Comparison between Two Adaptive Optics Methods for Imaging of Individual Retinal Pigmented Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Adaptive Optics Ophthalmoscopes
- Transscleral Flood Illumination (TFI) ophthalmoscope
- The TFI is an AO retinal camera with a transscleral flood illumination system (rtx1 TFI, Imagine Eyes, France). The ophthalmoscope uses two LED arrays at an 810 nm wavelength, which shine light through the sclera from both sides of the pupil (Figure 1) and generates images showing the boundaries of RPE cells as bright pixels and the centers of each cell as darker pixels. The acquisition time is 6 s per video.
- NIRAF Adaptive Optics Scanning Laser Ophthalmoscopes (AOSLO)
- Subjects 1 and 4 were imaged with the Paris near-infrared autofluorescence (NIRAF) AOSLO situated at the Quinze-Vingts Hospital, which has been previously described [11]. Subjects 2 and 3 were imaged with the NIRAF AOSLO situated at the Pittsburgh Vision Institute detailed in [21]. Both systems exploited near-infrared excitation of RPE fluorophores with light at similar wavelengths of 757 nm and 720 nm, respectively, which was used to generate images of the RPE cell mosaic in all subjects through detection of the autofluorescence. Image sequences from both systems were registered and corrected for distortions using a custom algorithm described in [22] and then averaged. Both NIRAF system characteristics can be found in Table 1.
2.2. Cohort Description and Image Acquisition
2.3. Image Processing and Analysis
3. Results
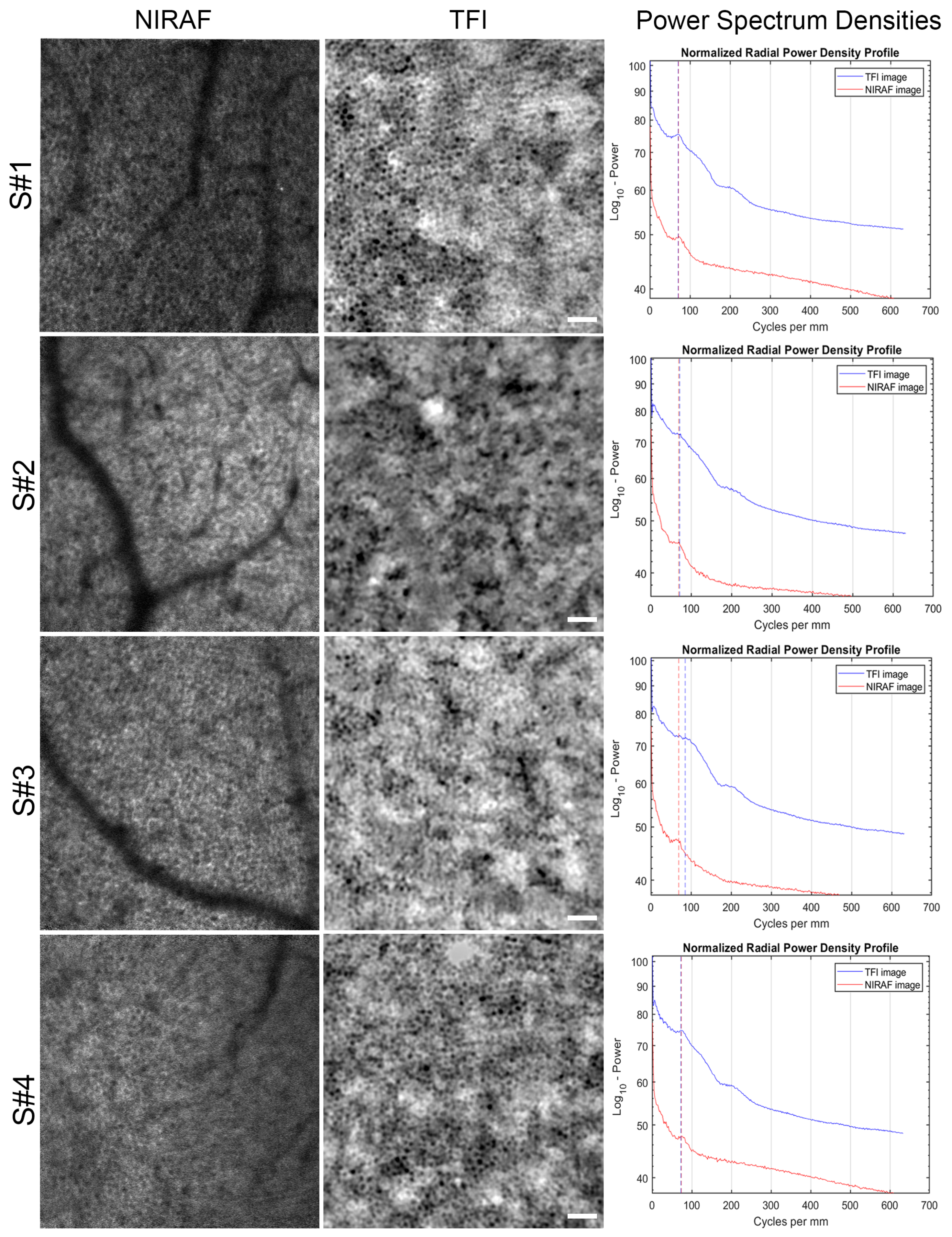
3.1. Images of TFI and NIRAF of Same Regions
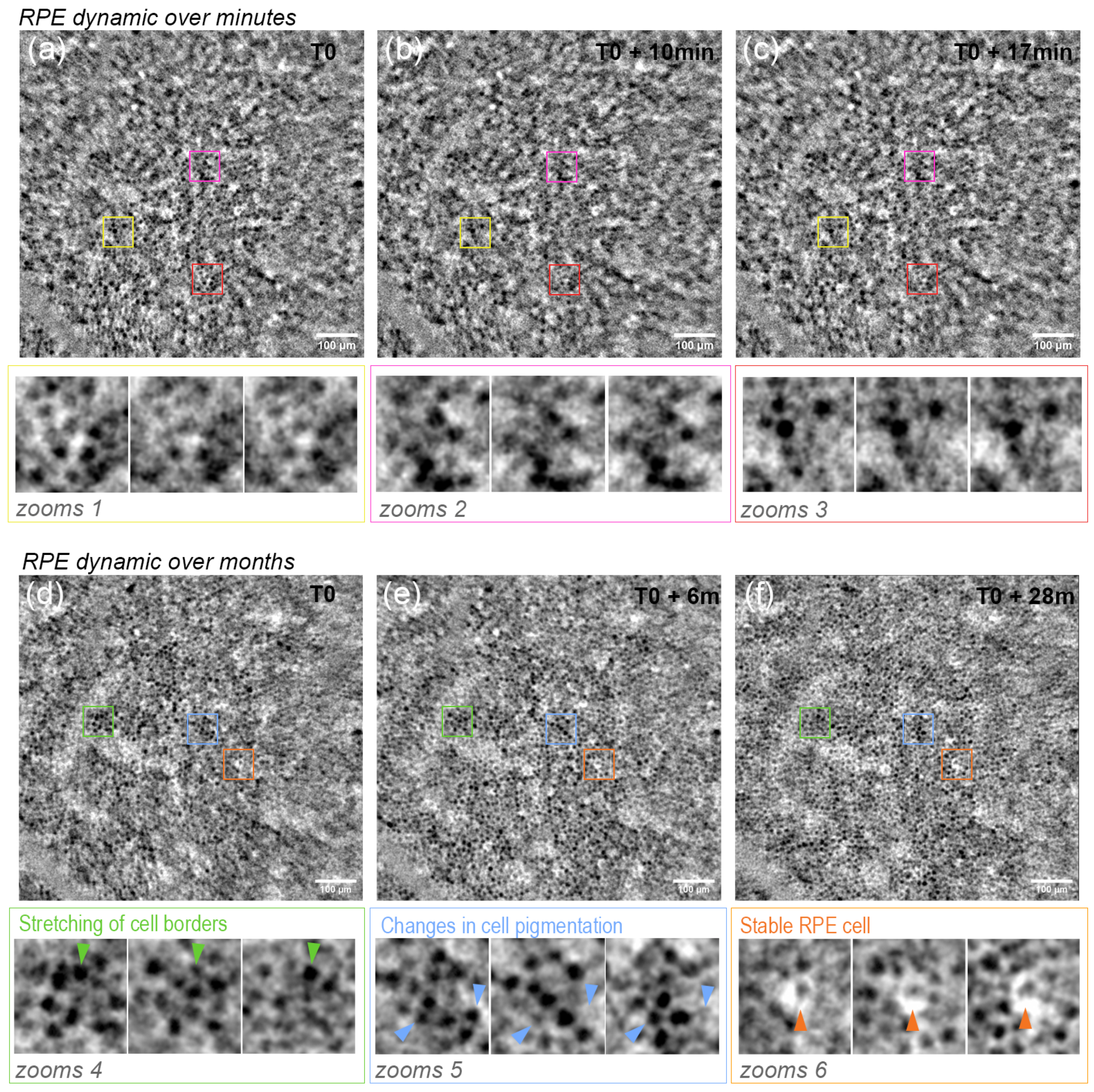
3.2. RPE Mosaic Variations with Time in Healthy Retina
4. Discussion
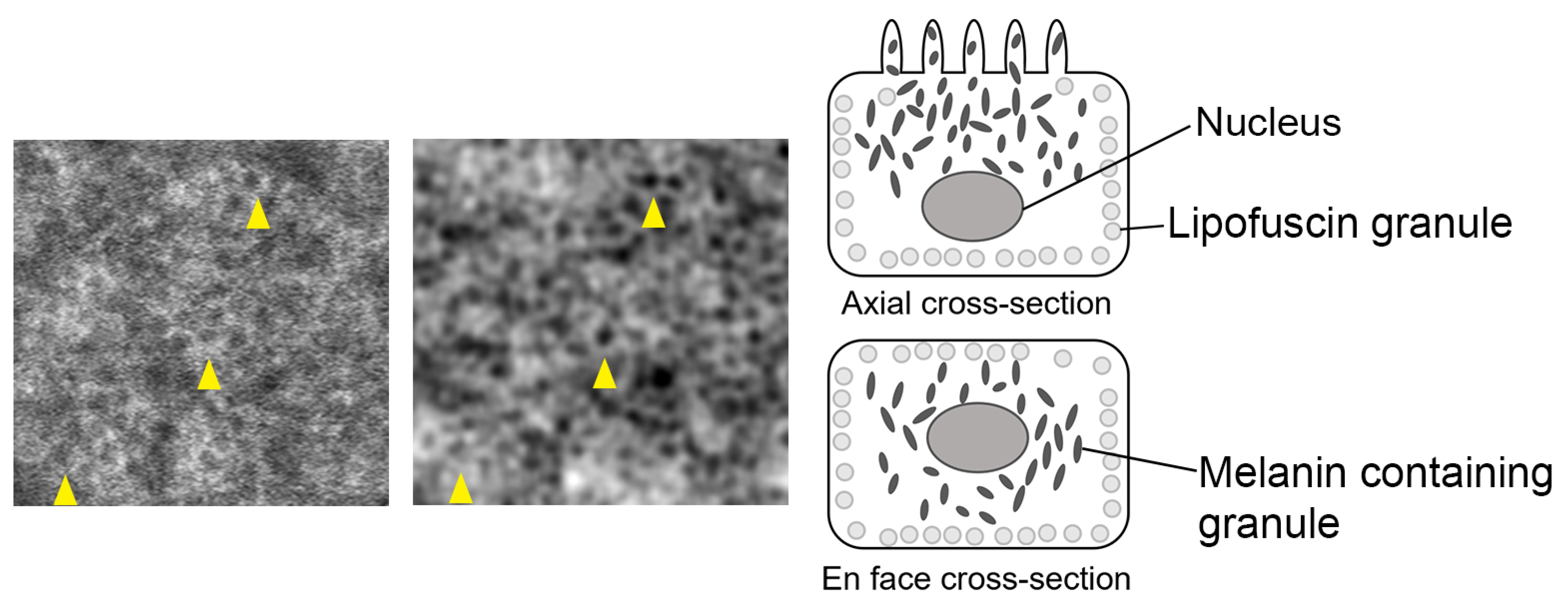
4.1. Origin of RPE Cell Contrast
4.2. RPE In Vivo Signal Variation with Eccentricity
4.3. RPE Pigmentation Dynamic
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Montages of TFI and NIRAF Images from Other Subjects
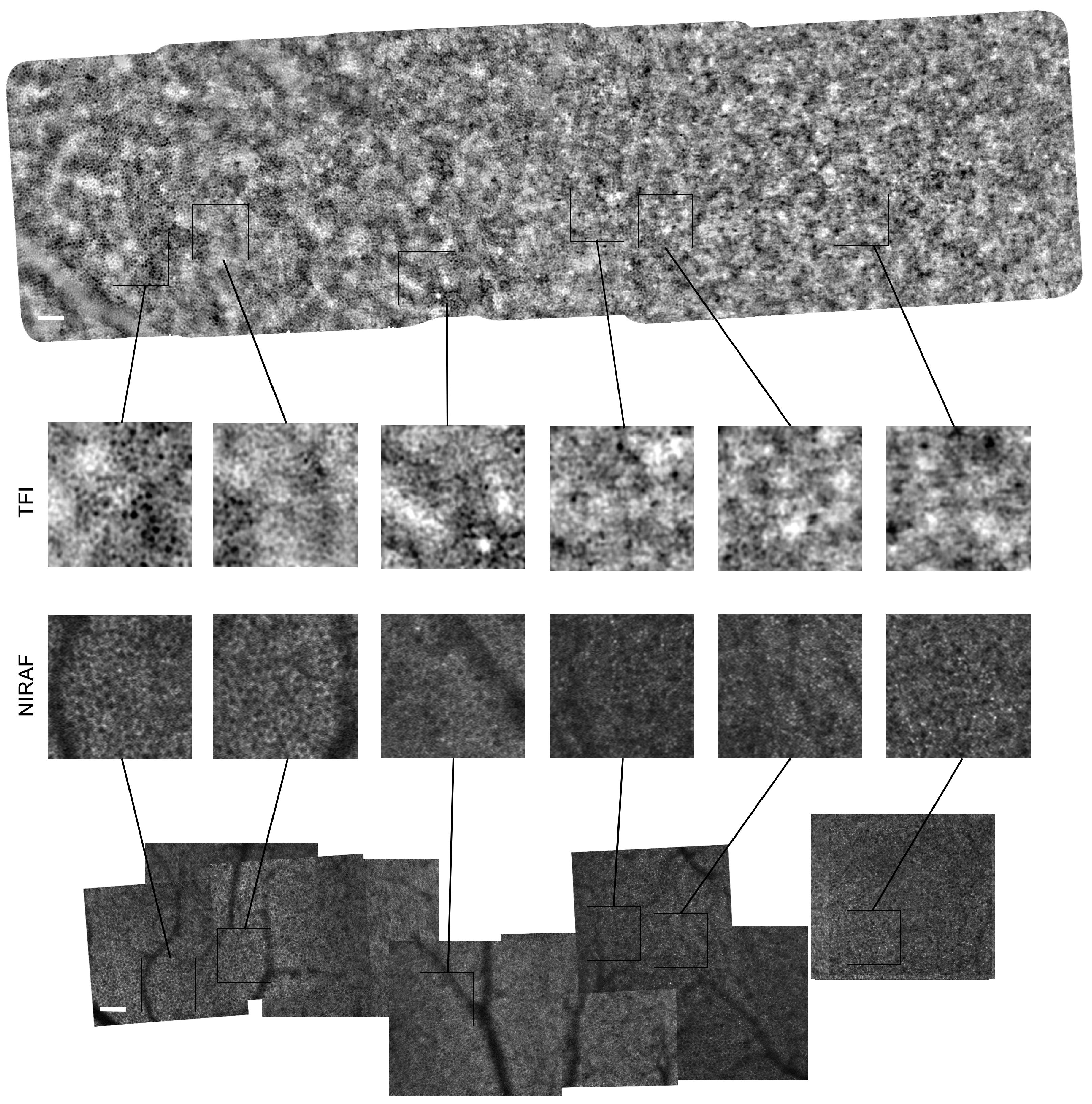
Appendix A.1. Montages of Subject #2
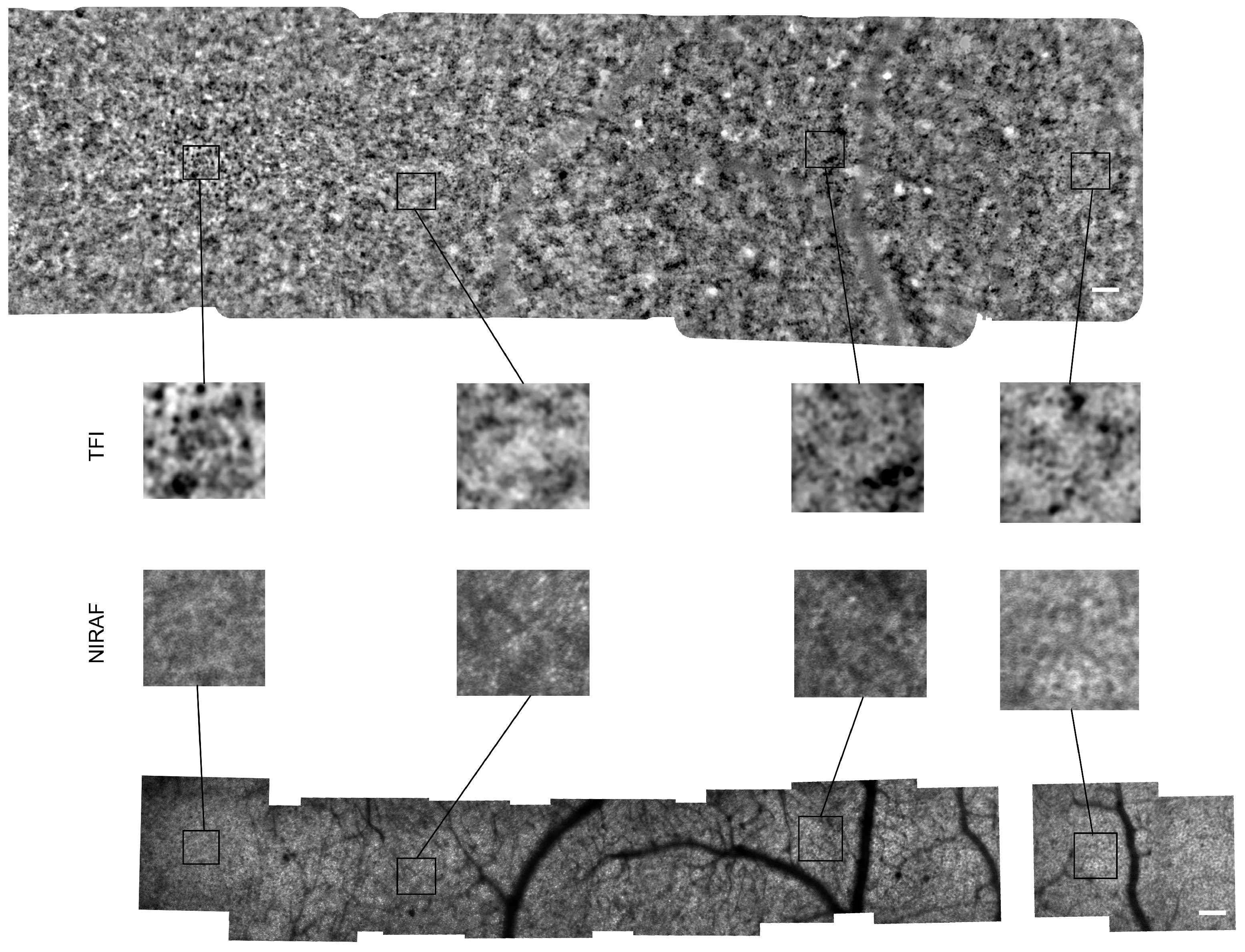
Appendix A.2. Montages of Subject #3

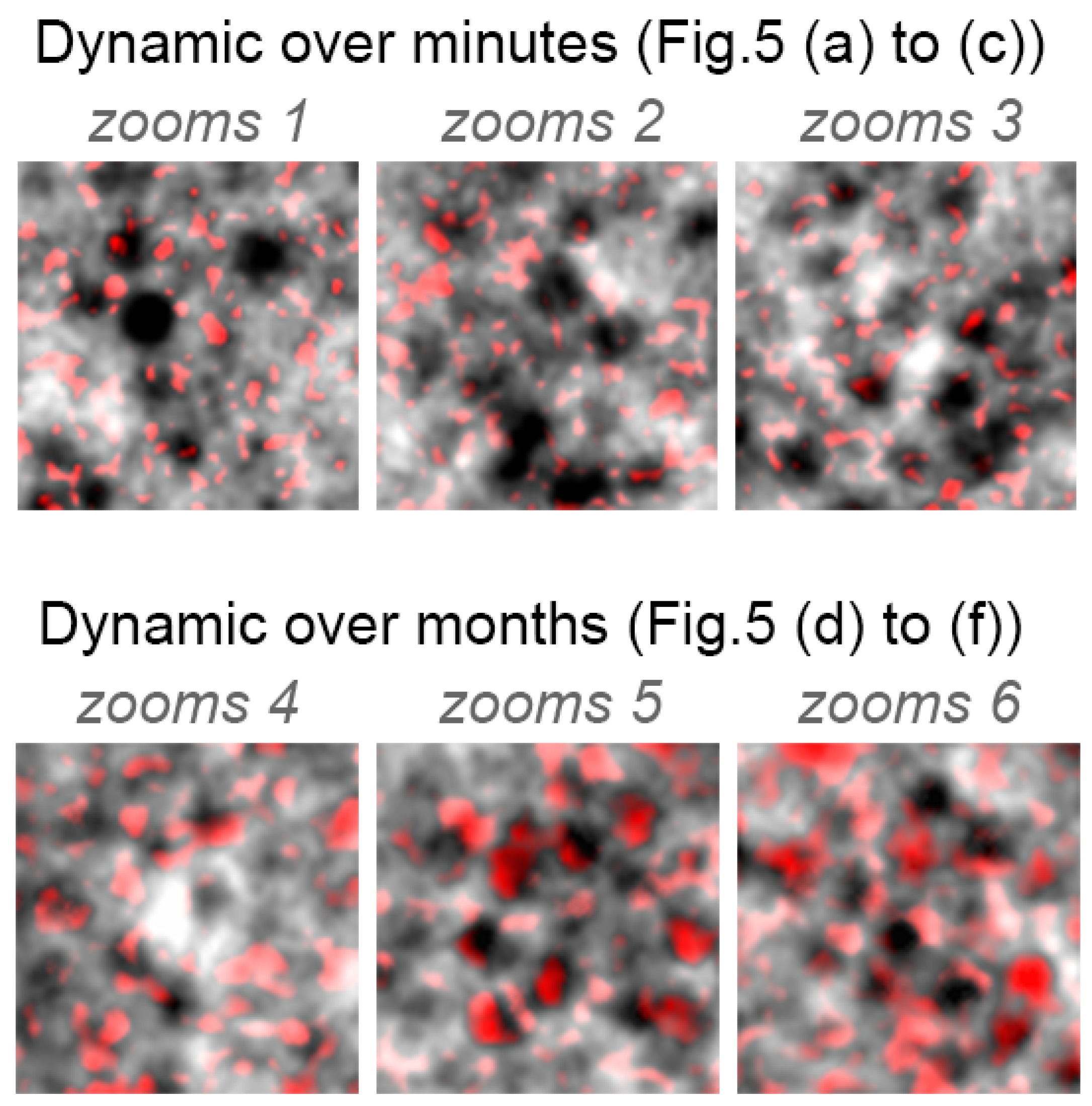
Appendix B. Colored Standard Deviations for Zooms 1–6 (from Figure 5)
References
- Prokofyeva, E.; Zrenner, E. Epidemiology of major eye diseases leading to blindness in Europe: A literature review. Ophthalmic Res. 2012, 47, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, P.; Dandona, R.; Prasad, M.N.; Kovai, V.; Dandona, L. Fear of blindness and perceptions about blind people. The Andhra Pradesh Eye Disease Study. Indian J. Ophthalmol. 2002, 50, 239–246. [Google Scholar]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelium in exudative age-related macular degeneration. Ophthalmol. Retin. 2019, 3, 850–859. [Google Scholar] [CrossRef]
- Ahlers, C.; Götzinger, E.; Pircher, M.; Golbaz, I.; Prager, F.; Schütze, C.; Baumann, B.; Hitzenberger, C.K.; Schmidt-Erfurth, U. Imaging of the retinal pigment epithelium in age-related macular degeneration using polarization-sensitive optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2149–2157. [Google Scholar] [CrossRef]
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995, 36, 718–729. [Google Scholar]
- Sparrow, J.R.; Duncker, T. Fundus autofluorescence and RPE lipofuscin in age-related macular degeneration. J. Clin. Med. 2014, 3, 1302–1321. [Google Scholar] [CrossRef]
- Morgan, J.I.; Hunter, J.J.; Masella, B.; Wolfe, R.; Gray, D.C.; Merigan, W.H.; Delori, F.C.; Williams, D.R. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3715–3729. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.I.; Dubra, A.; Wolfe, R.; Merigan, W.H.; Williams, D.R. In vivo autofluorescence imaging of the human and macaque retinal pigment epithelial cell mosaic. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Morgan, J.I.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef]
- Granger, C.E.; Yang, Q.; Song, H.; Saito, K.; Nozato, K.; Latchney, L.R.; Leonard, B.T.; Chung, M.M.; Williams, D.R.; Rossi, E.A. Human retinal pigment epithelium: In vivo cell morphometry, multispectral autofluorescence, and relationship to cone mosaic. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5705–5716. [Google Scholar] [CrossRef]
- Grieve, K.; Gofas-Salas, E.; Ferguson, R.D.; Sahel, J.A.; Paques, M.; Rossi, E.A. In vivo near-infrared autofluorescence imaging of retinal pigment epithelial cells with 757 nm excitation. Biomed. Opt. Express 2018, 9, 5946–5961. [Google Scholar] [CrossRef]
- Liu, T.; Jung, H.; Liu, J.; Droettboom, M.; Tam, J. Noninvasive near infrared autofluorescence imaging of retinal pigment epithelial cells in the human retina using adaptive optics. Biomed. Opt. Express 2017, 8, 4348–4360. [Google Scholar] [CrossRef]
- Bower, A.J.; Liu, T.; Aguilera, N.; Li, J.; Liu, J.; Lu, R.; Giannini, J.P.; Huryn, L.A.; Dubra, A.; Liu, Z.; et al. Integrating adaptive optics-SLO and OCT for multimodal visualization of the human retinal pigment epithelial mosaic. Biomed. Opt. Express 2021, 12, 1449–1466. [Google Scholar] [CrossRef]
- Shirazi, M.F.; Brunner, E.; Laslandes, M.; Pollreisz, A.; Hitzenberger, C.K.; Pircher, M. Visualizing human photoreceptor and retinal pigment epithelium cell mosaics in a single volume scan over an extended field of view with adaptive optics optical coherence tomography. Biomed. Opt. Express 2020, 11, 4520–4535. [Google Scholar] [CrossRef]
- Liu, Z.; Kocaoglu, O.P.; Miller, D.T. 3D imaging of retinal pigment epithelial cells in the living human retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT533–OCT543. [Google Scholar] [CrossRef]
- Liu, Z.; Kurokawa, K.; Hammer, D.X.; Miller, D.T. In vivo measurement of organelle motility in human retinal pigment epithelial cells. Biomed. Opt. Express 2019, 10, 4142–4158. [Google Scholar] [CrossRef]
- Taubitz, T.; Fang, Y.; Biesemeier, A.; Julien-Schraermeyer, S.; Schraermeyer, U. Age, lipofuscin and melanin oxidation affect fundus near-infrared autofluorescence. EBioMedicine 2019, 48, 592–604. [Google Scholar] [CrossRef]
- Laforest, T.; Künzi, M.; Kowalczuk, L.; Carpentras, D.; Behar-Cohen, F.; Moser, C. Transscleral optical phase imaging of the human retina. Nat. Photonics 2020, 14, 439–445. [Google Scholar] [CrossRef]
- Simon-Zoula, S.; Kowalczuk, L.; Kunzi, M.; Iskandar, A.; Misutkova, Z.; Gryczka, A.; Navarro, A.; Jeunet, F.; Mantel, I.; Behar-Cohen, F.F.; et al. Human retinal pigment epithelium cells can be imaged in vivo with a novel adaptive optics camera using transscleral illumination. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1056-F0303. [Google Scholar]
- Kowalczuk, L.; Dornier, R.; Kunzi, M.; Iskandar, A.; Misutkova, Z.; Gryczka, A.; Navarro, A.; Jeunet, F.; Mantel, I.; Behar-Cohen, F.; et al. In Vivo Retinal Pigment Epithelium Imaging using Transscleral Optical Imaging in Healthy Eyes. Ophthalmol. Sci. 2023, 3, 100234. [Google Scholar] [CrossRef]
- Vienola, K.V.; Zhang, M.; Snyder, V.C.; Sahel, J.A.; Dansingani, K.K.; Rossi, E.A. Microstructure of the retinal pigment epithelium near-infrared autofluorescence in healthy young eyes and in patients with AMD. Sci. Rep. 2020, 10, 9561. [Google Scholar] [CrossRef]
- Zhang, M.; Gofas-Salas, E.; Leonard, B.T.; Rui, Y.; Snyder, V.C.; Reecher, H.M.; Mecê, P.; Rossi, E.A. Strip-based digital image registration for distortion minimization and robust eye motion measurement from scanned ophthalmic imaging systems. Biomed. Opt. Express 2021, 12, 2353–2372. [Google Scholar] [CrossRef]
- Lee, D.M.; Zhang, M.; Snyder, V.C.; Rossi, E.A. Evaluating the multispectral autofluorescence (AF) of retinal pigmented epithelial (RPE) cells in healthy eyes with fluorescence adaptive optics scanning light ophthalmoscopy (AOSLO). Investig. Ophthalmol. Vis. Sci. 2023, 64, 1056. [Google Scholar]
- Chen, M.; Cooper, R.F.; Han, G.K.; Gee, J.; Brainard, D.H.; Morgan, J.I. Multi-modal automatic montaging of adaptive optics retinal images. Biomed. Opt. Express 2016, 7, 4899–4918. [Google Scholar] [CrossRef]
- Li, K.Y.; Tiruveedhula, P.; Roorda, A. Intersubject variability of foveal cone photoreceptor density in relation to eye length. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6858–6867. [Google Scholar] [CrossRef]
- Cooper, R.F.; Langlo, C.S.; Dubra, A.; Carroll, J. Automatic detection of modal spacing (Yellott’s ring) in adaptive optics scanning light ophthalmoscope images. Ophthalmic Physiol. Opt. 2013, 33, 540–549. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Rashid, A.; Chrenek, M.A.; Zhang, Q.; Bruce, B.B.; Klein, M.; Boatright, J.H.; Jiang, Y.; Grossniklaus, H.E.; Nickerson, J.M. Analysis of RPE morphometry in human eyes. Mol. Vis. 2016, 22, 898. [Google Scholar]
- Bermond, K.; Wobbe, C.; Tarau, I.S.; Heintzmann, R.; Hillenkamp, J.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent granules of the human retinal pigment epithelium: Phenotypes, intracellular distribution, and age-related topography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef]
- Bermond, K.; Berlin, A.; Tarau, I.S.; Wobbe, C.; Heintzmann, R.; Curcio, C.A.; Sloan, K.R.; Ach, T. Characteristics of normal human retinal pigment epithelium cells with extremes of autofluorescence or intracellular granule count. Ann. Eye Sci. 2021, 6, 3. [Google Scholar] [CrossRef]
- Starnes, A.C.; Huisingh, C.; MCGWIN, G.; Sloan, K.R.; Ablonczy, Z.; Smith, R.T.; Curcio, C.A.; Ach, T. Multi-nucleate retinal pigment epithelium cells of the human macula exhibit a characteristic and highly specific distribution. Vis. Neurosci. 2016, 33, E001. [Google Scholar] [CrossRef]
- Pollreisz, A.; Messinger, J.D.; Sloan, K.R.; Mittermueller, T.J.; Weinhandl, A.S.; Benson, E.K.; Kidd, G.J.; Schmidt-Erfurth, U.; Curcio, C.A. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp. Eye Res. 2018, 166, 131–139. [Google Scholar] [CrossRef]
- von der Emde, L.; Vaisband, M.; Hasenauer, J.; Bourauel, L.; Bermond, K.; Saßmannshausen, M.; Heintzmann, R.; Holz, F.G.; Curcio, C.A.; Sloan, K.R.; et al. Histologic cell shape descriptors for the retinal pigment epithelium in age-related macular degeneration: A comparison to unaffected eyes. Transl. Vis. Sci. Technol. 2022, 11, 19. [Google Scholar] [CrossRef]
- Bonilha, V.L. Age and disease-related structural changes in the retinal pigment epithelium. Clin. Ophthalmol. 2008, 2, 413–424. [Google Scholar] [CrossRef]
- Chen, M.; Rajapakse, D.; Fraczek, M.; Luo, C.; Forrester, J.V.; Xu, H. Retinal pigment epithelial cell multinucleation in the aging eye–a mechanism to repair damage and maintain homoeostasis. Aging Cell 2016, 15, 436–445. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R. Packing geometry of human cone photoreceptors: Variation with eccentricity and evidence for local anisotropy. Vis. Neurosci. 1992, 9, 169–180. [Google Scholar] [CrossRef]
- Feeney, L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Investig. Ophthalmol. Vis. Sci. 1978, 17, 583–600. [Google Scholar]
- Kwon, W.; Freeman, S.A. Phagocytosis by the retinal pigment epithelium: Recognition, resolution, recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]


| Paris NIRAF | Pittsburgh NIRAF | |
|---|---|---|
| Wavelength (nm) | 757 | 720 |
| Field of view (°) | 2 | 1.5 |
| Acquisition time (s) | 90 | 30 |
| Subjects | AO System | Regions Imaged | Year of Image Acquisition | Age of the Subject during Acquisition |
|---|---|---|---|---|
| Subject #1 | TFI | Fovea-10°T | 2023 | 31 yrs |
| 10°T | 2021 to 2023 | 29–31 yrs | ||
| Paris NIRAF | Fovea-10°T | 2017 | 25 yrs | |
| Subject #2 | TFI | Fovea-10°T | 2023 | 44 yrs |
| Pitt NIRAF | 8°–10°T | 2022 | 43 yrs | |
| Subject #3 | TFI | Fovea-10°T | 2023 | 25 yrs |
| Pitt NIRAF | 8°T | 2023 | 25 yrs | |
| Subject #4 | TFI | Fovea-10°T | 2023 | 28 yrs |
| Paris NIRAF | Fovea-10°T | 2017 | 22 yrs |
| S#1 | S#2 | S#3 | S#4 | Mean +/− SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TFI | NIRAF | TFI | NIRAF | TFI | NIRAF | TFI | NIRAF | TFI | NIRAF | |
| Modal spacing (cycles/deg) | 19.8 | 20.6 | 20.1 | 17.5 | 25.5 | 23.0 | 20.7 | 21.6 | 21.5 +/− 2.3 | 20.7 +/− 2 |
| Cell spacing (μm) | 16.9 | 16.3 | 16.7 | 19.2 | 13.2 | 14.6 | 16.2 | 15.5 | 15.7 +/− 1.5 | 16.4 +/− 1.7 |
| Cell density (cells/mm) | 4027 | 4327 | 4133 | 3117 | 6629 | 5411 | 4397 | 4777 | 4797 +/− 1066 | 4407 +/− 839 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gofas-Salas, E.; Lee, D.M.W.; Rondeau, C.; Grieve, K.; Rossi, E.A.; Paques, M.; Gocho, K. Comparison between Two Adaptive Optics Methods for Imaging of Individual Retinal Pigmented Epithelial Cells. Diagnostics 2024, 14, 768. https://doi.org/10.3390/diagnostics14070768
Gofas-Salas E, Lee DMW, Rondeau C, Grieve K, Rossi EA, Paques M, Gocho K. Comparison between Two Adaptive Optics Methods for Imaging of Individual Retinal Pigmented Epithelial Cells. Diagnostics. 2024; 14(7):768. https://doi.org/10.3390/diagnostics14070768
Chicago/Turabian StyleGofas-Salas, Elena, Daniel M. W. Lee, Christophe Rondeau, Kate Grieve, Ethan A. Rossi, Michel Paques, and Kiyoko Gocho. 2024. "Comparison between Two Adaptive Optics Methods for Imaging of Individual Retinal Pigmented Epithelial Cells" Diagnostics 14, no. 7: 768. https://doi.org/10.3390/diagnostics14070768
APA StyleGofas-Salas, E., Lee, D. M. W., Rondeau, C., Grieve, K., Rossi, E. A., Paques, M., & Gocho, K. (2024). Comparison between Two Adaptive Optics Methods for Imaging of Individual Retinal Pigmented Epithelial Cells. Diagnostics, 14(7), 768. https://doi.org/10.3390/diagnostics14070768






