Artificial Intelligence-Based Left Ventricular Ejection Fraction by Medical Students for Mortality and Readmission Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. The AI-Based Tool
2.3. Study Protocol
2.4. Sample Size Calculation
2.5. Statistical Analyses
3. Results
3.1. Baseline Demographic and Clinical Characteristics: AI-Based Preserved vs. Reduced LVEF (Table 1)
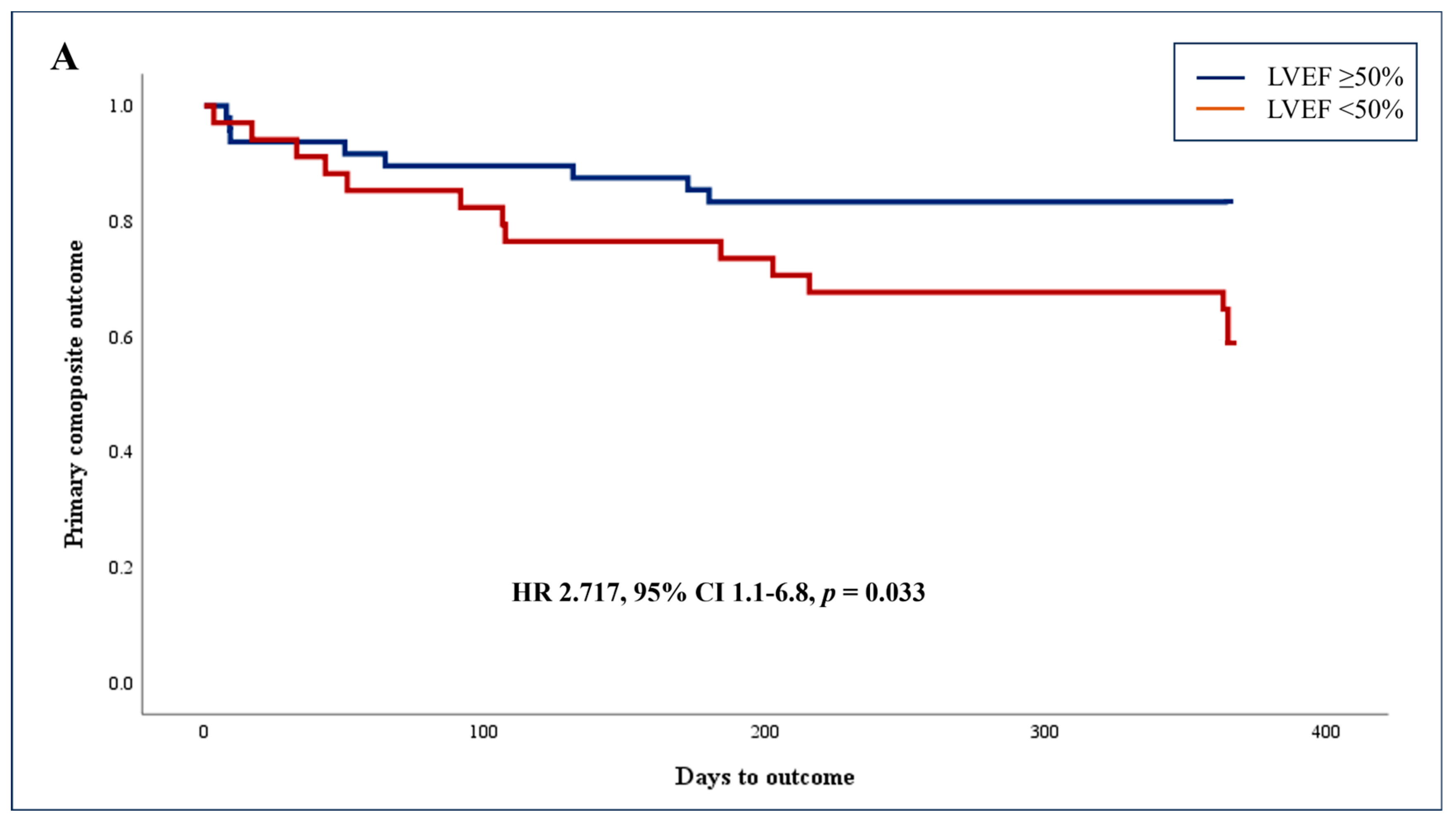
3.2. Primary Endpoint: Adjusted Association between AI-Based Reduced LV Systolic Function (LVEF < 50%) and Composite Outcome of 1-Year Mortality and Cardiovascular-Related Readmissions
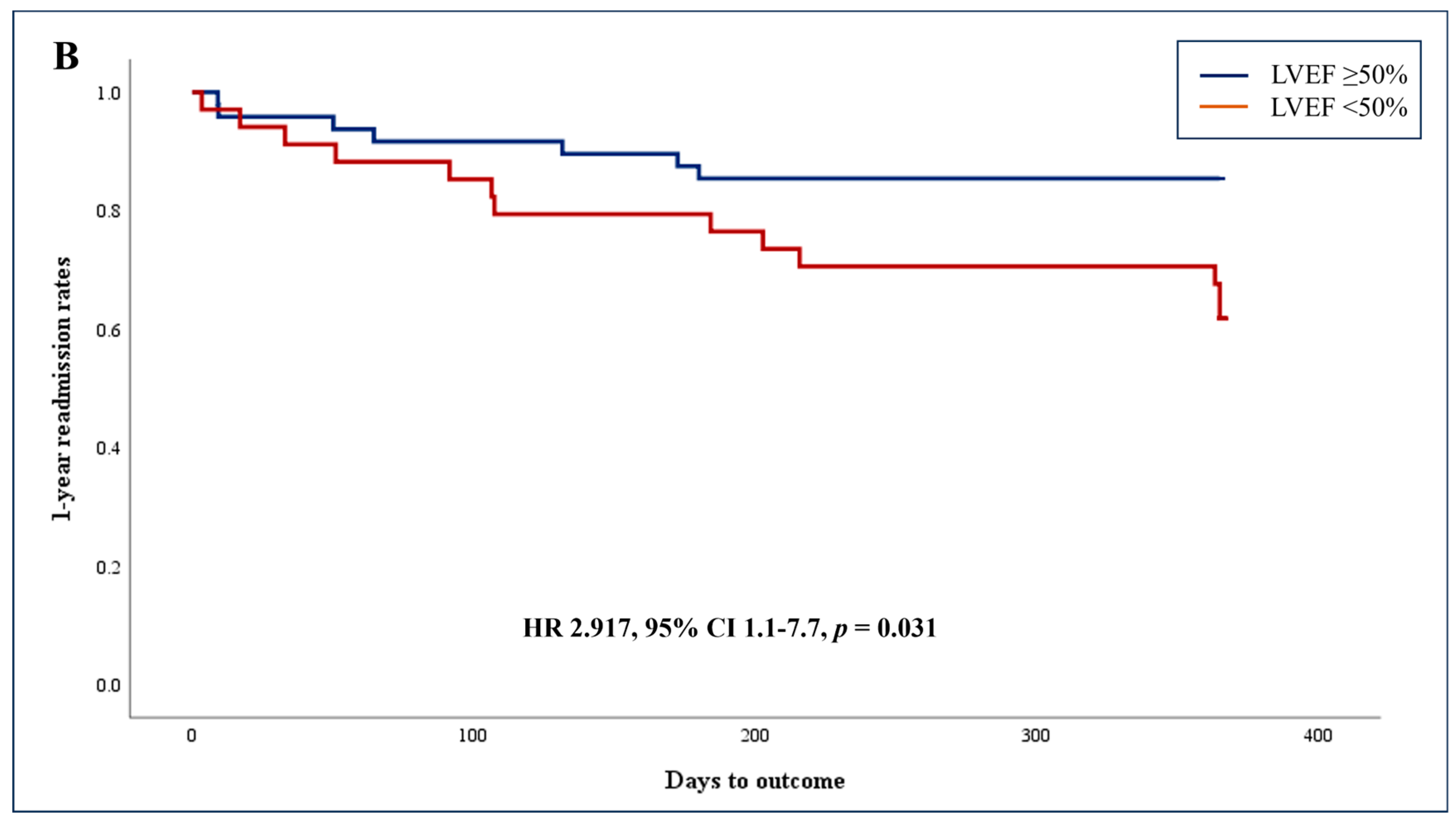
3.3. Unadjusted Association of AI-Based Reduced LVEF and Secondary Endpoints
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, L.; DeCara, J.M. Point-of-Care Ultrasound. Curr. Cardiol. Rep. 2020, 22, 149. [Google Scholar] [CrossRef]
- NCBI. Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann Emerg Med. 2017, 69, e27–e54. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; See, K.C. Point-of-care ultrasound for critically-ill patients: A mini-review of key diagnostic features and protocols. World J. Crit. Care Med. 2022, 11, 70–84. [Google Scholar] [CrossRef]
- Wong, A.; Chew, M.; Hernandez, G. Using ultrasound in ICU. Intensive Care Med. 2023, 49, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Rubin, D.; Baratta, J.L. Regional anesthesia and POCUS in the intensive care unit. Int. Anesthesiol. Clin. 2024, 62, 35–42. [Google Scholar] [CrossRef]
- Frantz, A.M.; Fahy, B.G. POCUS focus: Dilemmas of the technologically advanced device. J. Clin. Anesth. 2024, 92, 111305. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.M.; Zwemer, E.K.; Stephens, J.R.; Dancel, R. The State of Internal Medicine Point-of-Care Ultrasound (POCUS) Fellowships in the United States and Canada. Am. J. Med. 2023, 136, 830–836. [Google Scholar] [CrossRef]
- Tanner, C. Advancing Pocus Proficiency in Family Medicine Educators: A Comprehensive Overview of the STFM FM Pocus Educator Certificate Program. Ann. Fam. Med. 2023, 21, 559. [Google Scholar] [CrossRef]
- Gaspari, R.; Weekes, A.; Adhikari, S.; Noble, V.E.; Nomura, J.T.; Theodoro, D.; Woo, M.; Atkinson, P.; Blehar, D.; Brown, S.M.; et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 2016, 109, 33–39. [Google Scholar] [CrossRef]
- Gaspari, R.; Weekes, A.; Adhikari, S.; Noble, V.; Nomura, J.T.; Theodoro, D.; Woo, M.; Atkinson, P.; Blehar, D.; Brown, S.; et al. A retrospective study of pulseless electrical activity, bedside ultrasound identifies interventions during resuscitation associated with improved survival to hospital admission. A REASON Study. Resuscitation 2017, 120, 103–107. [Google Scholar] [CrossRef]
- Jones, A.E.; Tayal, V.S.; Sullivan, D.M.; Kline, J.A. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit. Care Med. 2004, 32, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, H.; Boniface, K.S.; Pourmand, A.; Liu, Y.T.; Davison, D.L.; Hawkins, K.D.; Buhumaid, R.E.; Salimian, M.; Yadav, K. Bedside Ultrasound Reduces Diagnostic Uncertainty and Guides Resuscitation in Patients With Undifferentiated Hypotension. Crit. Care Med. 2015, 43, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Stickles, S.P.; Carpenter, C.R.; Gekle, R.; Kraus, C.K.; Scoville, C.; Theodoro, D.; Tran, V.H.; Ubiñas, G.; Raio, C. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: A systematic review and meta-analysis. CJEM 2019, 21, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Canty, D.J.; Royse, C.F.; Kilpatrick, D.; Bowman, L.; Royse, A.G. The impact of focused transthoracic echocardiography in the pre-operative clinic. Anaesthesia 2012, 67, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Lenk, T.; Whittle, J.; Miller, T.E.; Williams, D.G.A.; Bronshteyn, Y.S. Focused cardiac ultrasound in preoperative assessment: The perioperative provider’s new stethoscope? Perioper. Med. 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Stokke, T.M.; Ruddox, V.; Sarvari, S.I.; Otterstad, J.E.; Aune, E.; Edvardsen, T. Brief group training of medical students in focused cardiac ultrasound may improve diagnostic accuracy of physical examination. J. Am. Soc. Echocardiogr. 2014, 27, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef]
- Unlüer, E.E.; Karagöz, A.; Akoğlu, H.; Bayata, S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J. Emerg. Med. 2014, 15, 221–226. [Google Scholar] [CrossRef]
- Johnson, B.K.; Tierney, D.M.; Rosborough, T.K.; Harris, K.M.; Newell, M.C. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J. Clin. Ultrasound 2016, 44, 92–99. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Belka, V.; Furlong, K.; Balasia, B.; Jacobs, L.D.; Corcoran, M.; Anderson, A.S.; Pastoret, A.; Spencer, K.T. Effectiveness of echocardiographic imaging by nurses to identify left ventricular systolic dysfunction in high-risk patients. Am. J. Cardiol. 2005, 95, 1271–1272. [Google Scholar] [CrossRef]
- Hope, M.D.; de la Pena, E.; Yang, P.C.; Liang, D.H.; McConnell, M.V.; Rosenthal, D.N. A visual approach for the accurate determination of echocardiographic left ventricular ejection fraction by medical students. J. Am. Soc. Echocardiogr. 2003, 16, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Söderström, L.; Alverlind, K.; Samuelsson, E.; Mooe, T. Hand-held cardiac ultrasound examinations performed in primary care patients by nonexperts to identify reduced ejection fraction. BMC Med. Educ. 2019, 19, 282. [Google Scholar] [CrossRef] [PubMed]
- Baribeau, Y.; Sharkey, A.; Chaudhary, O.; Krumm, S.; Fatima, H.; Mahmood, F.; Matyal, R. Handheld Point-of-Care Ultrasound Probes: The New Generation of POCUS. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Dadon, Z.; Levi, N.; Orlev, A.; Belman, D.; Alpert, E.A.; Glikson, M.; Gottlieb, S.; Butnaru, A. The Utility of Handheld Cardiac and Lung Ultrasound in Predicting Outcomes of Hospitalised Patients With COVID-19. Can. J. Cardiol. 2022, 38, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Dadon, Z.; Carasso, S.; Gottlieb, S. The Role of Hand-Held Cardiac Ultrasound in Patients with COVID-19. Biomedicines 2023, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Hughes, J.W.; Olgin, J.E.; Avram, R.; Abreau, S.A.; Sittler, T.; Radia, K.; Hsia, H.; Walters, T.; Lee, B.; Gonzalez, J.E.; et al. Performance of a Convolutional Neural Network and Explainability Technique for 12-Lead Electrocardiogram Interpretation. JAMA Cardiol. 2021, 6, 1285–1295. [Google Scholar] [CrossRef]
- Rao, S.; Li, Y.; Ramakrishnan, R.; Hassaine, A.; Canoy, D.; Cleland, J.; Lukasiewicz, T.; Salimi-Khorshidi, G.; Rahimi, K. An Explainable Transformer-Based Deep Learning Model for the Prediction of Incident Heart Failure. IEEE J. Biomed. Health Inform. 2022, 26, 3362–3372. [Google Scholar] [CrossRef]
- Stewart, J.E.; Goudie, A.; Mukherjee, A.; Dwivedi, G. Artificial intelligence-enhanced echocardiography in the emergency department. Emerg. Med. Australas. 2021, 33, 1117–1120. [Google Scholar] [CrossRef]
- Dadon, Z.; Orlev, A.; Butnaru, A.; Rosenmann, D.; Glikson, M.; Gottlieb, S.; Alpert, E.A. Empowering Medical Students: Harnessing Artificial Intelligence for Precision Point-of-Care Echocardiography Assessment of Left Ventricular Ejection Fraction. Int. J. Clin. Pract. 2023, 2023, 5225872. [Google Scholar] [CrossRef] [PubMed]
- Armato, S.G.; Drukker, K.; Hadjiiski, L. AI in medical imaging grand challenges: Translation from competition to research benefit and patient care. Br. J. Radiol. 2023, 96, 20221152. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.; Daluiski, A.; Chopra, S.; Lachapelle, A.; Mozer, M.; Sicular, S.; Hanel, D.; Gardner, M.; Gupta, A.; Hotchkiss, R.; et al. Deep neural network improves fracture detection by clinicians. Proc. Natl. Acad. Sci. USA 2018, 115, 11591–11596. [Google Scholar] [CrossRef] [PubMed]
- Krogue, J.D.; Cheng, K.V.; Hwang, K.M.; Toogood, P.; Meinberg, E.G.; Geiger, E.J.; Zaid, M.; McGill, K.C.; Patel, R.; Sohn, J.H.; et al. Automatic Hip Fracture Identification and Functional Subclassification with Deep Learning. Radiol. Artif. Intell. 2020, 2, e190023. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, L.A.M.; Sobol, G.L.; Langerhuizen, D.W.G.; Bulstra, A.E.J.; Hreha, J.; Sprague, S.; Sirkin, M.S.; Ring, D.; Kerkhoffs, G.M.M.J.; Jaarsma, R.L.; et al. A Machine Learning Algorithm to Predict the Probability of (Occult) Posterior Malleolar Fractures Associated With Tibial Shaft Fractures to Guide “Malleolus First” Fixation. J. Orthop. Trauma. 2020, 34, 131–138. [Google Scholar] [CrossRef]
- Machine Learning Consortium, on behalf of the SPRINT and FLOW Investigators. A Machine Learning Algorithm to Identify Patients with Tibial Shaft Fractures at Risk for Infection After Operative Treatment. J. Bone Jt. Surg. Am. 2021, 103, 532–540. [Google Scholar] [CrossRef] [PubMed]
- VanBerlo, B.; Wu, D.; Li, B.; Rahman, M.A.; Hogg, G.; VanBerlo, B.; Tschirhart, J.; Ford, A.; Ho, J.; McCauley, J.; et al. Accurate assessment of the lung sliding artefact on lung ultrasonography using a deep learning approach. Comput. Biol. Med. 2022, 148, 105953. [Google Scholar] [CrossRef] [PubMed]
- Nti, B.; Lehmann, A.S.; Haddad, A.; Kennedy, S.K.; Russell, F.M. Artificial Intelligence-Augmented Pediatric Lung POCUS: A Pilot Study of Novice Learners. J. Ultrasound Med. 2022, 41, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kaneko, T.; Yoshikawa, H.; Uchiyama, S.; Nagata, Y.; Matsushita, Y.; Hiki, M.; Minamino, T.; Takahashi, K.; Daida, H.; et al. Artificial intelligence-based point-of-care lung ultrasound for screening COVID-19 pneumoniae: Comparison with CT scans. PLoS ONE 2023, 18, e0281127. [Google Scholar] [CrossRef]
- Tan, G.F.L.; Du, T.; Liu, J.S.; Chai, C.C.; Nyein, C.M.; Liu, A.Y.L. Automated lung ultrasound image assessment using artificial intelligence to identify fluid overload in dialysis patients. BMC Nephrol. 2022, 23, 410. [Google Scholar] [CrossRef]
- Reinier, K.; Aro, A.L.; Uy-Evanado, A.; Rusinaru, C.; Chugh, H.S.; Shiota, T.; Jui, J.; Chugh, S.S. Electrical surrogate for detection of severe left ventricular systolic dysfunction. Ann. Noninvasive Electrocardiol. 2018, 23, e12591. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Dugan, J.; Rideout, A.; Maidens, J.N.; Venkatraman, S.; Guo, L.; Noseworthy, P.A.; Pellikka, P.A.; Pham, S.L.; Kapa, S.; et al. Automated detection of low ejection fraction from a one-lead electrocardiogram: Application of an AI algorithm to an electrocardiogram-enabled Digital Stethoscope. Eur. Heart J. Digit. Health 2022, 3, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Suzuki, S.; Arita, T.; Yagi, N.; Otsuka, T.; Kishi, M.; Semba, H.; Kano, H.; Matsuno, S.; Kato, Y.; et al. Prediction of current and new development of atrial fibrillation on electrocardiogram with sinus rhythm in patients without structural heart disease. Int. J. Cardiol. 2021, 327, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Takata, T.; Takechi, M.; Furukawa, A.; Iwasawa, J.; Kawamura, A.; Taniguchi, T.; Tamura, Y. Explainable Artificial Intelligence Model for Diagnosis of Atrial Fibrillation Using Holter Electrocardiogram Waveforms. Int. Heart J. 2021, 62, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Fiorina, L.; Maupain, C.; Gardella, C.; Manenti, V.; Salerno, F.; Socie, P.; Li, J.; Henry, C.; Plesse, A.; Narayanan, K.; et al. Evaluation of an Ambulatory ECG Analysis Platform Using Deep Neural Networks in Routine Clinical Practice. J. Am. Heart Assoc. 2022, 11, e026196. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Vaseli, H.; Mahdavi, M.; Taheri Dezaki, F.; Luong, C.; Yeung, D.; Gin, K.; Tsang, M.; Nair, P.; Jue, J.; et al. Automated Atrial Fibrillation Diagnosis by Echocardiography without ECG: Accuracy and Applications of a New Deep Learning Approach. Diseases 2024, 12, 35. [Google Scholar] [CrossRef]
- He, B.; Kwan, A.C.; Cho, J.H.; Yuan, N.; Pollick, C.; Shiota, T.; Ebinger, J.; Bello, N.A.; Wei, J.; Josan, K.; et al. Blinded, randomized trial of sonographer versus AI cardiac function assessment. Nature 2023, 616, 520–524. [Google Scholar] [CrossRef]
- Shaikh, F.; Kenny, J.E.; Awan, O.; Markovic, D.; Friedman, O.; He, T.; Singh, S.; Yan, P.; Qadir, N.; Barjaktarevic, I. Measuring the accuracy of cardiac output using POCUS: The introduction of artificial intelligence into routine care. Ultrasound J. 2022, 14, 47. [Google Scholar] [CrossRef]
- Arnaout, R.; Curran, L.; Zhao, Y.; Levine, J.C.; Chinn, E.; Moon-Grady, A.J. An ensemble of neural networks provides expert-level prenatal detection of complex congenital heart disease. Nat. Med. 2021, 27, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Weston, A.D.; Scott, C.G.; Kane, G.C.; Pellikka, P.A.; Carter, R.E. Machine Learning for Diagnosis of Pulmonary Hypertension by Echocardiography. Mayo Clin. Proc. 2024, 99, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Cinteza, E.; Vasile, C.M.; Busnatu, S.; Armat, I.; Spinu, A.D.; Vatasescu, R.; Duica, G.; Nicolescu, A. Can Artificial Intelligence Revolutionize the Diagnosis and Management of the Atrial Septal Defect in Children? Diagnostics 2024, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Roshanitabrizi, P.; Rwebembera, J.; Okello, E.; Beaton, A.; Linguraru, M.G.; Sable, C.A. Using Artificial Intelligence for Rheumatic Heart Disease Detection by Echocardiography: Focus on Mitral Regurgitation. J. Am. Heart Assoc. 2024, 13, e031257. [Google Scholar] [CrossRef] [PubMed]
- Steffner, K.R.; Christensen, M.; Gill, G.; Bowdish, M.; Rhee, J.; Kumaresan, A.; He, B.; Zou, J.; Ouyang, D. Deep learning for transesophageal echocardiography view classification. Sci. Rep. 2024, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Taskén, A.A.; Flade, H.M.; Skogvoll, E.; Berg, E.A.R.; Grenne, B.; Rimehaug, A.; Kirkeby-Garstad, I.; Kiss, G.; Aakhus, S. Automatic assessment of left ventricular function for hemodynamic monitoring using artificial intelligence and transesophageal echocardiography. J. Clin. Monit. Comput. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Firima, E.; Gonzalez, L.; Manthabiseng, M.; Bane, M.; Lukau, B.; Leigh, B.; Kaufmann, B.A.; Weisser, M.; Amstutz, A.; Tromp, J.; et al. Implementing focused echocardiography and AI-supported analysis in a population-based survey in Lesotho: Implications for community-based cardiovascular disease care models. Hypertens. Res. 2024, 47, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef]
- Curtis, J.P.; Sokol, S.I.; Wang, Y.; Rathore, S.S.; Ko, D.T.; Jadbabaie, F.; Portnay, E.L.; Marshalko, S.J.; Radford, M.J.; Krumholz, H.M. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J. Am. Coll. Cardiol. 2003, 42, 736–742. [Google Scholar] [CrossRef]
- Angaran, P.; Dorian, P.; Ha, A.C.T.; Thavendiranathan, P.; Tsang, W.; Leong-Poi, H.; Woo, A.; Dias, B.; Wang, X.; Austin, P.C.; et al. Association of Left Ventricular Ejection Fraction with Mortality and Hospitalizations. J. Am. Soc. Echocardiogr. 2020, 33, 802–811.e6. [Google Scholar] [CrossRef]

| Variable | All Cohort n = 82 | Preserved LVEF n = 48 | Reduced LVEF n = 34 | p-Value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, mean ± SD | 58.5 ± 16.8 | 56.8 ± 17.1 | 61.0 ± 16.3 | 0.266 |
| Male, n (%) | 72 (87.8) | 43 (89.6) | 29 (85.3) | 0.734 |
| Body mass index, mean ± SD | 28.2 ± 4.5 | 28.5 ± 4.2 | 27.8 ± 4.9 | 0.492 |
| Clinical characteristics | ||||
| Smoking, n (%) | 28 (34.1) | 15 (31.3) | 13 (38.2) | 0.637 |
| Diabetes mellitus, n (%) | 22 (26.8) | 11 (22.9) | 11 (32.4) | 0.342 |
| Hypertension, n (%) | 37 (45.1) | 22 (45.8) | 15 (44.1) | 0.878 |
| Hyperlipidemia, n(%) | 41 (50.0) | 24 (50.0) | 17 (50.0) | 1.000 |
| Ischemic heart disease, n (%) | 29 (35.4) | 16 (33.3) | 13 (38.2) | 0.647 |
| Revascularization, n (%) | 18 (22.0) | 9 (18.8) | 9 (26.5) | 0.342 |
| CKD, n (%) | 11 (13.4) | 6 (12.5) | 5 (14.7) | 0.773 |
| Atrial fibrillation, n (%) | 8 (9.8) | 5 (10.4) | 3 (8.8) | 1.000 |
| CHF, n (%) | 15 (18.3) | 5 (10.4) | 10 (29.4) | 0.028 |
| Hypothyroidism, n (%) | 7 (8.5) | 5 (10.4) | 2 (5.9) | 0.694 |
| Chronic medications | ||||
| Anti-platelets, n (%) | 35 (42.7) | 22 (45.8) | 13 (38.2) | 0.493 |
| Anticoagulation, n (%) | 6 (7.3) | 3 (6.3) | 3 (8.8) | 0.688 |
| Diuretics, n (%) | 13 (15.9) | 9 (18.8) | 4 (11.8) | 0.543 |
| β-blockers, n (%) | 26 (31.7) | 15 (31.3) | 11 (32.4) | 0.916 |
| ACE-I/ARB, n (%) | 20 (24.4) | 13 (27.1) | 7 (20.6) | 0.500 |
| Anti-diabetic drugs, n (%) | 21 (25.6) | 11 (22.9) | 10 (29.4) | 0.507 |
| Statins, n (%) | 33 (40.2) | 21 (43.8) | 12 (35.3) | 0.442 |
| Hospitalization’s main diagnosis | 0.040 | |||
| Acute coronary syndrome, n (%) | 48 (58.5) | 29 (60.4) | 19 (55.9) | |
| Heart failure, n (%) | 9 (11.0) | 2 (4.2) | 7 (20.6) | |
| Arrhythmias, n (%) | 11 (13.4) | 5 (10.4) | 6 (16.7) | |
| Perimyocarditis, n (%) | 8 (9.8) | 7 (14.6) | 1 (2.9) | |
| Others, n (%) | 6 (7.3) | 5 (10.4) | 1 (2.9) | |
| In-hospital vitals and laboratory workup | ||||
| AI-based LVEF, mean ± SD | 49.5 ± 11.4 | 57.3 ± 5.3 | 38.5 ± 8.1 | <0.001 |
| HR (admission; bpm), mean ± SD | 73.9 ± 13.3 | 72.1 ± 14.2 | 76.4 ± 11.4 | 0.144 |
| SBP (admission; mmHg), mean ± SD | 141.0 ± 28.7 | 144.2 ± 30.6 | 136.6 ± 25.8 | 0.248 |
| DBP (admission; mmHg), mean ± SD | 82.3 ± 16.7 | 82.3 ± 17.1 | 82.2 ± 16.5 | 0.964 |
| O2 saturation (admission; %), mean ± SD | 95.2 ± 3.1 | 95.5 ± 2.9 | 94.8 ± 3.4 | 0.333 |
| Lowest SBP (mmHg), mean ± SD | 102.5 ± 14.8 | 105.5 ± 12.1 | 98.4 ± 17.1 | 0.033 |
| Creatinine (admission; mg/dL), mean ± SD | 1.0 ± 0.3 | 1.0 ± 0.4 | 1.0 ± 0.2 | 0.809 |
| Peak Hs-cTnI > 10,000 ng/L, n (%) | 15 (18.3) | 2 (4.2) | 13 (38.2) | <0.001 |
| Potassium (admission; mEq/L), mean ± SD | 3.9 ± 0.4 | 3.9 ± 0.4 | 4.0 ± 0.4 | 0.847 |
| Sodium (admission; mEq/L), mean ± SD | 138.6 ± 2.5 | 138.8 ± 2.3 | 138.4 ± 2.8 | 0.591 |
| LDL (admission; mg/dL), mean ± SD | 111.0 ± 41.0 | 111.6 ± 42.7 | 110.3 ± 39.8 | 0.905 |
| Hemoglobin (admission; g/dL), mean ± SD | 13.8 ± 2.1 | 13.6 ± 2.0 | 14.1 ± 2.2 | 0.267 |
| TSH (mIU/L), mean ± SD | 1.9 ± 1.5 | 1.9 ± 1.5 | 2.0 ± 1.5 | 0.670 |
| CRP (peak; mg/L), median [IQR] | 0.5 [0.2, 2.3] | 0.5 [0.2,2.3] | 0.3 [0.2, 2.0] | 0.847 |
| Variable | Unadjusted Analysis | Adjusted Anslysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.008 | 0.982–1.034 | 0.566 | 0.977 | 0.947–1.007 | 0.133 |
| Reduced LVEF | 2.700 | 1.132–6.441 | 0.025 | 2.717 | 1.083–6.817 | 0.033 |
| Congestive heart failure | 2.555 | 1.039–6.286 | 0.041 | 1.375 | 0.497–3.806 | 0.540 |
| Ischemic heart disease | 2.526 | 1.091–5.852 | 0.031 | 0.494 | 0.129–1.890 | 0.303 |
| Hypertension | 2.885 | 1.175–7.082 | 0.021 | 3.183 | 1.018–9.957 | 0.047 |
| Previous revascularization | 6.074 | 2.606–14.155 | <0.001 | 7.555 | 1.849–30.867 | 0.005 |
| Variable | All Cohort n = 82 | AI-Based Preserved LVEF n = 48 | AI-Based Reduced LVEF n = 34 | p-Value | Unadjusted OR (95% CI) |
|---|---|---|---|---|---|
| Secondary composite outcome *, n (%) | 22 (26.8) | 7 (14.6) | 15 (44.1) | 0.004 | 4.624 (1.619–13.203) |
| In-hospital death, n (%) | 2 (2.4) | 1 (2.1) | 1 (2.9) | 1.000 | 1.424 (0.086–23.593) |
| Advanced ventilatory support, n (%) | 8 (9.8) | 3 (6.3) | 5 (14.7) | 0.266 | 2.586 (0.574–11.655) |
| Shock, n (%) | 8 (9.8) | 1 (2.1) | 7 (20.6) | 0.008 | 12.185 (1.422–104.407) |
| ADHF, n (%) | 20 (24.4) | 6 (12.6) | 14 (41.2) | 0.003 | 7.077 (1.397–35.846) |
| LV/LAA thrombus, n (%) | 6 (7.3) | 1 (2.1) | 5 (14.7) | 0.077 | 8.103 (0.901–72.867) |
| Acute kidney injury, n (%) | 10 (12.2) | 3 (6.3) | 7 (20.6) | 0.084 | 3.889 (0.927–16.319) |
| Any ventilatory support, n (%) | 25 (30.5) | 11 (22.9) | 14 (41.2) | 0.077 | 2.355 (0.903–6.143) |
| Cardioversion, n (%) | 5 (6.1) | 1 (2.1) | 4 (11.8) | 0.155 | 6.267 (0.668–58.786) |
| CIED insertion, n (%) | 3 (3.7) | 3 (6.3) | 0 (0.0) | 0.260 | |
| LOS (days), median [IQR] | 3.6 [2.0, 7.0] | 3.0 [1.7, 6.5] | 4.7 [2.8, 7.5] | 0.019 | |
| LOS > 3.6 days, n (%) | 42 (51.2) | 20 (41.7) | 22 (64.7) | 0.042 | 2.567 (1.035–6.362) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadon, Z.; Rav Acha, M.; Orlev, A.; Carasso, S.; Glikson, M.; Gottlieb, S.; Alpert, E.A. Artificial Intelligence-Based Left Ventricular Ejection Fraction by Medical Students for Mortality and Readmission Prediction. Diagnostics 2024, 14, 767. https://doi.org/10.3390/diagnostics14070767
Dadon Z, Rav Acha M, Orlev A, Carasso S, Glikson M, Gottlieb S, Alpert EA. Artificial Intelligence-Based Left Ventricular Ejection Fraction by Medical Students for Mortality and Readmission Prediction. Diagnostics. 2024; 14(7):767. https://doi.org/10.3390/diagnostics14070767
Chicago/Turabian StyleDadon, Ziv, Moshe Rav Acha, Amir Orlev, Shemy Carasso, Michael Glikson, Shmuel Gottlieb, and Evan Avraham Alpert. 2024. "Artificial Intelligence-Based Left Ventricular Ejection Fraction by Medical Students for Mortality and Readmission Prediction" Diagnostics 14, no. 7: 767. https://doi.org/10.3390/diagnostics14070767
APA StyleDadon, Z., Rav Acha, M., Orlev, A., Carasso, S., Glikson, M., Gottlieb, S., & Alpert, E. A. (2024). Artificial Intelligence-Based Left Ventricular Ejection Fraction by Medical Students for Mortality and Readmission Prediction. Diagnostics, 14(7), 767. https://doi.org/10.3390/diagnostics14070767






