Susceptibility-Weighted MRI for Predicting NF-2 Mutations and S100 Protein Expression in Meningiomas
Abstract
1. Introduction
2. Materials and Methods
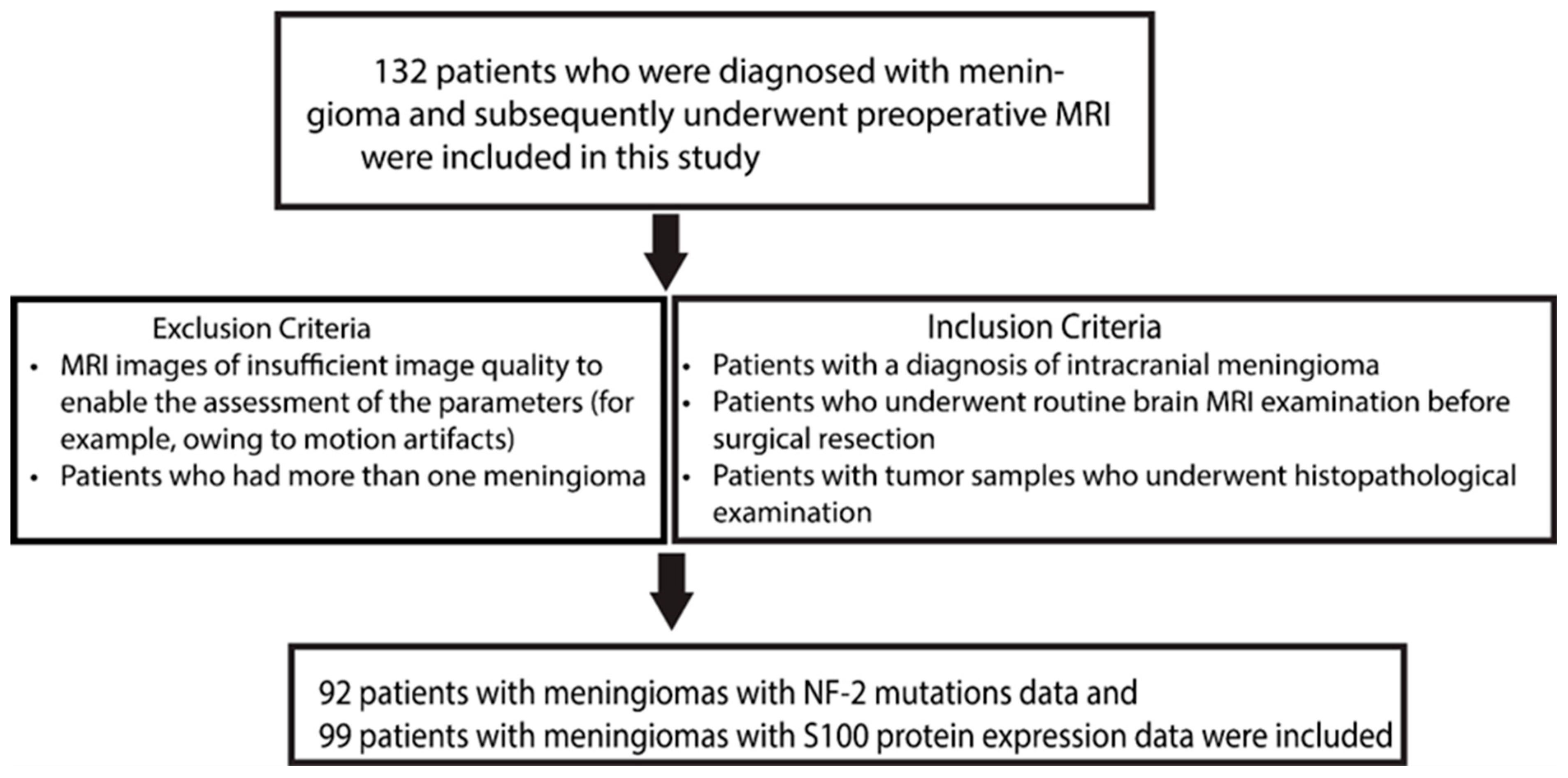
2.1. Clinical Cohort and Data Acquisition
2.2. Histopathological and Genetic Analyses
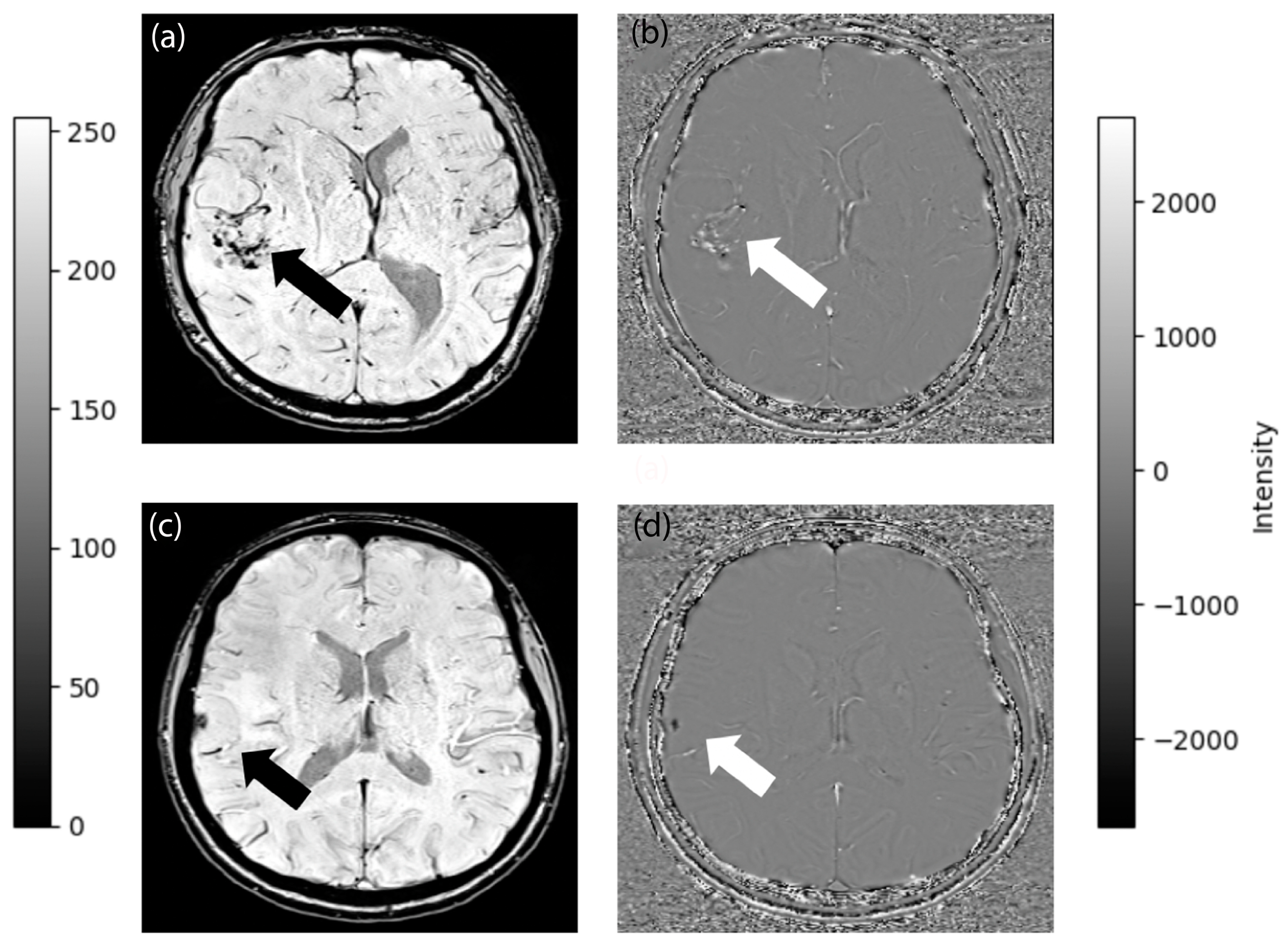
2.3. MRI Protocol
2.4. Semi-Quantitative Evaluation of Morphological Characteristics
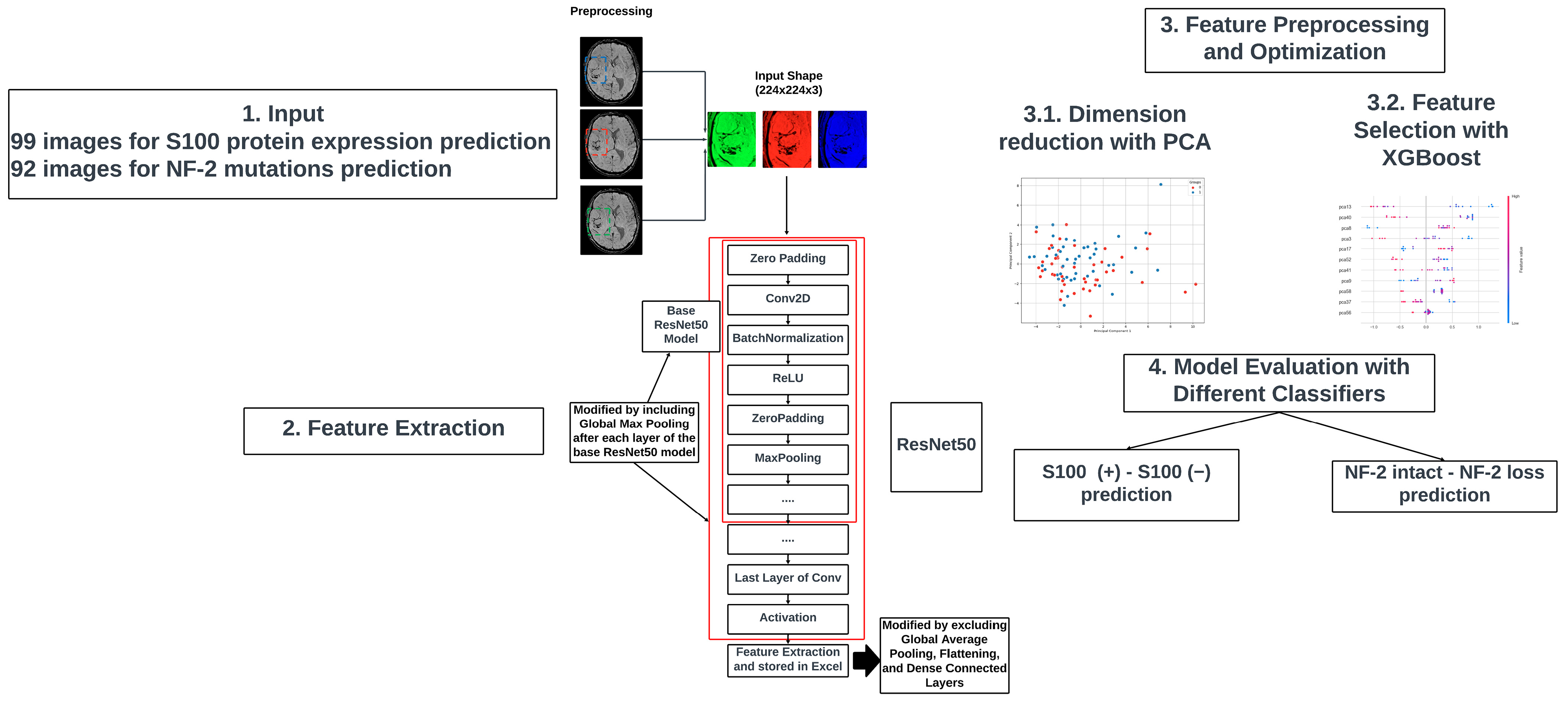
2.5. Image Processing
2.6. Statistical Analysis
2.7. Deep Learning-Based Feature Extraction
3. Results
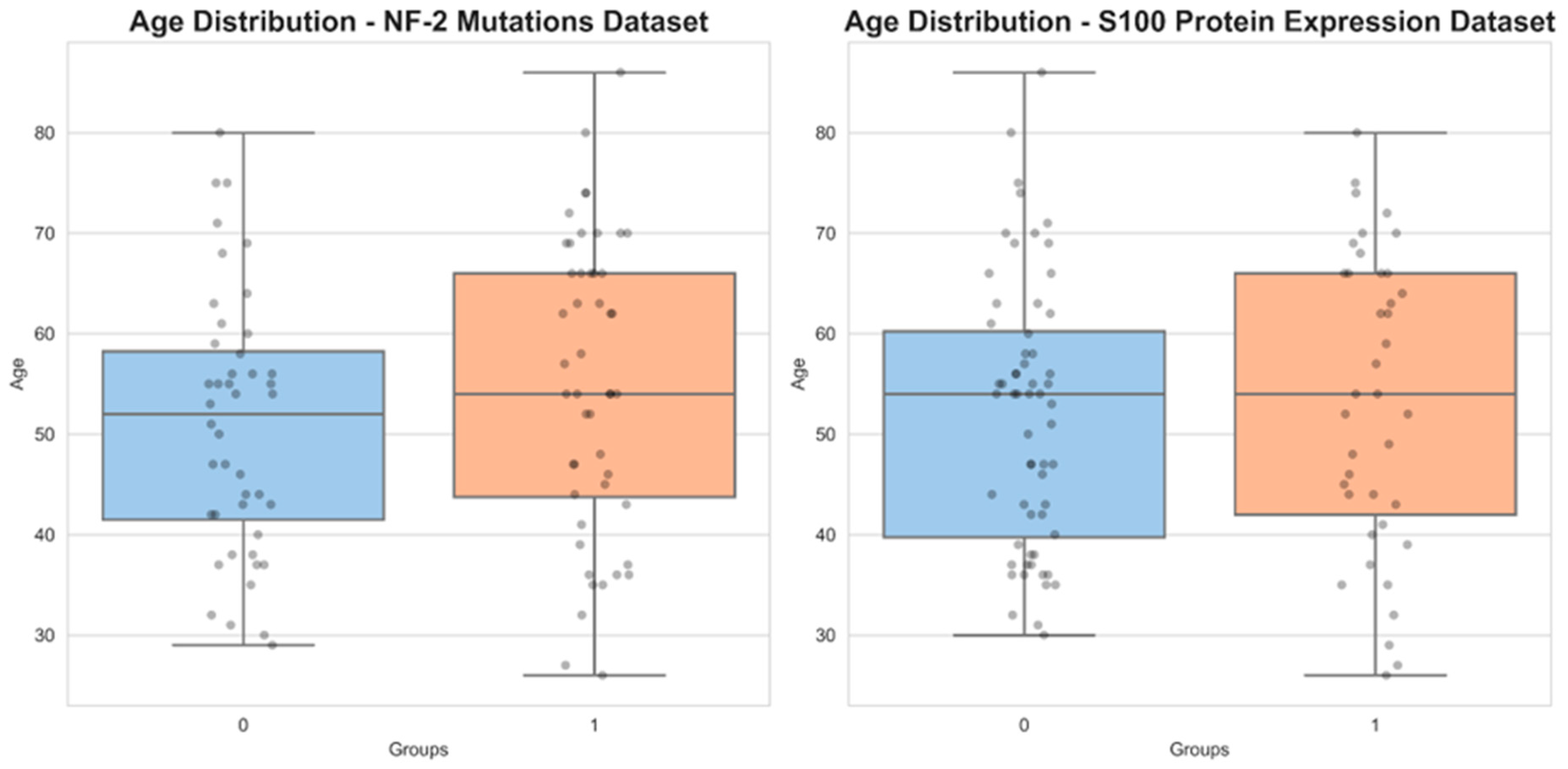
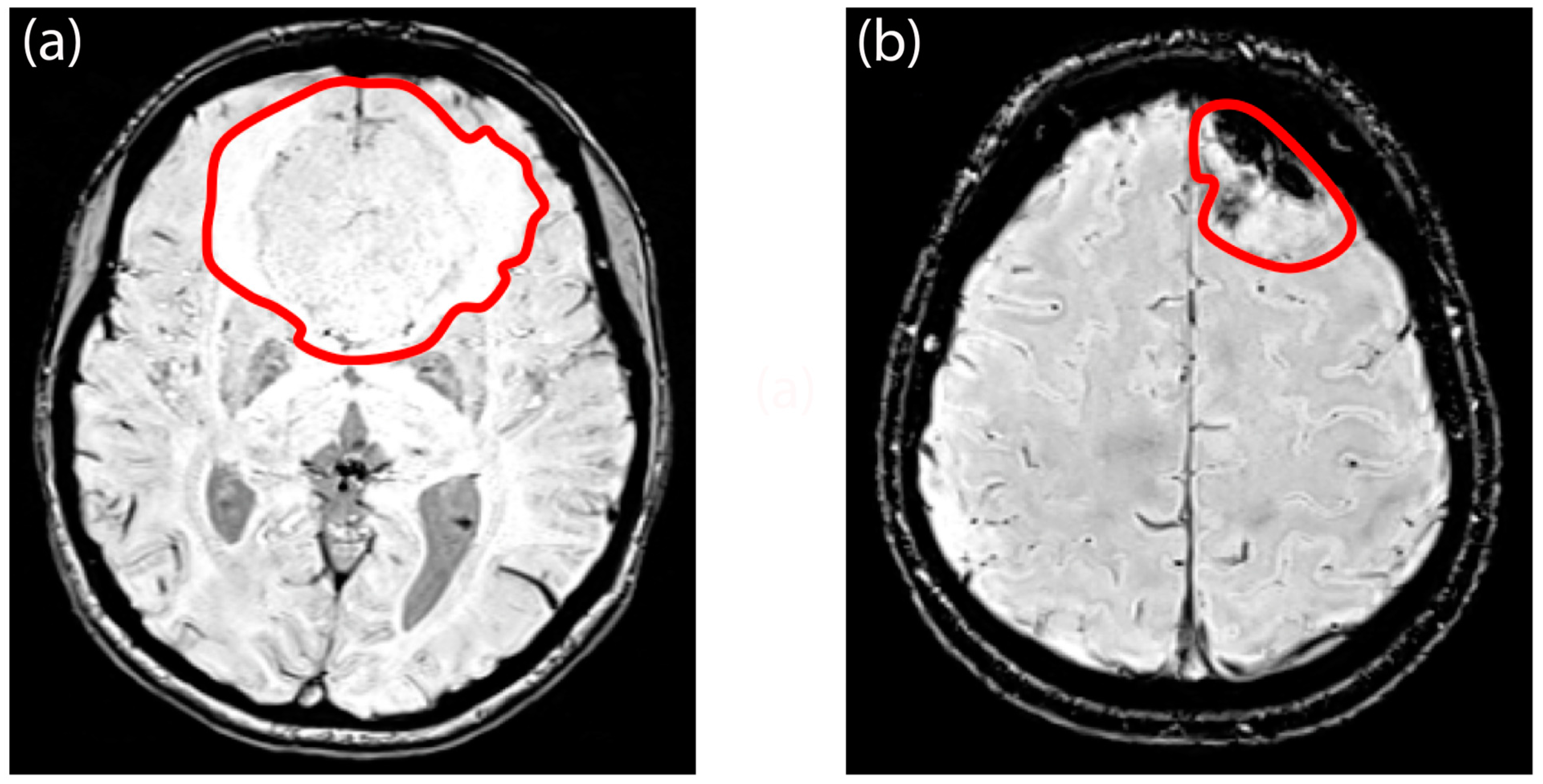
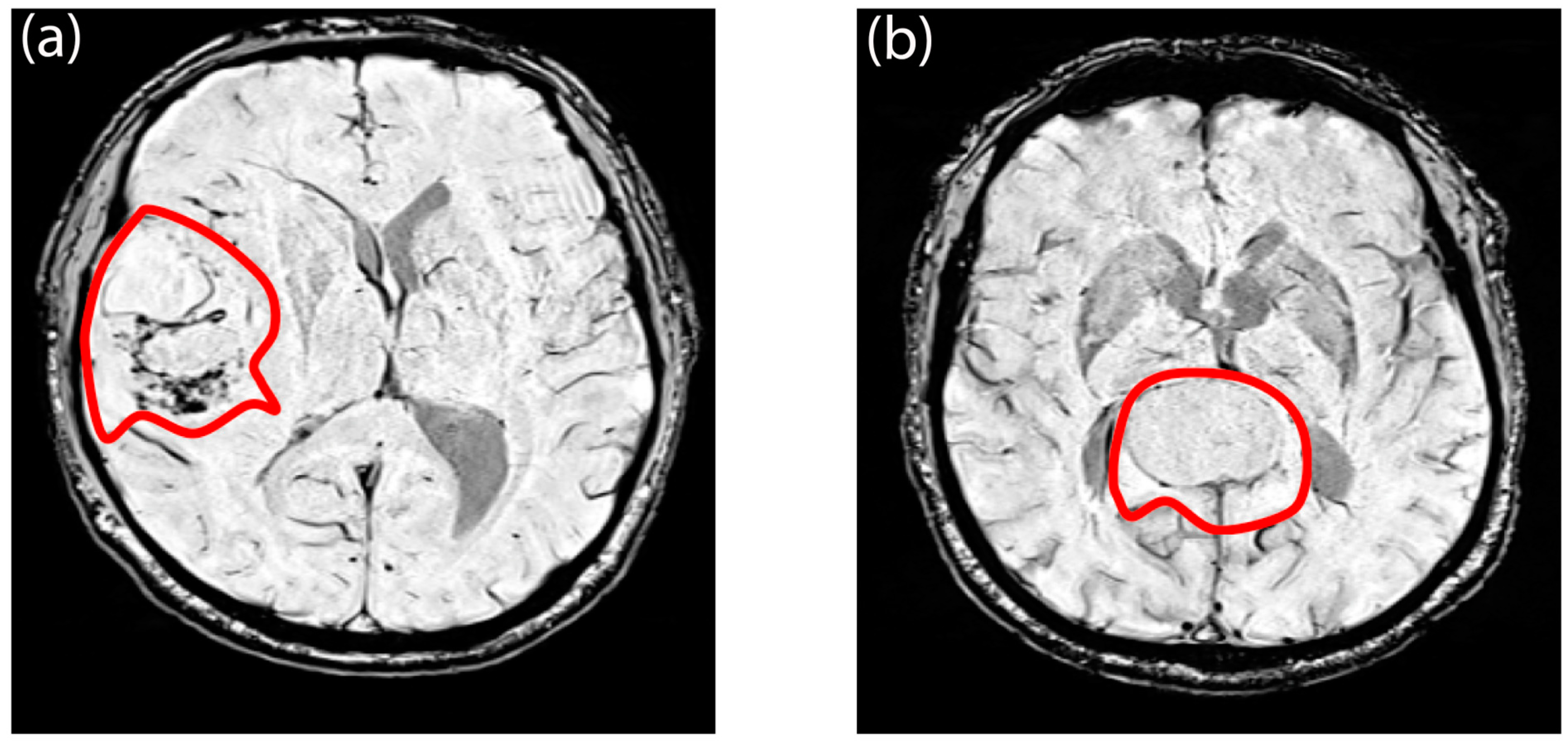
3.1. Demographic and Radiological Characteristics
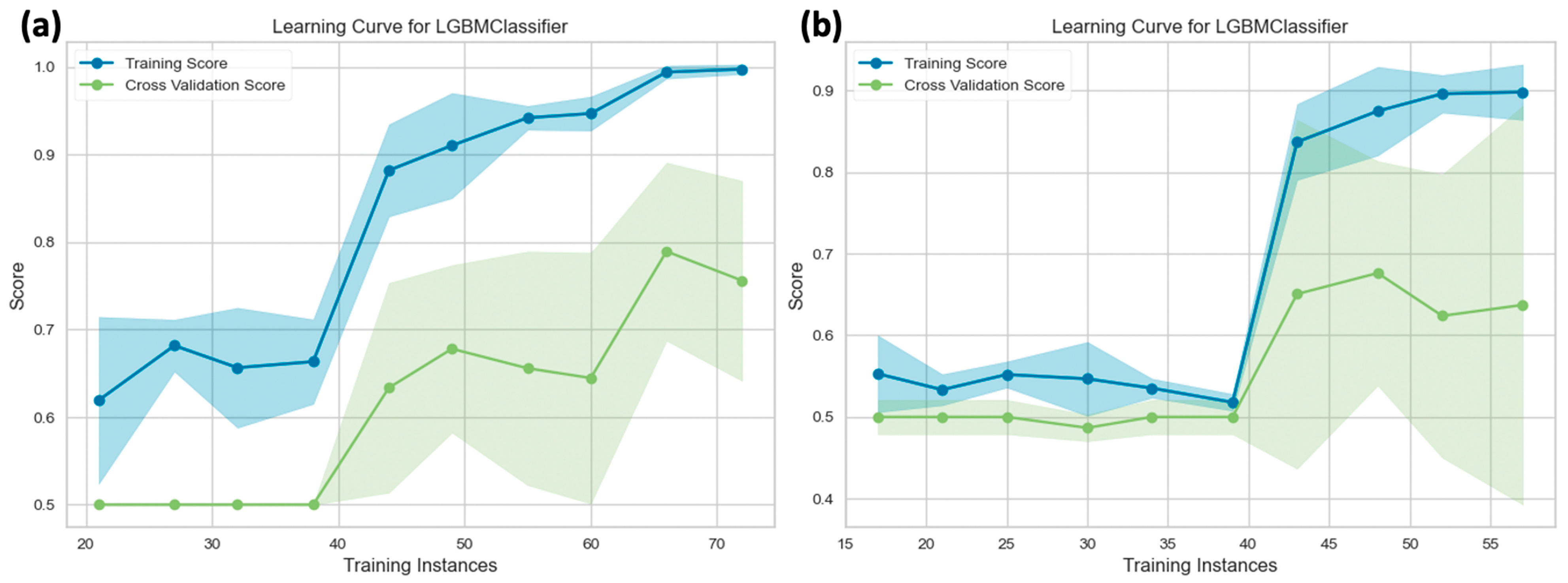
3.2. Diagnostic Performance of Deep Learning Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, J.T.; Ostrom, Q.T.; Cioffi, G.; Neff, C.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. Primary Brain and Other Central Nervous System Tumors in the United States (2014–2018): A Summary of the CBTRUS Statistical Report for Clinicians. Neurooncol. Pract. 2022, 9, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, M.P.; Aldape, K.D. Molecular Classification and Grading of Meningioma. J. Neurooncol. 2023, 161, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, Y.; Li, M.; Liu, J.; Wang, F.; Weng, Q.; Wang, X.; Cao, D. Machine Learning-Based Radiomics Analysis in Predicting the Meningioma Grade Using Multiparametric MRI. Eur. J. Radiol. 2020, 131, 109251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Man, C.; Gong, L.; Dong, D.; Yu, X.; Wang, S.; Fang, M.; Wang, S.; Fang, X.; Chen, X.; et al. A Deep Learning Radiomics Model for Preoperative Grading in Meningioma. Eur. J. Radiol. 2019, 116, 128–134. [Google Scholar] [CrossRef]
- Zhang, S.; Chiang, G.C.-Y.; Knapp, J.M.; Zecca, C.M.; He, D.; Ramakrishna, R.; Magge, R.S.; Pisapia, D.J.; Fine, H.A.; Tsiouris, A.J.; et al. Grading Meningiomas Utilizing Multiparametric MRI with Inclusion of Susceptibility Weighted Imaging and Quantitative Susceptibility Mapping. J. Neuroradiol. 2020, 47, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, M.W.; Miyagishima, D.F.; Jin, L.; Gupte, T.; Li, C.; Duran, D.; Montejo, J.D.; Zhao, A.; Sheth, A.; Tyrtova, E.; et al. Associations of Meningioma Molecular Subgroup and Tumor Recurrence. Neuro-Oncology 2021, 23, 783–794. [Google Scholar] [CrossRef]
- Moazzam, A.A.; Wagle, N.; Zada, G. Recent Developments in Chemotherapy for Meningiomas: A Review. Neurosurg. Focus 2013, 35, E18. [Google Scholar] [CrossRef]
- Smith, M.J.; Higgs, J.E.; Bowers, N.L.; Halliday, D.; Paterson, J.; Gillespie, J.; Huson, S.M.; Freeman, S.R.; Lloyd, S.; Rutherford, S.A.; et al. Cranial Meningiomas in 411 Neurofibromatosis Type 2 (NF2) Patients with Proven Gene Mutations: Clear Positional Effect of Mutations, but Absence of Female Severity Effect on Age at Onset. J. Med. Genet. 2011, 48, 261–265. [Google Scholar] [CrossRef]
- Ülgen, E.; Bektaşoğlu, P.K.; Sav, M.A.; Can, Ö.; Danyeli, A.E.; Hızal, D.B.; Pamir, M.N.; Özduman, K. Meningiomas Display a Specific Immunoexpression Pattern in a Rostrocaudal Gradient: An Analysis of 366 Patients. World Neurosurg. 2019, 123, e520–e535. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A Clinically Applicable Integrative Molecular Classification of Meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Hancq, S.; Salmon, I.; Brotchi, J.; Gabius, H.-J.; Heizmann, C.W.; Kiss, R.; Decaestecker, C. Detection of S100B, S100A6 and Galectin-3 Ligands in Meningiomas as Markers of Aggressiveness. Int. J. Oncol. 2004, 25, 1233–1240. [Google Scholar] [CrossRef]
- Hancq, S.; Salmon, I.; Brotchi, J.; De Witte, O.; Gabius, H.-J.; Heizmann, C.W.; Kiss, R.; Decaestecker, C. S100A5: A Marker of Recurrence in WHO Grade I Meningiomas. Neuropathol. Appl. Neurobiol. 2004, 30, 178–187. [Google Scholar] [CrossRef]
- Robert, S.M.; Vetsa, S.; Nadar, A.; Vasandani, S.; Youngblood, M.W.; Gorelick, E.; Jin, L.; Marianayagam, N.; Erson-Omay, E.Z.; Günel, M.; et al. The Integrated Multiomic Diagnosis of Sporadic Meningiomas: A Review of Its Clinical Implications. J. Neurooncol. 2022, 156, 205–214. [Google Scholar] [CrossRef]
- Sehgal, V.; Delproposto, Z.; Haacke, E.M. Clinical Applications of Neuroimaging with Susceptibility-weighted Imaging. Reson. Imaging 2005, 22, 439–450. [Google Scholar] [CrossRef]
- Haller, S.; Haacke, E.M.; Thurnher, M.M.; Barkhof, F. Susceptibility-Weighted Imaging: Technical Essentials and Clinical Neurologic Applications. Radiology 2021, 299, 3–26. [Google Scholar] [CrossRef]
- Kong, L.-W.; Chen, J.; Zhao, H.; Yao, K.; Fang, S.-Y.; Wang, Z.; Wang, Y.-Y.; Li, S.-W. Intratumoral Susceptibility Signals Reflect Biomarker Status in Gliomas. Sci. Rep. 2019, 9, 17080. [Google Scholar] [CrossRef]
- Hsu, C.C.-T.; Watkins, T.W.; Kwan, G.N.C.; Haacke, E.M. Susceptibility-Weighted Imaging of Glioma: Update on Current Imaging Status and Future Directions. J. Neuroimaging 2016, 26, 383–390. [Google Scholar] [CrossRef]
- Gaudino, S.; Marziali, G.; Pezzullo, G.; Guadalupi, P.; Giordano, C.; Infante, A.; Benenati, M.; Ramaglia, A.; Massimi, L.; Gessi, M.; et al. Role of Susceptibility-Weighted Imaging and Intratumoral Susceptibility Signals in Grading and Differentiating Pediatric Brain Tumors at 1.5 T: A Preliminary Study. Neuroradiology 2020, 62, 705–713. [Google Scholar] [CrossRef]
- Pinker, K.; Noebauer-Huhmann, I.M.; Stavrou, I.; Hoeftberger, R.; Szomolanyi, P.; Karanikas, G.; Weber, M.; Stadlbauer, A.; Knosp, E.; Friedrich, K.; et al. High-Resolution Contrast-Enhanced, Susceptibility-Weighted MR Imaging at 3T in Patients with Brain Tumors: Correlation with Positron-Emission Tomography and Histopathologic Findings. AJNR Am. J. Neuroradiol. 2007, 28, 1280–1286. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, H.S.; Jahng, G.-H.; Ryu, C.-W.; Park, S.M.; Kim, S.Y. Semiquantitative Assessment of Intratumoral Susceptibility Signals Using Non-Contrast-Enhanced High-Field High-Resolution Susceptibility-Weighted Imaging in Patients with Gliomas: Comparison with MR Perfusion Imaging. AJNR Am. J. Neuroradiol. 2009, 30, 1402–1408. [Google Scholar] [CrossRef]
- Schmainda, K.M.; Rand, S.D.; Joseph, A.M.; Lund, R.; Ward, B.D.; Pathak, A.P.; Ulmer, J.L.; Badruddoja, M.A.; Krouwer, H.G.J. Characterization of a First-Pass Gradient-Echo Spin-Echo Method to Predict Brain Tumor Grade and Angiogenesis. AJNR Am. J. Neuroradiol. 2004, 25, 1524–1532. [Google Scholar]
- Bachir, S.; Shah, S.; Shapiro, S.; Koehler, A.; Mahammedi, A.; Samy, R.N.; Zuccarello, M.; Schorry, E.; Sengupta, S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int. J. Mol. Sci. 2021, 22, 690. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Han, T.; Liu, X.; Long, C.; Xu, Z.; Geng, Y.; Zhang, B.; Deng, L.; Jing, M.; Zhou, J. Prediction of Meningioma Grade by Constructing a Clinical Radiomics Model Nomogram Based on Magnetic Resonance Imaging. Magn. Reson. Imaging 2023, 104, 16–22. [Google Scholar] [CrossRef]
- Patel, R.V.; Yao, S.; Huang, R.Y.; Bi, W.L. Application of Radiomics to Meningiomas: A Systematic Review. Neuro-Oncology 2023, 25, 1166–1176. [Google Scholar] [CrossRef]
- Prakash, B.V.; Kannan, A.R.; Santhiyakumari, N.; Kumarganesh, S.; Raja, D.S.S.; Hephzipah, J.J.; MartinSagayam, K.; Pomplun, M.; Dang, H. Meningioma Brain Tumor Detection and Classification Using Hybrid CNN Method and RIDGELET Transform. Sci. Rep. 2023, 13, 14522. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Watts, J.; Box, G.; Galvin, A.; Brotchie, P.; Trost, N.; Sutherland, T. Magnetic Resonance Imaging of Meningiomas: A Pictorial Review. Insights Imaging 2014, 5, 113–122. [Google Scholar] [CrossRef]
- Wu, Z.; Mittal, S.; Kish, K.; Yu, Y.; Hu, J.; Haacke, E.M. Identification of Calcification with MRI Using Susceptibility-Weighted Imaging: A Case Study. J. Magn. Reson. Imaging 2009, 29, 177–182. [Google Scholar] [CrossRef]
- Hale, A.T.; Wang, L.; Strother, M.K.; Chambless, L.B. Differentiating Meningioma Grade by Imaging Features on Magnetic Resonance Imaging. J. Clin. Neurosci. 2018, 48, 71–75. [Google Scholar] [CrossRef]
- Lee, K.-J.; Joo, W.-I.; Rha, H.-K.; Park, H.-K.; Chough, J.-K.; Hong, Y.-K.; Park, C.-K. Peritumoral Brain Edema in Meningiomas: Correlations between Magnetic Resonance Imaging, Angiography, and Pathology. Surg. Neurol. 2008, 69, 350–355; discussion 355. [Google Scholar] [CrossRef]
- Simis, A.; Pires de Aguiar, P.H.; Leite, C.C.; Santana, P.A., Jr.; Rosemberg, S.; Teixeira, M.J. Peritumoral Brain Edema in Benign Meningiomas: Correlation with Clinical, Radiologic, and Surgical Factors and Possible Role on Recurrence. Surg. Neurol. 2008, 70, 471–477; discussion 477. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 770–778. [Google Scholar]
- Dhawan, A.P. Medical Image Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9780470922897. [Google Scholar]
- PyCaret 3.0. Available online: https://pycaret.gitbook.io/docs/ (accessed on 27 November 2023).
- Liu, F.T.; Ting, K.M.; Zhou, Z.-H. Isolation Forest. In Proceedings of the 2008 Eighth IEEE International Conference on Data Mining, Pisa, Italy, 15–19 December 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 413–422. [Google Scholar]
- Hastie, T.; Friedman, J.; Tibshirani, R. The Elements of Statistical Learning; Springer: New York, NY, USA, 2009. [Google Scholar]
- Richardson, M. Principal Component Analysis. 2009. Available online: http://people.maths.ox.ac.uk/richardsonm/SignalProcPCA.pdf (accessed on 3 May 2013).
- Raschka, S.; Liu, Y.; Mirjalili, V.; Dzhulgakov, D. Machine Learning with PyTorch and Scikit-Learn: Develop Machine Learning and Deep Learning Models with Python; Packt Publishing Ltd.: Birmingham, UK, 2022; ISBN 9781801816380. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-Sampling Technique. Jair 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Fisher, R.A. The Use of Multiple Measurements in Taxonomic Problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9780470582473. [Google Scholar]
- Swain, P.H.; Hauska, H. The Decision Tree Classifier: Design and Potential. IEEE Trans. Geosci. Remote Sens. 1977, 15, 142–147. [Google Scholar] [CrossRef]
- Zhang, H. The optimality of Naive Bayes. In Proceedings of the Seventeenth International Florida Artificial Intelligence Research Society Conference, FLAIRS 2004, Miami Beach, FL, USA, 12–15 May 2004; pp. 562–567. [Google Scholar]
- Rojas, R. AdaBoost and the Super Bowl of Classifiers a Tutorial Introduction to Adaptive Boosting. Freie Univ. Berl. Tech. Rep. 2009, 1, 1–6. [Google Scholar]
- Blagus, R.; Lusa, L. Gradient Boosting for High-Dimensional Prediction of Rare Events. Comput. Stat. Data Anal. 2017, 113, 19–37. [Google Scholar] [CrossRef]
- Alzamzami, F.; Hoda, M.; El Saddik, A. Light Gradient Boosting Machine for General Sentiment Classification on Short Texts: A Comparative Evaluation. IEEE Access 2020, 8, 101840–101858. [Google Scholar] [CrossRef]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V. Xgboost: Extreme Gradient Boosting. R Package Version 0.4-2 2015, 1, 1–4. [Google Scholar]
- Parmar, A.; Katariya, R.; Patel, V. A Review on Random Forest: An Ensemble Classifier. In Proceedings of the International Conference on Intelligent Data Communication Technologies and Internet of Things (ICICI) 2018, Coimbatore, India, 7–8 August 2018; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Bhati, B.S.; Rai, C.S. Ensemble Based Approach for Intrusion Detection Using Extra Tree Classifier. In Intelligent Computing in Engineering; Springer: Singapore, 2020; pp. 213–220. [Google Scholar]
- Donato, R. S100: A Multigenic Family of Calcium-Modulated Proteins of the EF-Hand Type with Intracellular and Extracellular Functional Roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N. Merlin, the NF2 Gene Product. Pathol. Oncol. Res. 2013, 19, 365–373. [Google Scholar] [CrossRef]
- Xiao, G.-H.; Chernoff, J.; Testa, J.R. NF2: The Wizardry of Merlin. Genes Chromosomes Cancer 2003, 38, 389–399. [Google Scholar] [CrossRef]
- Kluwe, L.; Friedrich, R.E.; Hagel, C.; Lindenau, M.; Mautner, V.F. Mutations and Allelic Loss of the NF2 Gene in Neurofibromatosis 2-Associated Skin Tumors. J. Investig. Dermatol. 2000, 114, 1017–1021. [Google Scholar] [CrossRef][Green Version]
- Pemov, A.; Dewan, R.; Hansen, N.F.; Chandrasekharappa, S.C.; Ray-Chaudhury, A.; Jones, K.; Luo, W.; Heiss, J.D.; Mullikin, J.C.; Chittiboina, P.; et al. Comparative Clinical and Genomic Analysis of Neurofibromatosis Type 2-Associated Cranial and Spinal Meningiomas. Sci. Rep. 2020, 10, 12563. [Google Scholar] [CrossRef]
- Goutagny, S.; Bah, A.B.; Henin, D.; Parfait, B.; Grayeli, A.B.; Sterkers, O.; Kalamarides, M. Long-Term Follow-up of 287 Meningiomas in Neurofibromatosis Type 2 Patients: Clinical, Radiological, and Molecular Features. Neuro-Oncology 2012, 14, 1090–1096. [Google Scholar] [CrossRef]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schumacher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic Landscape of High-Grade Meningiomas. NPJ Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef]
- Das, D.K. Psammoma Body: A Product of Dystrophic Calcification or of a Biologically Active Process That Aims at Limiting the Growth and Spread of Tumor? Diagn. Cytopathol. 2009, 37, 534–541. [Google Scholar] [CrossRef]
- Morin, O.; Chen, W.C.; Nassiri, F.; Susko, M.; Magill, S.T.; Vasudevan, H.N.; Wu, A.; Vallières, M.; Gennatas, E.D.; Valdes, G.; et al. Integrated Models Incorporating Radiologic and Radiomic Features Predict Meningioma Grade, Local Failure, and Overall Survival. Neurooncol. Adv. 2019, 1, vdz011. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Thiele, F.; Shakirin, G.; Zopfs, D.; Faymonville, A.; Timmer, M.; Maintz, D.; Perkuhn, M.; Borggrefe, J. Fully Automated Detection and Segmentation of Meningiomas Using Deep Learning on Routine Multiparametric MRI. Eur. Radiol. 2019, 29, 124–132. [Google Scholar] [CrossRef]
- Mohsen, H.; El-Dahshan, E.-S.A.; El-Horbaty, E.-S.M.; Salem, A.-B.M. Classification Using Deep Learning Neural Networks for Brain Tumors. Future Comput. Inform. J. 2018, 3, 68–71. [Google Scholar] [CrossRef]
- Zuo, Q.; Zou, L.; Fan, C.; Li, D.; Jiang, H.; Liu, Y. Whole and Part Adaptive Fusion Graph Convolutional Networks for Skeleton-Based Action Recognition. Sensors 2020, 20, 7149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, C.; Guo, H.; Wang, J.; Zhao, X.; Lu, H. Attention CoupleNet: Fully Convolutional Attention Coupling Network for Object Detection. IEEE Trans. Image Process. 2019, 28, 113–126. [Google Scholar] [CrossRef]
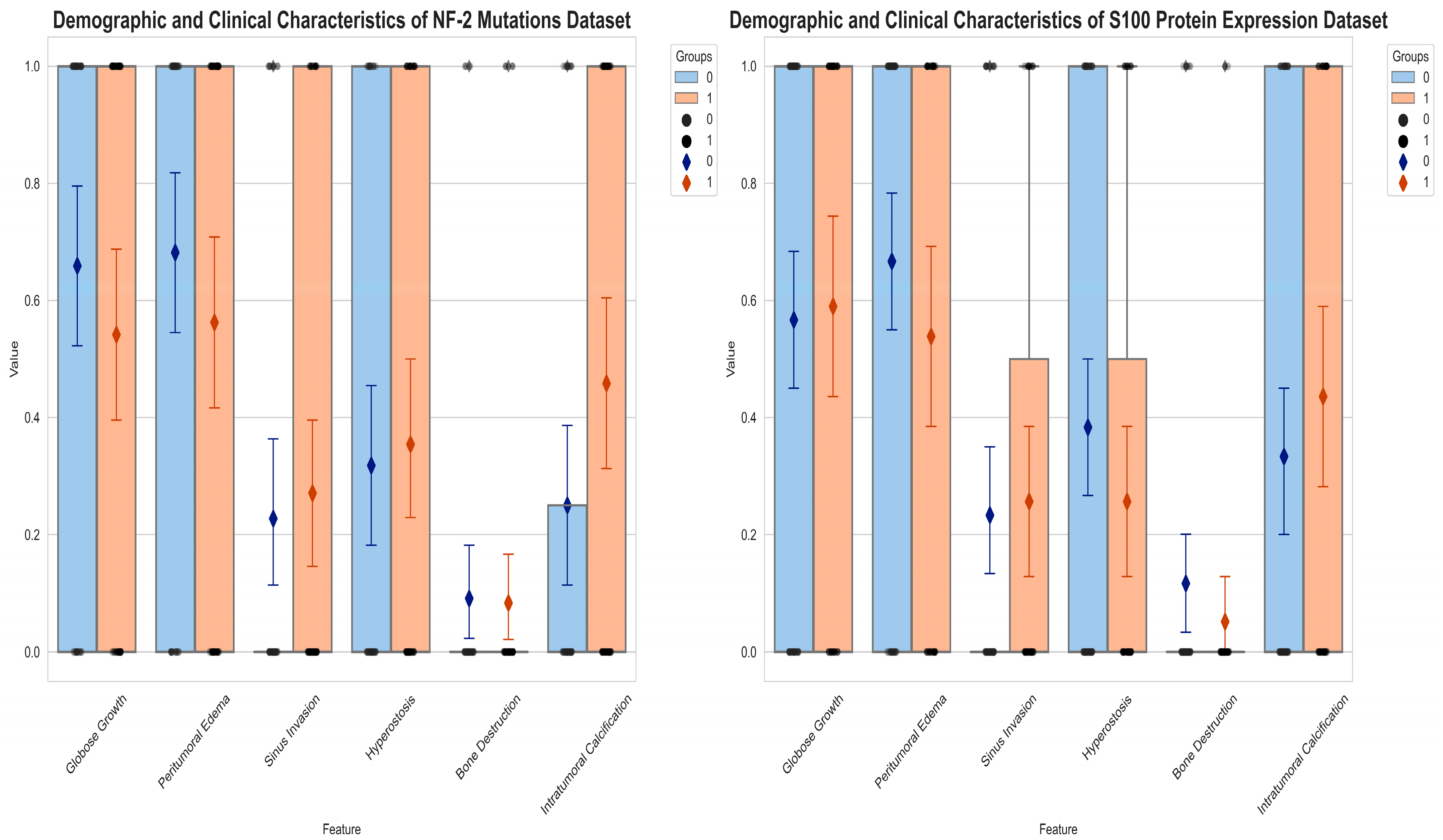

| Molecular Analysis | High Grade a | Peritumoral Edema a | Bone Destruction b | Hyperostosis a | Sinus Invasion a | Tumoral Calcification a | Globose Growth a |
|---|---|---|---|---|---|---|---|
| NF-2 Loss | 33/48 | 26/48 | 4/48 | 17/48 | 13/48 | 22/48 | 26/48 |
| NF-2 Intact | 19/44 | 13/44 | 4/44 | 14/44 | 10/44 | 11/44 | 29/44 |
| S100 (+) | 22/39 | 21/39 | 2/39 | 10/39 | 10/39 | 17/39 | 23/39 |
| S100 (−) | 34/60 | 40/60 | 7/60 | 23/60 | 14/60 | 20/60 | 34/60 |
| Algorithms | AUC | Accuracy | Recall | Precision | F1 | Kappa |
|---|---|---|---|---|---|---|
| Light Gradient Boosting Machine ** | 0.84 ± 0.10 | 0.75 ± 0.13 | 0.70 ± 0.17 | 0.79 ± 0.11 | 0.74 ± 0.13 | 0.51 ± 0.26 |
| Extreme Gradient Boosting | 0.78 ± 0.05 | 0.68 ± 0.09 | 0.63 ± 0.05 | 0.75 ± 0.16 | 0.68 ± 0.07 | 0.37 ± 0.19 |
| Extra Trees Classifier | 0.65 ± 0.11 | 0.57 ± 0.11 | 0.67 ± 0.16 | 0.58 ± 0.08 | 0.61 ± 0.10 | 0.15 ± 0.23 |
| Logistic Regression | 0.65 ± 0.10 | 0.61 ± 0.10 | 0.66 ± 0.11 | 0.66 ± 0.18 | 0.64 ± 0.06 | 0.21 ± 0.21 |
| Random Forest Classifier | 0.62 ± 0.12 | 0.56 ± 0.07 | 0.64 ± 0.23 | 0.57 ± 0.05 | 0.58 ± 0.11 | 0.12 ± 0.16 |
| AdaBoost Classifier | 0.61 ± 0.19 | 0.56 ± 0.18 | 0.65 ± 0.32 | 0.53 ± 0.16 | 0.57 ± 023 | 0.14 ± 0.35 |
| Gradient Boosting Classifier | 0.60 ± 0.07 | 0.59 ± 0.10 | 0.58 ± 0.18 | 0.66 ± 0.19 | 0.58 ± 0.11 | 0.19 ± 0.21 |
| Linear Discriminant Analysis | 0.58 ± 0.15 | 0.53 ± 0.03 | 0.60 ± 0.10 | 0.54 ± 0.04 | 0.56 ± 0.04 | 0.06 ± 0.09 |
| K-Neighbors Classifier | 0.54 ± 0.20 | 0.53 ± 0.12 | 0.66 ± 0.12 | 0.53 ± 0.11 | 0.59 ± 0.11 | 0.05 ± 0.24 |
| Naive Bayes | 0.53 ± 0.13 | 0.55 ± 0.15 | 0.54 ± 0.15 | 0.61 ± 0.21 | 0.55 ± 0.12 | 0.09 ± 0.30 |
| Decision Tree Classifier | 0.53 ± 0.08 | 0.52 ± 0.08 | 0.60 ± 0.11 | 0.54 ± 0.08 | 0.56 ± 0.07 | 0.05 ± 0.16 |
| Quadratic Discriminant Analysis | 0.49 ± 0.09 | 0.49 ± 0.09 | 0.50 ± 0.20 | 0.48 ± 0.14 | 0.49 ± 0.17 | −0.01 ± 0.20 |
| Algorithms | AUC | Accuracy | Recall | Precision | F1 | Kappa |
|---|---|---|---|---|---|---|
| Extra Trees Classifier | 0.80 ± 0.12 | 0.72 ± 0.11 | 0.46 ± 0.22 | 0.79 ± 0.19 | 0.54 ± 0.19 | 0.38 ± 0.24 |
| Naive Bayes | 0.79 ± 0.11 | 0.69 ± 0.06 | 0.37 ± 0.17 | 0.77 ± 0.20 | 0.47 ± 0.15 | 0.29 ± 0.15 |
| Linear Discriminant Analysis | 0.78 ± 0.15 | 0.71 ± 0.12 | 0.61 ± 0.28 | 0.70 ± 0.19 | 0.59 ± 0.21 | 0.39 ± 0.27 |
| Light Gradient Boosting Machine ** | 0.78 ± 0.002 | 0.78 ± 0.08 | 0.77 ± 0.09 | 0.70 ± 0.09 | 0.74 ± 0.08 | 0.55 ± 0.15 |
| Logistic Regression | 0.75 ± 0.13 | 0.68 ± 0.11 | 0.56 ± 0.24 | 0.58 ± 0.13 | 0.55 ± 0.20 | 0.32 ± 0.25 |
| K-Neighbors Classifier | 0.72 ± 0.12 | 0.45 ± 0.04 | 0.96 ± 0.06 | 0.41 ± 0.02 | 0.57 ± 0.03 | 0.06 ± 0.08 |
| Random Forest Classifier | 0.72 ± 0.11 | 0.63 ± 0.06 | 0.38 ± 0.20 | 0.45 ± 0.23 | 0.40 ± 0.21 | 0.19 ± 0.13 |
| Extreme Gradient Boosting | 0.64 ± 0.16 | 0.64 ± 0.10 | 0.48 ± 0.10 | 0.58 ± 0.21 | 0.51 ± 0.12 | 0.24 ± 0.21 |
| Quadratic Discriminant Analysis | 0.56 ± 0.13 | 0.58 ± 0.04 | 0.10 ± 0.08 | 0.35 ± 0.37 | 0.15 ± 0.12 | −0.01 ± 0.12 |
| AdaBoost Classifier | 0.54 ± 0.10 | 0.53 ± 0.10 | 0.37 ± 0.16 | 0.41 ± 0.15 | 0.37 ± 0.15 | 0.02 ± 0.20 |
| Decision Tree Classifier | 0.51 ± 0.10 | 0.52 ± 0.11 | 0.52 ± 0.07 | 0.41 ± 0.07 | 0.46 ± 0.07 | 0.03 ± 0.19 |
| Gradient Boosting Classifier | 0.45 ± 0.05 | 0.52 ± 0.06 | 0.52 ± 0.18 | 0.41 ± 0.05 | 0.44 ± 0.07 | 0.03 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azamat, S.; Buz-Yalug, B.; Dindar, S.S.; Yilmaz Tan, K.; Ozcan, A.; Can, O.; Ersen Danyeli, A.; Pamir, M.N.; Dincer, A.; Ozduman, K.; et al. Susceptibility-Weighted MRI for Predicting NF-2 Mutations and S100 Protein Expression in Meningiomas. Diagnostics 2024, 14, 748. https://doi.org/10.3390/diagnostics14070748
Azamat S, Buz-Yalug B, Dindar SS, Yilmaz Tan K, Ozcan A, Can O, Ersen Danyeli A, Pamir MN, Dincer A, Ozduman K, et al. Susceptibility-Weighted MRI for Predicting NF-2 Mutations and S100 Protein Expression in Meningiomas. Diagnostics. 2024; 14(7):748. https://doi.org/10.3390/diagnostics14070748
Chicago/Turabian StyleAzamat, Sena, Buse Buz-Yalug, Sukru Samet Dindar, Kubra Yilmaz Tan, Alpay Ozcan, Ozge Can, Ayca Ersen Danyeli, M. Necmettin Pamir, Alp Dincer, Koray Ozduman, and et al. 2024. "Susceptibility-Weighted MRI for Predicting NF-2 Mutations and S100 Protein Expression in Meningiomas" Diagnostics 14, no. 7: 748. https://doi.org/10.3390/diagnostics14070748
APA StyleAzamat, S., Buz-Yalug, B., Dindar, S. S., Yilmaz Tan, K., Ozcan, A., Can, O., Ersen Danyeli, A., Pamir, M. N., Dincer, A., Ozduman, K., & Ozturk-Isik, E. (2024). Susceptibility-Weighted MRI for Predicting NF-2 Mutations and S100 Protein Expression in Meningiomas. Diagnostics, 14(7), 748. https://doi.org/10.3390/diagnostics14070748






