Epidemiological Characterization of Respiratory Pathogens Using the Multiplex PCR FilmArray™ Respiratory Panel
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Pathogen Identification with the FilmArray™ RP
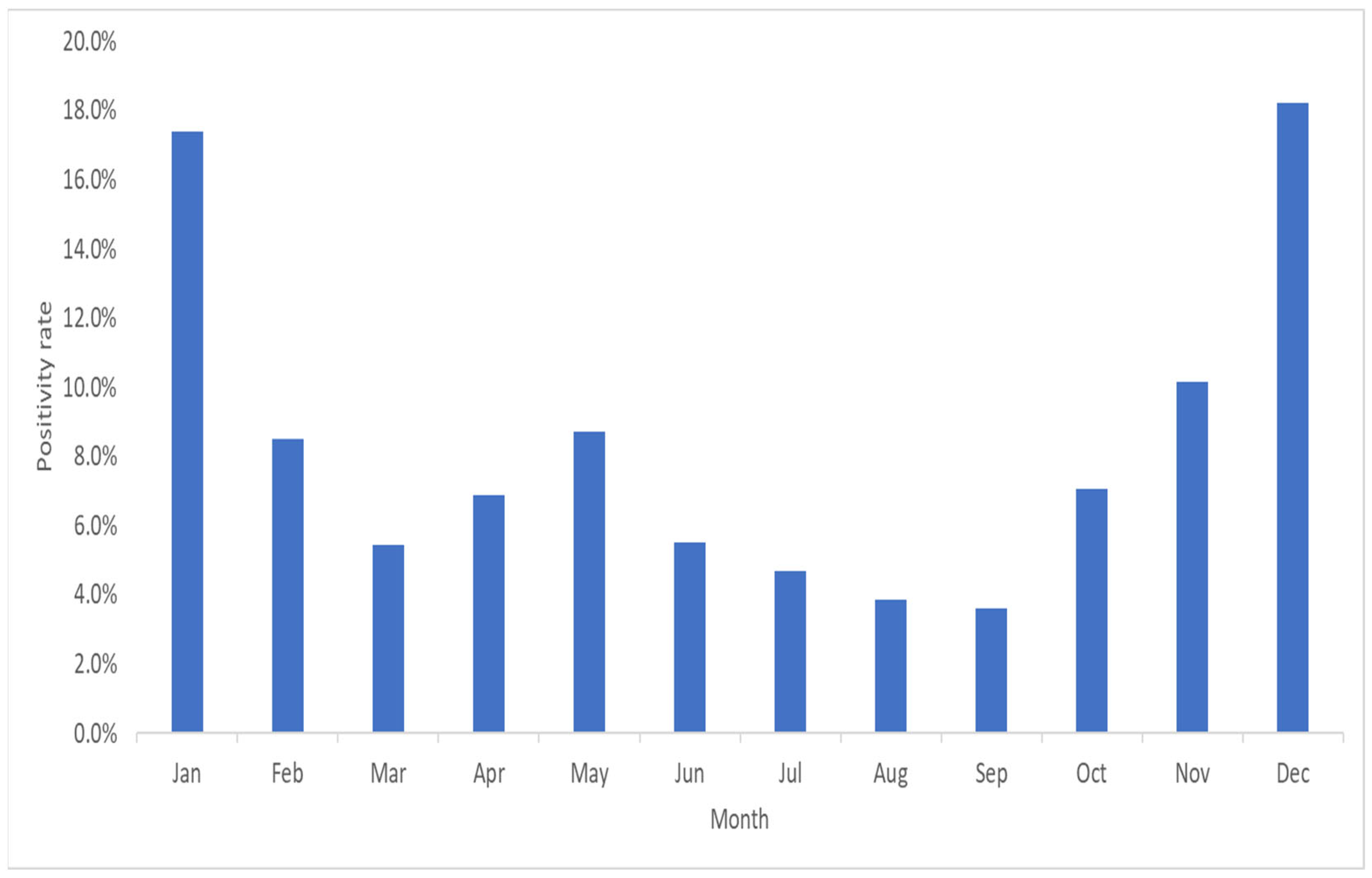
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The economic burden of non–influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef]
- Pillet, S.; Lardeux, M.; Dina, J.; Grattard, F.; Verhoeven, P.; Le Goff, J.; Vabret, A.; Pozzetto, B. Comparative evaluation of six commercialized multiplex PCR kits for the diagnosis of respiratory infections. PLoS ONE 2013, 8, e72174. [Google Scholar] [CrossRef] [PubMed]
- Pitrez, P.M.; Pitrez, J.L. Acute upper respiratory tract infections: Outpatient diagnosis and treatment. J. Pediatr. 2003, 79, S77–S86. [Google Scholar] [CrossRef]
- Ruopp, M.; Chiswell, K.; Thaden, J.T.; Merchant, K.; Tsalik, E.L. Respiratory tract infection clinical trials from 2007 to 2012. A systematic review of ClinicalTrials.gov. Ann. Am. Thorac. Soc. 2015, 12, 1852–1863. [Google Scholar] [CrossRef] [PubMed]
- Kuchar, E.; Miśkiewicz, K.; Nitsch-Osuch, A.; Szenborn, L. Pathophysiology of clinical symptoms in acute viral respiratory tract infections. Adv. Exp. Med. Biol. 2015, 857, 25–38. [Google Scholar]
- Hersh, A.L.; Shapiro, D.J.; Pavia, A.T.; Shah, S.S. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011, 128, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, A.-C.; Gonzales, R.; Steiner, J.F.; Sande, M.A. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA 1998, 279, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Evaluation of 11 commercially available rapid influenza diagnostic tests—United States, 2011–2012. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 873–876. [Google Scholar]
- Salez, N.; Nougairede, A.; Ninove, L.; Zandotti, C.; De Lamballerie, X.; Charrel, R.N. Xpert Flu for point-of-care diagnosis of human influenza in industrialized countries. Expert Rev. Mol. Diagn. 2014, 14, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.P.; Gagne, L.S.; Stock, S.R.; Marty, F.M.; Gelman, R.S.; Marasco, W.A.; Poritz, M.A.; Baden, L.R. Respiratory virus detection in immunocompromised patients with FilmArray Respiratory Panel compared to conventional methods. J. Clin. Microbiol. 2012, 50, 3216–3221. [Google Scholar] [CrossRef] [PubMed]
- Kaku, N.; Hashiguchi, K.; Iwanaga, Y.; Akamatsu, N.; Matsuda, J.; Kosai, K.; Uno, N.; Morinaga, Y.; Kitazaki, T.; Hasegawa, H.; et al. Evaluation of FilmArray Respiratory Panel multiplex polymerase chain reaction assay for detection of pathogens in adult outpatients with acute respiratory tract infection. J. Infect. Chemother. 2018, 24, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper respiratory tract infections. Indian J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Gonzalez, R.; Wang, Z.; Xiang, Z.; Wang, Y.; Zhou, H.; Li, J.; Xiao, Y.; Yang, Q.; Zhang, J.; et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin. Microbiol. Infect. 2009, 15, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, Y.; Wang, H.; Zhang, L.; Bao, Y.; Zhou, X. High incidence of multiple viral infections identified in upper respiratory tract infected children under three years of age in Shanghai, China. PLoS ONE 2012, 7, e44568. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L.; Everhart, K.; Daly, J.A.; Hopper, A.; Harrington, A.; Schreckenberger, P.; McKinley, K.; Jones, M.; Holmberg, K.; Kensinger, B. Multicenter evaluation of BioFire FilmArray Respiratory Panel 2 for detection of viruses and bacteria in nasopharyngeal swab samples. J. Clin. Microbiol. 2018, 56, e01945-17. [Google Scholar] [CrossRef]
- Li, J.; Tao, Y.; Tang, M.; Du, B.; Xia, Y.; Mo, X.; Cao, Q. Rapid detection of respiratory organisms with the FilmArray Respiratory Panel in a large Children’s Hospital in China. BMC Infect. Dis. 2018, 18, 510. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L.; Lisby, J.G.; Hansen, G.; Relich, R.F.; Schneider, U.V.; Granato, P.; Young, S.; Pareja, J.; Hannet, I. Multicenter evaluation of the QIAstat-Dx Respiratory Panel for detection of viruses and bacteria in nasopharyngeal swab specimens. J. Clin. Microbiol. 2020, 58, e00155-20. [Google Scholar] [CrossRef] [PubMed]
- Loeffelholz, M.J.; Pong, D.L.; Pyles, R.B.; Xiong, Y.; Miller, A.L.; Bufton, K.K.; Chonmaitree, T. Comparison of the FilmArray Respiratory Panel and Prodesse real-time PCR assays for detection of respiratory pathogens. J. Clin. Microbiol. 2011, 49, 4083–4088. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L.; Everhart, K.; Balada-Llasat, J.M.; Cullison, J.; Daly, J.; Holt, S.; Lephart, P.; Salimnia, H.; Schreckenberger, P.C.; DesJarlais, S.; et al. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J. Clin. Microbiol. 2016, 54, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Piralla, A.; Lunghi, G.; Ardissino, G.; Girello, A.; Premoli, M.; Bava, E.; Arghittu, M.; Colombo, M.R.; Cognetto, A.; Bono, P.; et al. FilmArray™ GI panel performance for the diagnosis of acute gastroenteritis or hemorragic diarrhea. BMC Microbiol. 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Busson, L.; Bartiaux, M.; Brahim, S.; Konopnicki, D.; Dauby, N.; Gérard, M.; De Backer, P.; Van Vaerenbergh, K.; Mahadeb, B.; Mekkaoui, L.; et al. Contribution of the FilmArray Respiratory Panel in the management of adult and pediatric patients attending the emergency room during 2015–2016 influenza epidemics: An interventional study. Int. J. Infect. Dis. 2019, 83, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Parčina, M.; Schneider, U.V.; Visseaux, B.; Jozić, R.; Hannet, I.; Lisby, J.G. Multicenter evaluation of the QIAstat Respiratory Panel—A new rapid highly multiplexed PCR based assay for diagnosis of acute respiratory tract infections. PLoS ONE 2020, 15, e0230183. [Google Scholar] [CrossRef]
- Sanghavi, S.K.; Bullotta, A.; Husain, S.; Rinaldo, C.R. Clinical evaluation of multiplex real-time PCR panels for rapid detection of respiratory viral infections. J. Med. Virol. 2012, 84, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.C.; Slamon, N.B. The impact of multiple viral respiratory infections on outcomes for critically ill children. Pediatr. Crit. Care Med. 2017, 18, e333–e338. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Komurian-Pradel, F.; Javouhey, E.; Perret, M.; Rajoharison, A.; Bagnaud, A.; Billaud, G.; Vernet, G.; Lina, B.; Floret, D.; et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr. Infect. Dis. J. 2008, 27, 213–217. [Google Scholar] [CrossRef]
- Scotta, M.C.; Chakr, V.C.; de Moura, A.; Becker, R.G.; de Souza, A.P.D.; Jones, M.H.; Pinto, L.A.; Sarria, E.E.; Pitrez, P.M.; Stein, R.T.; et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta-analysis. J. Clin. Virol. 2016, 80, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wishaupt, J.O.; van der Ploeg, T.; de Groot, R.; Versteegh, F.G.; Hartwig, N.G. Single-and multiple viral respiratory infections in children: Disease and management cannot be related to a specific pathogen. BMC Infect. Dis. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, H.; Tang, Y.; Yu, F.; Ma, C.; Zhang, H.; Pang, L.; Zhao, H.; Wang, L. Multiplex tests for respiratory tract infections: The direct utility of the FilmArray Respiratory Panel in emergency department. Can. Respir. J. 2020, 2020, 6014563. [Google Scholar] [CrossRef]
- Lamrani Hanchi, A.; Guennouni, M.; Rachidi, M.; Benhoumich, T.; Bennani, H.; Bourrous, M.; Maoulainine, F.M.R.; Younous, S.; Bouskraoui, M.; Soraa, N. Epidemiology of respiratory pathogens in children with severe acute respiratory infection and impact of the multiplex PCR Film Array Respiratory Panel: A 2-year study. Int. J. Microbiol. 2021, 2021, 2276261. [Google Scholar] [CrossRef] [PubMed]
- Visseaux, B.; Burdet, C.; Voiriot, G.; Lescure, F.X.; Chougar, T.; Brugière, O.; Crestani, B.; Casalino, E.; Charpentier, C.; Descamps, D.; et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS ONE 2017, 12, e0180888. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.C.; Edouard, S.; Pagnier, I.; Mediannikov, O.; Drancourt, M.; Raoult, D. Current and Past Strategies for Bacterial Culture in Clinical Microbiology. Clin. Microbiol. Rev. 2015, 28, 208–236. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.K.; Falsey, A.R. The diagnosis of viral respiratory disease in older adults. Clin. Infect. Dis. 2010, 50, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Panasiuk, L.; Lukas, W.; Paprzycki, P.; Verheij, T.; Godycki-Ćwirko, M.; Chlabicz, S. Antibiotics in the treatment of upper respiratory tract infections in Poland. Is there any improvement? J. Clin. Pharm. Ther. 2010, 35, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Koo, S.H.; Jiang, B.; Lim, P.Q.; Tan, T.Y. Comparison of the BioFire FilmArray Respiratory Panel, Seegene AnyplexII RV16, and Argene for the detection of respiratory viruses. J. Clin. Virol. 2018, 106, 13–17. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Positivity | |
|---|---|---|
| Number of Samples | % of Total | |
| All samples (N = 6367) | ||
| Negative samples | 4.829 | 75.8% |
| Positive samples | 1538 | 24.1% |
| Single detections | 1351 | 21.2% |
| Co-infections | 187 | 2.9% |
| Co-infections (n = 187) | ||
| Double infection | 169 | 90.3% |
| Triple infection | 17 | 9.1% |
| Quadruple infection | 1 | 0.6% |
| Detected | Detection Rate (%) | Co-Infection | Co-Infection Rate (%) | |
|---|---|---|---|---|
| AdV | 329 | 18.9 | 93 | 28.3 |
| CoV-229E | 40 | 2.3 | 16 | 40.0 |
| CoV-HKU1 | 5 | 0.3 | 3 | 60.0 |
| CoV-NL63 | 68 | 3.9 | 26 | 38.2 |
| CoV-OC43 | 101 | 5.8 | 31 | 30.7 |
| SARS-CoV-2 | 28 | 1.6 | 6 | 21.4 |
| hMPV | 170 | 9.7 | 29 | 17.1 |
| HRV/EV | 181 | 10.4 | 57 | 31.5 |
| FluA | 289 | 16.6 | 38 | 13.1 |
| FluB | 107 | 6.1 | 15 | 14.0 |
| PIV1 | 77 | 4.4 | 15 | 19.5 |
| PIV2 | 29 | 1.7 | 4 | 13.8 |
| PIV3 | 214 | 12.3 | 29 | 13.6 |
| PIV4 | 9 | 0.5 | 5 | 55.6 |
| RSV | 97 | 5.6 | 26 | 26.8 |
| Total | 1744 | 100.0 | 393 | 22.5 |
| Single Infection | Double Infection | Triple Infection | |||||
|---|---|---|---|---|---|---|---|
| AdV | 236 (17.5) | AdV | CoV-229E | 2 (1.2) | AdV & CoV-NL63 | CoV-229E | 1 (5.9) |
| CoV-229E | 24 (1.8) | CoV-OL63 | 4 (2.4) | CoV-OC43 | 1 (5.9) | ||
| CoV-HKU1 | 2 (0.1) | CoV-OC43 | 11 (6.5) | PIV3 | 1 (5.9) | ||
| CoV-NL63 | 42 (3.1) | hMPV | 13 (7.7) | HRV/EV | 1 (5.9) | ||
| CoV-OC43 | 70 (5.2) | HRV/EV | 14 (8.3) | AdV & HRV/EV | PIV1 | 1 (5.9) | |
| SARS-CoV-2 | 22 (1.6) | FluA | 10 (5.9) | RSV | 2 (11.8) | ||
| HRV/EV | 141 (10.4) | FluB | 2 (1.2) | CoV-OC43 | 1 (5.9) | ||
| RV/EV | 124 (9.2) | PIV1 | 2 (1.2) | PIV3 | 3 (17.6) | ||
| FluA | 251 (18.6) | PIV2 | 1 (0.6) | AdV & CoV-OC43 | CoV-HKU1 | 1 (5.9) | |
| FluB | 92 (6.8) | PIV3 | 13 (7.7) | PIV3 | 1 (5.9) | ||
| PIV1 | 62 (4.6) | PIV4 | 1 (0.6) | AdV & RSV | SARS-CoV-2 | 1 (5.9) | |
| PIV2 | 25 (1.9) | RSV | 5 (3.0) | CoV-NL63 & PIV1 | PIV3 | 1 (5.9) | |
| PIV3 | 185 (13.7) | CoV-229E | CoV-OL63 | 2 (1.2) | CoV-NL63 & HRV/EV | RSV | 1 (5.9) |
| PIV4 | 4 (0.3) | FluA | 8 (4.7) | CoV-OC43 & hMPV | FluA | 1 (5.9) | |
| RSV | 71 (5.3) | FluB | 3 (1.8) | total | 17 (100) | ||
| Total | 1351 (100) | CoV-HKU1 | hMPV | 1 (0.6) | |||
| FluA | 1 (0.6) | ||||||
| CoV-OL63 | CoV-OC43 | 2 (1.2) | |||||
| hMPV | 2 (1.2) | ||||||
| HRV/EV | 1 (0.6) | ||||||
| FluA | 4 (2.4) | ||||||
| FluB | 1 (0.6) | ||||||
| PIV1 | 1 (0.6) | ||||||
| PIV3 | 2 (1.2) | ||||||
| C.OC43 | HRV/EV | 1 (0.6) | |||||
| FluA | 4 (2.4) | ||||||
| FluB | 3 (1.8) | ||||||
| PIV3 | 3 (1.8) | ||||||
| RSV | 1 (0.6) | ||||||
| SARS | hMPV | 1 (0.6) | |||||
| HRV/EV | 2 (1.2) | ||||||
| FluA | 1 (0.6) | ||||||
| RSV | 1 (0.6) | ||||||
| MV | HRV/EV | 4 (2.4) | |||||
| FluB | 1 (0.6) | ||||||
| PIV1 | 2 (1.2) | ||||||
| PIV2 | 1 (0.6) | ||||||
| PIV3 | 3 (1.8) | ||||||
| HRV/EV | FluA | 1 (0.6) | |||||
| PIV1 | 5 (3.0) | ||||||
| PIV2 | 1 (0.6) | ||||||
| PIV3 | 4 (2.4) | ||||||
| RSV | 14 (8.3) | ||||||
| FluA | FluB | 5 (3.0) | |||||
| PIV1 | 1 (0.6) | ||||||
| PIV2 | 1 (0.6) | ||||||
| PIV4 | 1 (0.6) | ||||||
| PIV1 | PIV3 | 1 (0.6) | |||||
| RSV | 1 (0.6) | ||||||
| Total | 169 (100) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.J.; Jung, B.K.; Kim, J.K. Epidemiological Characterization of Respiratory Pathogens Using the Multiplex PCR FilmArray™ Respiratory Panel. Diagnostics 2024, 14, 734. https://doi.org/10.3390/diagnostics14070734
Hong YJ, Jung BK, Kim JK. Epidemiological Characterization of Respiratory Pathogens Using the Multiplex PCR FilmArray™ Respiratory Panel. Diagnostics. 2024; 14(7):734. https://doi.org/10.3390/diagnostics14070734
Chicago/Turabian StyleHong, Young Jun, Bo Kyeung Jung, and Jae Kyung Kim. 2024. "Epidemiological Characterization of Respiratory Pathogens Using the Multiplex PCR FilmArray™ Respiratory Panel" Diagnostics 14, no. 7: 734. https://doi.org/10.3390/diagnostics14070734
APA StyleHong, Y. J., Jung, B. K., & Kim, J. K. (2024). Epidemiological Characterization of Respiratory Pathogens Using the Multiplex PCR FilmArray™ Respiratory Panel. Diagnostics, 14(7), 734. https://doi.org/10.3390/diagnostics14070734






