multiparametric Cardiac Magnetic Resonance in Sickle Cell Patients. Ann. Hematol. 2023, 102, 261–270. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Maggio, A.; Sorrentino, F.; Filosa, A.; Rosso, R.; et al. National Networking in Rare Diseases and Reduction of Cardiac Burden in Thalassemia Major. Eur. Heart J. 2022, 43, 2482–2492. [Google Scholar] [CrossRef]
- Varat, M.A.; Adolph, R.J.; Fowler, N.O. Cardiovascular Effects of Anemia. Am. Heart J. 1972, 83, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Borlaug, B.A. High-Output Heart Failure in Sickle Cell Anemia. JACC Cardiovasc. Imaging 2016, 9, 1122–1123. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Ricchi, P.; Gerardi, C.; Restaino, G.; Righi, R.; et al. Multi-Parametric Cardiac Magnetic Resonance for Prediction of Heart Failure Death in Thalassemia Major. Diagnostics 2023, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Gordeuk, V.R.; Bacon, B.R.; Brittenham, G.M. Iron Overload: Causes and Consequences. Annu. Rev. Nutr. 1987, 7, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Ballas, S.K. Iron Overload Is a Determinant of Morbidity and Mortality in Adult Patients with Sickle Cell Disease. Semin. Hematol. 2001, 38 (Suppl. S1), 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. β-Thalassemia Cardiomyopathy. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-Star (T2*) Magnetic Resonance for the Early Diagnosis of Myocardial Iron Overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- Gladwin, M.T. Cardiovascular Complications and Risk of Death in Sickle-Cell Disease. Lancet 2016, 387, 2565–2574. [Google Scholar] [CrossRef]
- Wood, K.C.; Gladwin, M.T.; Straub, A.C. Sickle Cell Disease: At the Crossroads of Pulmonary Hypertension and Diastolic Heart Failure. Heart 2020, 106, 562–568. [Google Scholar] [CrossRef]
- Karyofyllis, P.; Tsiapras, D.; Demerouti, E.; Armenis, I.; Papadopoulou, V.; Voudris, V. Sickle Cell Disease Related Chronic Thromboembolic Pulmonary Hypertension: Challenging Clinical Scenario. J. Thromb. Thrombolysis 2022, 53, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Sachdev, V.; Jison, M.L.; Shizukuda, Y.; Plehn, J.F.; Minter, K.; Brown, B.; Coles, W.A.; Nichols, J.S.; Ernst, I.; et al. Pulmonary Hypertension as a Risk Factor for Death in Patients with Sickle Cell Disease. N. Engl. J. Med. 2004, 350, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Marker, P.H.; Weber, J.P.; Hebbel, R.P.; Vercellotti, G.M. Activated Monocytes in Sickle Cell Disease: Potential Role in the Activation of Vascular Endothelium and Vaso-Occlusion. Blood 2000, 96, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Hebbel, R.P.; Steinberg, M.H.; Gladwin, M.T. Vasculopathy in Sickle Cell Disease: Biology, Pathophysiology, Genetics, Translational Medicine, and New Research Directions. Am. J. Hematol. 2009, 84, 618–625. [Google Scholar] [CrossRef]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle Cell Disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Niss, O.; Taylor, M.D. Applications of Cardiac Magnetic Resonance Imaging in Sickle Cell Disease. Blood Cells Mol. Dis. 2017, 67, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Tsironi, M.; Aessopos, A. The Heart in Sickle Cell Disease. Acta Cardiol. 2005, 60, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.A.; Patel, A.R.; Ahmad, H.; Groth, J.V.; Thiruvoipati, T.; Turner, K.; Yodwut, C.; Czobor, P.; Artz, N.; Machado, R.F.; et al. Mechanistic Insights and Characterization of Sickle Cell Disease-Associated Cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 430–437. [Google Scholar] [CrossRef]
- Raman, S.V.; Simonetti, O.P.; Cataland, S.R.; Kraut, E.H. Myocardial Ischemia and Right Ventricular Dysfunction in Adult Patients with Sickle Cell Disease. Haematologica 2006, 91, 1329–1335. [Google Scholar]
- Aquaro, G.D.; Camastra, G.; Monti, L.; Lombardi, M.; Pepe, A.; Castelletti, S.; Maestrini, V.; Todiere, G.; Masci, P.; di Giovine, G.; et al. Reference Values of Cardiac Volumes, Dimensions, and New Functional Parameters by MR: A Multicenter, Multivendor Study. J. Magn. Reson. Imaging 2017, 45, 1055–1067. [Google Scholar] [CrossRef]
- Meloni, A.; Puliyel, M.; Pepe, A.; Berdoukas, V.; Coates, T.D.; Wood, J.C. Cardiac Iron Overload in Sickle-Cell Disease. Am. J. Hematol. 2014, 89, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, F.P.; Fernandes, J.L.; Cunha, G.M.; TAKubo, T.; MAOLima, C.; BPLima, D.; Uellendhal, M.; Sales, S.R.; ASCunha, C.; LR de Pessoa, V.; et al. Right and Left Ventricular Function and Myocardial Scarring in Adult Patients with Sickle Cell Disease: A Comprehensive Magnetic Resonance Assessment of Hepatic and Myocardial Iron Overload. J. Cardiovasc. Magn. Reson. 2013, 15, 83. [Google Scholar] [CrossRef]
- Wu, E.; Judd, R.M.; Vargas, J.D.; Klocke, F.J.; Bonow, R.O.; Kim, R.J. Visualisation of Presence, Location, and Transmural Extent of Healed Q-Wave and Non-Q-Wave Myocardial Infarction. Lancet 2001, 357, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Niss, O.; Fleck, R.; Makue, F.; Alsaied, T.; Desai, P.; Towbin, J.A.; Malik, P.; Taylor, M.D.; Quinn, C.T. Association between Diffuse Myocardial Fibrosis and Diastolic Dysfunction in Sickle Cell Anemia. Blood 2017, 130, 205–213. [Google Scholar] [CrossRef]
- Moyssakis, I.; Tzanetea, R.; Tsaftaridis, P.; Rombos, I.; Papadopoulos, D.P.; Kalotychou, V.; Aessopos, A. Systolic and Diastolic Function in Middle Aged Patients with Sickle Beta Thalassaemia. An Echocardiographic Study. Postgrad. Med. J. 2005, 81, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Benites, B.D.; Cisneiros, I.S.; Bastos, S.O.; Lino, A.P.B.L.; Costa, F.F.; Gilli, S.C.O.; Saad, S.T.O. Echocardiografic Abnormalities in Patients with Sickle Cell/β-Thalassemia Do Not Depend on the β-Thalassemia Phenotype. Hematol. Transfus. Cell Ther. 2019, 41, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular Function and Treatment in β-Thalassemia Major: A Consensus Statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.H.J.; Benites, B.D.; Fertrin, K.Y. Myocardial Iron Overload in Sickle Cell Disease: A Rare But Potentially Fatal Complication of Transfusion. Transfus. Med. Rev. 2019, 33, 170–175. [Google Scholar] [CrossRef]
- Ghoti, H.; Goitein, O.; Koren, A.; Levin, C.; Kushnir, T.; Rachmilewitz, E.; Konen, E. No Evidence for Myocardial Iron Overload and Free Iron Species in Multitransfused Patients with Sickle/Beta-Thalassaemia. Eur. J. Haematol. 2010, 84, 59–63. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Sorrentino, F.; D’Ascola, D.G.; Spasiano, A.; Filosa, A.; et al. Prediction of Cardiac Complications for Thalassemia Major in the Widespread Cardiac Magnetic Resonance Era: A Prospective Multicentre Study by a Multi-Parametric Approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef]
- Meloni, A.; Ramazzotti, A.; Positano, V.; Salvatori, C.; Mangione, M.; Marcheschi, P.; Favilli, B.; De Marchi, D.; Prato, S.; Pepe, A.; et al. Evaluation of a Web-Based Network for Reproducible T2* MRI Assessment of Iron Overload in Thalassemia. Int. J. Med. Inform. 2009, 78, 503–512. [Google Scholar] [CrossRef]
- Mooij, C.F.; de Wit, C.J.; Graham, D.A.; Powell, A.J.; Geva, T. Reproducibility of MRI Measurements of Right Ventricular Size and Function in Patients with Normal and Dilated Ventricles. J. Magn. Reson. Imaging 2008, 28, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V.; et al. Cardiac Iron and Cardiac Disease in Males and Females with Transfusion-Dependent Thalassemia Major: A T2* Magnetic Resonance Imaging Study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Moss, J.; Thisted, R. Predictors of Body Surface Area. J. Clin. Anesth. 1992, 4, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Positano, V.; Meloni, A.; Santarelli, M.F.; Gerardi, C.; Bitti, P.P.; Cirotto, C.; De Marchi, D.; Salvatori, C.; Landini, L.; Pepe, A. Fast Generation of T2* Maps in the Entire Range of Clinical Interest: Application to Thalassemia Major Patients. Comput. Biol. Med. 2015, 56, 200–210. [Google Scholar] [CrossRef]
- Meloni, A.; Maggio, A.; Positano, V.; Leto, F.; Angelini, A.; Putti, M.C.; Maresi, E.; Pucci, A.; Basso, C.; Marra, M.P.; et al. CMR for Myocardial Iron Overload Quantification: Calibration Curve from the MIOT Network. Eur. Radiol. 2020, 30, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Positano, V.; Pepe, A.; Rossi, G.; Dell’Amico, M.; Salvatori, C.; Keilberg, P.; Filosa, A.; Sallustio, G.; Midiri, M.; et al. Preferential Patterns of Myocardial Iron Overload by Multislice Multiecho T*2 CMR in Thalassemia Major Patients. Magn. Reson. Med. 2010, 64, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Luciani, A.; Positano, V.; De Marchi, D.; Valeri, G.; Restaino, G.; Cracolici, E.; Caruso, V.; Dell’amico, M.C.; Favilli, B.; et al. Single Region of Interest versus Multislice T2* MRI Approach for the Quantification of Hepatic Iron Overload. J. Magn. Reson. Imaging 2011, 33, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Positano, V.; Pepe, A.; Santarelli, M.F.; Scattini, B.; De Marchi, D.; Ramazzotti, A.; Forni, G.; Borgna-Pignatti, C.; Lai, M.E.; Midiri, M.; et al. Standardized T2* Map of Normal Human Heart In Vivo to Correct T2* Segmental Artefacts. NMR Biomed. 2007, 20, 578–590. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. A Statement for Healthcare Professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* Mapping Accurately Estimates Hepatic Iron Concentration in Transfusion-Dependent Thalassemia and Sickle Cell Disease Patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Positano, V.; Capra, M.; Maggio, A.; Pinto, C.L.; Spasiano, A.; Forni, G.; Derchi, G.; Favilli, B.; Rossi, G.; et al. Myocardial Scarring by Delayed Enhancement Cardiovascular Magnetic Resonance in Thalassaemia Major. Heart 2009, 95, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, E.; Brittenham, G.M.; McLaren, C.E.; Ripalti, M.; Baronciani, D.; Giardini, C.; Galimberti, M.; Polchi, P.; Lucarelli, G. Hepatic Iron Concentration and Total Body Iron Stores in Thalassemia Major. N. Engl. J. Med. 2000, 343, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Righi, R.; Missere, M.; Renne, S.; Schicchi, N.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Spasiano, A.; Roberti, M.G.; et al. Biventricular Reference Values by Body Surface Area, Age, and Gender in a Large Cohort of Well-Treated Thalassemia Major Patients without Heart Damage Using a Multiparametric CMR Approach. J. Magn. Reson. Imaging 2021, 53, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Chen, T.; Johnson, S.C.; Szeto, H.; Rabinovitch, P.S. Cardiac Aging: From Molecular Mechanisms to Significance in Human Health and Disease. Antioxid. Redox Signal. 2012, 16, 1492–1526. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Bodez, D.; Habibi, A.; Guellich, A.; Rappeneau, S.; Inamo, J.; Guendouz, S.; Gellen-Dautremer, J.; Pissard, S.; Loric, S.; et al. Haematological Determinants of Cardiac Involvement in Adults with Sickle Cell Disease. Eur. Heart J. 2016, 37, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Liem, R.I.; Rigsby, C.K.; Labotka, R.J.; DeFreitas, R.A.; Thompson, A.A. Assessing Cardiac and Liver Iron Overload in Chronically Transfused Patients with Sickle Cell Disease. Br. J. Haematol. 2016, 175, 705–713. [Google Scholar] [CrossRef]
- Meloni, A.; Martini, N.; Positano, V.; De Luca, A.; Pistoia, L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Allò, M.; et al. Myocardial Iron Overload by Cardiovascular Magnetic Resonance Native Segmental T1 Mapping: A Sensitive Approach That Correlates with Cardiac Complications. J. Cardiovasc. Magn. Reson. 2021, 23, 70. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Positano, V.; Martini, N.; Borrello, R.L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Putti, M.C.; et al. Myocardial Tissue Characterization by Segmental T2 Mapping in Thalassaemia Major: Detecting Inflammation beyond Iron. Eur. Heart J. Cardiovasc. Imaging 2023, 24, jead068. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Positano, V.; De Luca, A.; Martini, N.; Spasiano, A.; Fotzi, I.; Bitti, P.P.; Visceglie, D.; Alberini, G.; et al. Increased Myocardial Extracellular Volume Is Associated with Myocardial Iron Overload and Heart Failure in Thalassemia Major. Eur. Radiol. 2023, 33, 1266–1276. [Google Scholar] [CrossRef]
| NTD Sβ-Thal Patients N = 57 | TD Sβ-Thal Patients N = 54 | p Value | |
|---|---|---|---|
| Sex, M/F | 31/26 | 26/28 | 0.51 |
| Age, years | 37.3 ± 11.1 | 35.6 ± 17.7 | 0.62 |
| Hemoglobin, g/dL | 9.6 ± 1.6 | 9.3 ± 1.1 | 0.25 |
| Hemoglobin F, % | 15.3 ± 9.6 | 7.6 ± 6.2 | <0.0001 |
| Hemoglobin S, % | 67.3 ± 9.1 | 43.3 ± 15.8 | <0.0001 |
| Serum ferritin, ng/mL | 753.7 ± 893.0 | 1505.4 ± 1558.5 | <0.0001 |
| Lactate dehydrogenase, mg/dL | 663.0 ± 237.6 | 650.7 ± 311.1 | 0.84 |
| Serum creatinine, mg/dL | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.65 |
| Splenectomy, N (%) | 35 (61.4) | 38 (70.4) | 0.32 |
| Chelation therapy, N (%) | 16 (30.2) | 43 (79.6) | <0.0001 |
| Hydroxyurea therapy, N (%) | 40 (85.1) | 17 (36.2) | <0.0001 |
| History of pulmonary hypertension, N (%) | 1 (1.8) | 0 (0.0) | 0.32 |
| NTD Sβ-Thal Patients | TD Sβ-Thal Patients | p Value | |
|---|---|---|---|
| Left atrial area (cm2/m2) | 12.3 ± 1.9 | 13.0 ± 3.1 | 0.65 |
| Right atrial area (cm2/m2) | 11.7 ± 2.0 | 12.0 ± 2.1 | 0.59 |
| LV EDVI (mL/m2) | 91.7 ± 22.3 | 88.2 ± 16.6 | 0.38 |
| LV ESVI (mL/m2) | 36.7 ± 15.9 | 35.1 ± 10.5 | 0.56 |
| LV mass index (g/m2) | 63.2 ± 14.8 | 59.3 ± 14.8 | 0.18 |
| SVI (mL/m2) | 55.4 ± 14.0 | 52.93 ± 11.0 | 0.31 |
| Cardiac index (L/min/m2) | 4.0 ± 1.1 | 4.0 ± 1.3 | 0.47 |
| LV EF (%) | 62.2 ± 6.4 | 59.8 ± 7.9 | 0.09 |
| RV EDVI (mL/m2) | 83.8 ± 22.9 | 83.2 ± 15.2 | 0.96 |
| RV ESVI (mL/m2) | 32.1 ± 15.2 | 31.8 ± 7.7 | 0.37 |
| RV mass index (g/m2) | 27.9 ± 8.8 | 21.6 ± 8.4 | 0.001 |
| RV EF (%) | 63.2 ± 9.3 | 60.8 ± 6.8 | 0.14 |
| Global Heart T2* (ms) | 37.3 ± 5.2 | 35.9 ± 7.9 | 0.59 |
| Global Heart T2* < 20 ms. N (%) | 0 (0.0) | 2 (3.7) | 0.14 |
| At least 1 segment with T2* < 20 ms. N (%) | 16 (28.1) | 24 (44.4) | 0.07 |
| No MIO. N (%) | 41 (71.9) | 30 (55.6) | 0.07 |
| Heterogeneous MIO and no significant global heart iron. N (%) | 16 (28.1) | 22 (40.7) | 0.16 |
| Heterogeneous MIO and significant global heart iron. N (%) | 0 (0.0) | 1 (1.9) | 0.30 |
| Homogeneous MIO. N (%) | 0 (0.0) | 1 (1.9) | 0.30 |
| MRI LIC (mg/g dw) | 3.9 ± 3.5 | 7.5 ± 7.5 | 0.017 |
| MRI LIC > 3 mg/g dw. N (%) | 24 (42.1) | 35 (64.8) | 0.017 |
| Myocardial fibrosis by LGE. N (%) (N = 67) | 7 (17.5) | 5 (18.5) | 0.92 |
| Sβ-Thal Patients N = 111 | SCA-Patients N = 46 | p Value | |
|---|---|---|---|
| Sex, M/F | 57/54 | 24/22 | 0.93 |
| Age, years | 36.5 ± 14.6 | 29.0 ± 14.3 | 0.004 |
| Hemoglobin, g/dL | 9.4 ± 1.4 | 9.7 ± 1.3 | 0.49 |
| Hemoglobin F, % | 11.5 ± 9.0 | 10.3 ± 9.8 | 0.36 |
| Hemoglobin S, % | 55.4 ± 17.6 | 51.5 ± 17.6 | 0.33 |
| Serum ferritin, ng/mL | 1122.7 ± 1313.8 | 1353.3 ± 1501.1 | 0.41 |
| Lactate dehydrogenase, mg/dL | 657.0 ± 274.3 | 663.1 ± 269.0 | 0.99 |
| Serum creatinine, mg/dL | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.68 |
| Splenectomy, N (%) | 73 (65.8) | 13 (28.9) | <0.0001 |
| Chelation therapy, N (%) | 59 (55.1) | 22 (55.0) | 0.99 |
| Hydroxyurea, N (%) | 57 (60.6) | 14 (34.1) | 0.005 |
| Transfusion dependent patients, N (%) | 54 (48.6) | 30 (65.2) | 0.06 |
| History of pulmonary hypertension, N (%) | 1 (0.9) | 0 (0.0) | 0.53 |
| Sβ-Thal Patients | SCA Patients | p Value | |
|---|---|---|---|
| Left atrial area (cm2/m2) | 12.6 ± 2.5 | 14.0 ± 3.3 | 0.023 |
| Right atrial area (cm2/m2) | 11.8 ± 2.0 | 12.2 ± 2.5 | 0.38 |
| LV EDVI (mL/m2) | 90.1 ± 19.8 | 93.4 ± 23.0 | 0.39 |
| LV ESVI (mL/m2) | 35.9 ± 13.6 | 34.6 ± 13.1 | 0.43 |
| LV mass index (g/m2) | 61.4 ± 14.8 | 64.6 ± 20.4 | 0.68 |
| SVI (mL/m2) | 54.3 ± 12.7 | 59.2 ± 14.6 | 0.05 |
| Cardiac index (L/min/m2) | 3.9 ± 1.2 | 3.9 ± 1.1 | 0.94 |
| LV EF (%) | 61.1 ± 7.2 | 63.1 ± 7.4 | 0.12 |
| RV EDVI (mL/m2) | 83.5 ± 19.6 | 86.3 ± 21.9 | 0.45 |
| RV ESVI (mL/m2) | 32.0 ± 12.2 | 33.3 ± 13.7 | 0.94 |
| RV mass index (g/m2) | 25.1 ± 9.1 | 25.5 ± 12.1 | 0.83 |
| RV EF (%) | 62.1 ± 8.3 | 62.1 ± 8.8 | 1.0 |
| Global Heart T2* (ms) | 36.7 ± 6.7 | 38.4 ± 7.2 | 0.15 |
| Global Heart T2* < 20 ms, N (%) | 2 (1.8) | 0 (0.0) | 0.36 |
| At least 1 segment with T2* < 20 ms, N (%) | 40 (36) | 10 (21.7) | 0.08 |
| No MIO, N (%) | 71 (64) | 36 (78.3) | 0.33 |
| Heterogeneous MIO and no significant global heart iron, N (%) | 38 (34.2) | 10 (21.7) | |
| Heterogeneous MIO and significant global heart iron, N (%) | 1 (0.9) | 0 (0.0) | |
| Homogeneous MIO, N (%) | 1 (0.9) | 0 (0.0) | |
| MRI LIC (mg/g dw) | 5.6 ± 6.0 | 7.2 ± 12.9 | 0.73 |
| MRI LIC > 3 mg/g dw, N (%) | 59 (53.2) | 24 (52.2) | 0.91 |
| Myocardial fibrosis by LGE, N (%) (N = 93) | 12 (17.9) | 3 (11.5) | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistoia, L.; Meloni, A.; Positano, V.; Longo, F.; Borsellino, Z.; Spasiano, A.; Righi, R.; Renne, S.; Izzo, D.; Savino, K.; et al. Multiparametric Cardiac Magnetic Resonance Assessment in Sickle Beta Thalassemia. Diagnostics 2024, 14, 691. https://doi.org/10.3390/diagnostics14070691
Pistoia L, Meloni A, Positano V, Longo F, Borsellino Z, Spasiano A, Righi R, Renne S, Izzo D, Savino K, et al. Multiparametric Cardiac Magnetic Resonance Assessment in Sickle Beta Thalassemia. Diagnostics. 2024; 14(7):691. https://doi.org/10.3390/diagnostics14070691
Chicago/Turabian StylePistoia, Laura, Antonella Meloni, Vincenzo Positano, Filomena Longo, Zelia Borsellino, Anna Spasiano, Riccardo Righi, Stefania Renne, Daniela Izzo, Ketty Savino, and et al. 2024. "Multiparametric Cardiac Magnetic Resonance Assessment in Sickle Beta Thalassemia" Diagnostics 14, no. 7: 691. https://doi.org/10.3390/diagnostics14070691
APA StylePistoia, L., Meloni, A., Positano, V., Longo, F., Borsellino, Z., Spasiano, A., Righi, R., Renne, S., Izzo, D., Savino, K., Mavrogeni, S., Quaia, E., Cademartiri, F., & Pepe, A. (2024). Multiparametric Cardiac Magnetic Resonance Assessment in Sickle Beta Thalassemia. Diagnostics, 14(7), 691. https://doi.org/10.3390/diagnostics14070691













