Ambulatory Risk Stratification for Worsening Heart Failure in Patients with Reduced and Preserved Ejection Fraction Using Diagnostic Parameters Available in Implantable Cardiac Monitors
Abstract
1. Background
2. Methods
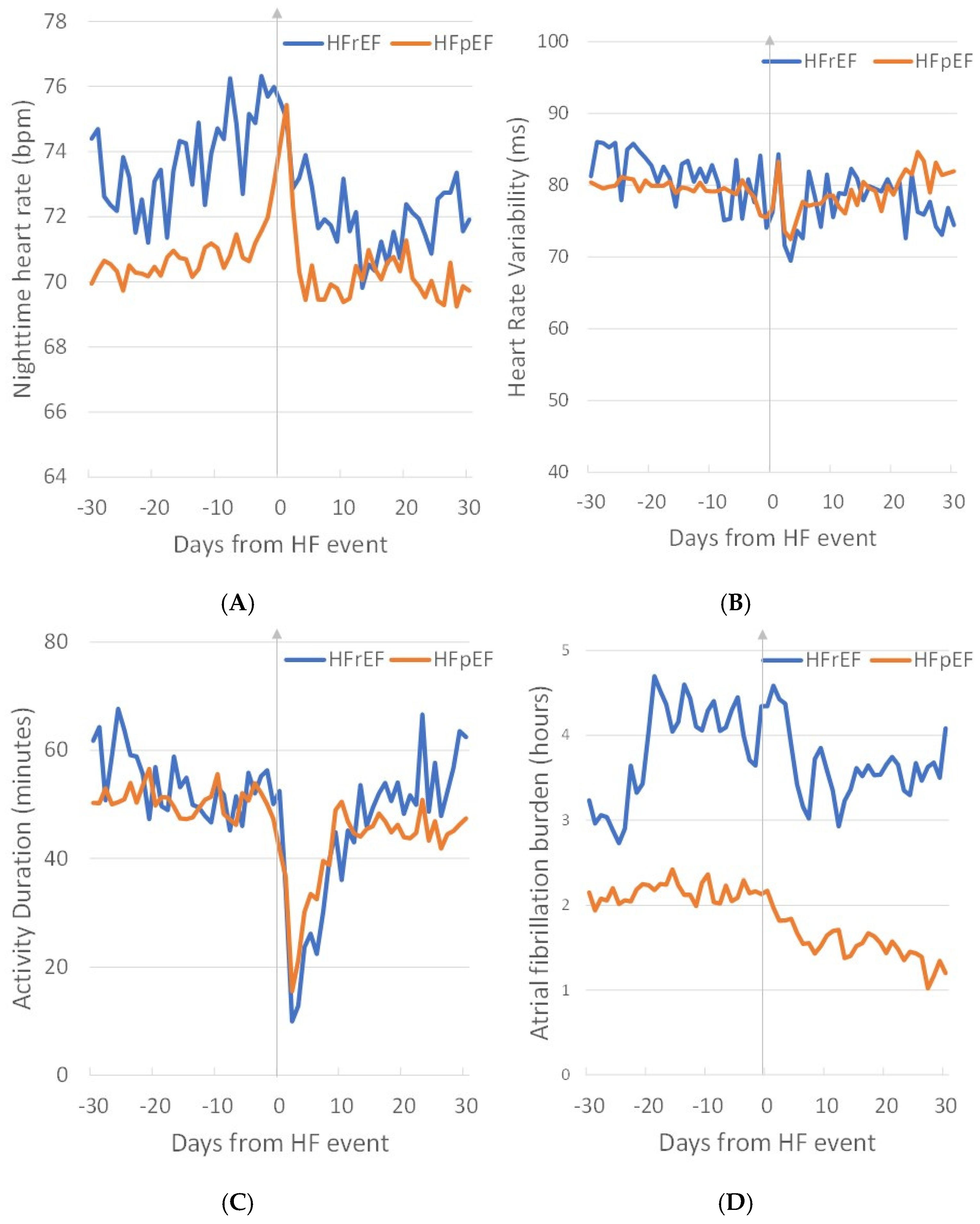
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Zile, M.R.; Desai, A.S.; Bhatt, K.; Ducharme, A.; Horstmanshof, D.; Krim, S.R.; Maisel, A.; Mehra, M.R.; Paul, S.; et al. Haemodynamic-guided management of heart failure (GUIDE-HF): A randomised controlled trial. Lancet 2021, 398, 991–1001. [Google Scholar] [CrossRef]
- Zile, M.R.; Mehra, M.R.; Ducharme, A.; Sears, S.F.; Desai, A.S.; Maisel, A.; Paul, S.; Smart, F.; Grafton, G.; Kumar, S.; et al. Hemodynamically-Guided Management of Heart Failure Across the Ejection Fraction Spectrum: The GUIDE-HF Trial. JACC Heart Fail. 2022, 10, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Costanzo, M.R.; Zile, M.R.; Ducharme, A.; Troughton, R.; Maisel, A.; Mehra, M.R.; Paul, S.; Sears, S.F.; Smart, F.; et al. Implantable Hemodynamic Monitors Improve Survival in Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2024, 83, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Wang, L.I.; Chau, E.; Chan, R.H.W.; Kong, S.L.; Tang, M.O.; Christensen, J.; Stadler, R.W.; Lau, C.P. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005, 112, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Vanderheyden, M.; Houben, R.; Verstreken, S.; Ståhlberg, M.; Reiters, P.; Kessels, R.; Braunschweig, F. Continuous monitoring of intrathoracic impedance and right ventricular pressures in patients with heart failure. Circ. Heart Fail. 2010, 3, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Compton, S.; Haas, G.; Foreman, B.; Canby, R.C.; Fishel, R.; McRae, S.; Toledo, G.B.; Sarkar, S. Superior Performance of Intrathoracic Impedance-Derived fluid Index versus Daily Weight monitoring in Heart Failure Patients: Results of the Fluid Accumulation Status Trial (FAST). Congest. Heart Fail. 2011, 17, 51–55. [Google Scholar] [CrossRef]
- Whellan, D.J.; Ousdigian, K.T.; Al-Khatib, S.M.; Pu, W.; Sarkar, S.; Porter, C.B.; Pavri, B.B.; O’Connor, C.M. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: Results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study. J. Am. Coll. Cardiol. 2010, 55, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Sarkar, S.; Koehler, J.; Whellan, D.J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Sharma, V.; Santini, M. Development and validation of an integrated diagnostic algorithm derived from pa-rameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur. Heart J. 2013, 34, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multi-Sensor Algorithm Predicts Heart Failure Events in Patients with Implanted Devices: Results from the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Solimene, F.; Calò, L.; Calvi, V.; Viscusi, M.; Melissano, D.; Russo, V.; Rapacciuolo, A.; Campana, A.; Caravati, F.; et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: Results from the SELENE HF study. Europace 2022, 24, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Kahwash, R.; Sarkar, S.; Koehler, J.; Butler, J. Temporal Characteristics of Device-Based Individual and Integrated Risk Metrics in Patients With Chronic Heart Failure. JACC Heart Fail. 2023, 11, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Kahwash, R.; Sarkar, S.; Koehler, J.; Zielinski, T.; Mehra, M.R.; Fonarow, G.C.; Gulati, S.; Butler, J. A Novel Heart Failure Diagnostic Risk Score Using a Minimally Invasive Subcutaneous Insertable Cardiac Monitor. JACC Hearth Fail. 2024, 12, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Klein, G.J.; Yee, R.; Takle-Newhouse, T.; Norris, C. Use of an Extended Monitoring Strategy in Patients With Problematic Syncope. Circulation 1999, 99, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pürerfellner, H.; Sanders, P.; Pokushalov, E.; Di Bacco, M.; Bergemann, T.; Dekker, L.R. Miniaturized Reveal LINQ insertable cardiac monitoring system: First-in-human experience. Heart Rhythm 2015, 12, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Klein, G.J.; Yee, R.; Skanes, A.C. Detection of asymptomatic arrhythmias in unexplained syncope. Am. Hearth J. 2004, 148, 326–332. [Google Scholar] [CrossRef]
- Farwell, D.; Freemantle, N.; Sulke, A. Use of implantable loop recorders in the diagnosis and management of syncope. Eur. Hearth J. 2004, 25, 1257–1263. [Google Scholar] [CrossRef]
- Verma, A.; Champagne, J.; Sapp, J.; Essebag, V.; Novak, P.; Skanes, A.; Morillo, C.A.; Khaykin, Y.; Birnie, D. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): A prospective, multicenter study. JAMA Intern. Med. 2013, 173, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Sanna, T.; Diener, H.-C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Rogers, J.; Sarkar, S.; Koehler, J.; Passman, R.S. Real-World Incidence of Pacemaker and Defibrillator Implantation Following Diagnostic Monitoring with an Insertable Cardiac Monitor. Am. J. Cardiol. 2019, 123, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.D.; Sanders, P.; Piorkowski, C.; Sohail, M.R.; Anand, R.; Crossen, K.; Khairallah, F.S.; Kaplon, R.E.; Stromberg, K.; Kowal, R.C. In-office insertion of a miniaturized insertable cardiac monitor: Results from the Reveal LINQ In-Office 2 randomized study. Hearth Rhythm. 2017, 14, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Beinart, S.C.; Natale, A.; Verma, A.; Amin, A.; Kasner, S.; Diener, H.C.; Del Greco, M.; Wilkoff, B.L.; Pouliot, E.; Franco, N.; et al. Real-world comparison of in-hospital Reveal LINQ insertable cardiac monitor insertion inside and outside of the cardiac catheterization or electrophysiology laboratory. Am. Heart J. 2019, 207, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Black, C.L.B.; Thomsen, P.E.B.; Sutton, R.; Moya, A.; Stadler, R.W.; Cao, J.; Messier, M.; Huikuri, H.V. Improved arrhythmia detection in implantable loop recorders. J. Cardiovasc. Electrophysiol. 2008, 19, 928–934. [Google Scholar] [CrossRef]
- Sarkar, S.; Ritscher, D.; Mehra, R. A Detector for a Chronic Implantable Atrial Tachyarrhythmia Monitor. IEEE Trans. Biomed. Eng. 2008, 55, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Pürerfellner, H.; Sanders, P.; Sarkar, S.; Reisfeld, E.; Reiland, J.; Koehler, J.; Pokushalov, E.; Urban, Ľ.; Dekker, L.R.C. Adapting detection sensitivity based on evidence of irregular sinus arrhythmia to improve atrial fibrillation detection in insertable cardiac monitors. EP Europace 2018, 20, f321–f328. [Google Scholar] [CrossRef]
- Adamson, P.B.; Smith, A.L.; Abraham, W.T.; Kleckner, K.J.; Stadler, R.W.; Shih, A.; Rhodes, M.M. InSync III Model 8042 and Attain OTW Lead Model 4193 Clinical Trial Investigators. Continuous autonomic assessment in patients with symptomatic heart failure: Prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation 2004, 110, 2389–2394. [Google Scholar]
- Singh, J.P.; Rosenthal, L.S.; Hranitzky, P.M.; Berg, K.C.; Mullin, C.M.; Thackeray, L.; Kaplan, A. Device diagnostics and long-term clinical outcome in patients receiving cardiac resynchronization therapy. Eur. 2009, 11, 1647–1653. [Google Scholar] [CrossRef]
- Page, E.; Cazeau, S.; Ritter, P.; Galley, D.; Casset, C. Physiological approach to monitor patients in congestive heart failure: Application of a new implantable device-based system to monitor daily life activity and ventilation. Europace 2007, 9, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Gasparini, M.; Landolina, M.; Lunati, M.; Proclemer, A.; Lonardi, G.; Iacopino, S.; Rahue, W.; Biffi, M.; DiStefano, P.; et al. Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur. J. Hearth Fail. 2011, 13, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Koehler, J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Whellan, D.J. Burden of Atrial Fibrillation and Poor Rate Control Detected by Continuous Monitoring via Implanted Devices Identifies When a Patient Is at Risk for Heart Failure Hospitalization. J. Am. Coll. Cardiol. 2011, 57, E107. [Google Scholar] [CrossRef]
- Ruwald, A.C.; Pietrasik, G.; Goldenberg, I.; Kutyifa, V.; Daubert, J.P.; Ruwald, M.H.; Jons, C.; McNitt, S.; Wang, P.; Zareba, W.; et al. The Effect of Intermittent Atrial Tachyarrhythmia on Heart Failure or Death in Cardiac Resynchronization Therapy With Defibrillator Versus Implantable Cardioverter-Defibrillator Patients A MADIT-CRT Substudy (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy). J. Am. Coll. Cardiol. 2014, 63, 1190–1197. [Google Scholar] [PubMed]
- Nakajima, I.; Noda, T.; Kanzaski, H.; Kamakura, T.; Wada, M.; Ishibashi, K.; Inoue, Y.; Miyamoto, K.; Okamura, H.; Nagase, S.; et al. Development of Heart Failure From Transient Atrial Fibrillation Attacks in Responders to Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2018, 4, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Li, Z.; O’brien, E.C.; Pritchard, J.; Chew, D.S.; Bunch, T.J.; Mark, D.B.; Nabutovsky, Y.; Greiner, M.A.; Piccini, J.P. Atrial fibrillation burden and heart failure: Data from 39,710 individuals with cardiac implanted electronic devices. Hearth Rhythm. 2021, 18, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Capucci, A.; Wong, J.A.; Gold, M.R.; Boehmer, J.; Ahmed, R.; Kwan, B.; Thakur, P.H.; Zhang, Y.; Jones, P.W.; Healey, J.S. Temporal Association of Atrial Fibrillation with Cardiac Implanted Electronic Device Detected Heart Failure Status. JACC Clin. Electrophysiol. 2022, 8, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Conraads, V.M.; Spruit, M.A.; Braunschweig, F.; Cowie, M.R.; Tavazzi, L.; Borggrefe, M.; Hill, M.R.; Jacobs, S.; Gerritse, B.; van Veldhuisen, D.J. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ. Hearth Fail. 2014, 7, 279–287. [Google Scholar] [CrossRef]
- Rosman, L.; Lampert, R.; Sears, S.F.; Burg, M.M. Measuring Physical Activity With Implanted Cardiac Devices: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e008663. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef]
- Borlaug, B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef] [PubMed]

| Diagnostic Evidence | Feature Set | Rationale | |
|---|---|---|---|
| AF | H | [{avgAFB7 > 12.5 h OR avgAFB30 > 12 h OR avgAFB7—avgAFB30 > 0.6 h OR ND(AFB30 > 6 h) ≥ 1 OR ND(AFB7 > 6 h) ≥ 1} AND ND(AFB30 > 23 h) < 30] | Presence of paroxysmal AF |
| OR max2minAFB7 > 6.5 h OR max2minAFB30 > 7 h | Change in AF burden | ||
| OR ND(AFB30 > 6 h AND VRAF30 ≥ 90 bpm) ≥ 1 OR ND(AFB30 > 23 h AND VRAF30 ≥ 90 bpm) ≥ 15 | Poor rate control | ||
| OR maxVRAF(AFB30 > 6 h) ≥ 80 bpm OR maxVRAF(AFB7 > 6 h) ≥ 70 bpm | Higher rates during AF | ||
| L | Not ‘H’ | ||
| NHR | H | maxNHR30 > 95 bpm OR minNHR30 < 40 bpm OR minNHR30 ≥ 80 bpm OR ND(NHR30 ≥ 90 bpm) ≥ 10 | High resting heart rate |
| OR {avgNHR7—avgNHR30} ≥ 8 bpm | Increasing resting HR | ||
| OR max2avgNHR30 ≥ 33% | Change in resting HR | ||
| M | Avg(DHR-NHR)30 < 9 bpm} AND Not ‘H’ | Similar daytime and nighttime HR | |
| L | {Not ‘H’ OR ‘M’} | ||
| HRV | H | ND(HRV30 ≤ 60 ms) ≥ 25 OR minHRV7 < 35 ms OR CSFRHRV30 < −12 | Very low HRV or high sympathetic tone |
| OR max2avgHRV30 ≥ 85% | Change in HRV | ||
| M | {ND(HRV7 ≤ 60 ms) ≥2 OR ND(HRV30 ≤ 60 ms) ≥ 6 OR minHRV30 < 55 ms OR avgHRV30 < 65 ms OR avgHRV7 < 75 ms OR CSFRHRV7 < −2 | Lower HRV or higher sympathetic tone | |
| OR max2avgHRV30 ≥ 65% } | Change in HRV | ||
| AND Not ‘H’ | |||
| L | {Not ‘H’ OR ‘M’} | ||
| ACT | H | ND(ACT7 ≤ 30 min) ≥ 7 OR ND(ACT30 ≤ 30 min) ≥ 27 OR avgACT7 < 10 min OR CSFRACT7 < −43 | Very low ACT or low functional capacity |
| M | {ND(ACT30 ≤ 30 min) ≥ 11 OR avgACT30 < 30 min OR CSFRACT30 < −3 | Lower ACT or lower functional capacity | |
| OR max2avgACT30 ≥ 150% } | Change in ACT | ||
| AND Not ‘H’ | |||
| L | Not ‘H’ OR ‘M’ | ||
| All Patients with a History of HF Events | Patients with a History of HF Events and A Median LVEF ≤ 40 | Patients with a History of HF Events and A Median LVEF > 40 | |
|---|---|---|---|
| Number of patients | 1020 | 267 | 622 |
| Mean age (SD) | 68 (13) | 64 (13) | 69 (12) |
| Male gender | 535 (52%) | 178 (67%) | 294 (47%) |
| Hypertension | 967 (95%) | 249 (93%) | 599 (96%) |
| Diabetes | 571 (56%) | 133 (50%) | 362 (58%) |
| CAD | 766 (75%) | 212 (79%) | 464 (75%) |
| MI | 403 (40%) | 105 (39%) | 264 (42%) |
| Vascular disease | 312 (31%) | 78 (29%) | 205 (33%) |
| Atrial fibrillation | 586 (57%) | 158 (59%) | 358 (58%) |
| Renal dysfunction | 539 (53%) | 130 (49%) | 352 (57%) |
| Stroke/TIA | 525 (51%) | 117 (44%) | 353 (57%) |
| Medications | |||
| ACE-I/ARB | 855 (84%) | 245 (92%) | 512 (82%) |
| Beta-Blockers | 797 (78%) | 202 (76%) | 510 (82%) |
| Diuretics | 915 (90%) | 250 (94%) | 557 (90%) |
| Spironolactone | 532 (52%) | 142 (53%) | 336 (54%) |
| Sacubitril/valsartan | 26 (3%) | 24 (9%) | 2 (0.3%) |
| Vasodilator/Nitrate | 256 (25%) | 104 (39%) | 130 (21%) |
| AAD Class I | 72 (7%) | 11 (4%) | 52 (8%) |
| AAD Class III/IV | 382 (37%) | 123 (46%) | 228 (37%) |
| Anticoagulation | 547 (54%) | 156 (58%) | 332 (53%) |
| ICM Reason for monitoring | |||
| AF ablation monitoring | 52 (5%) | 19 (7%) | 23 (4%) |
| AF management | 172 (17%) | 55 (21%) | 99 (16%) |
| Cryptogenic stroke | 240 (24%) | 59 (22%) | 160 (26%) |
| Palpitations | 47 (5%) | 10 (4%) | 29 (5%) |
| Suspected AF | 71 (7%) | 21 (8%) | 46 (7%) |
| Syncope | 388 (38%) | 83 (31%) | 240 (39%) |
| Ventricular tachycardia | 27 (3%) | 13 (5%) | 13 (2%) |
| Other/unknown | 23 (2%) | 7 (3%) | 12 (2%) |
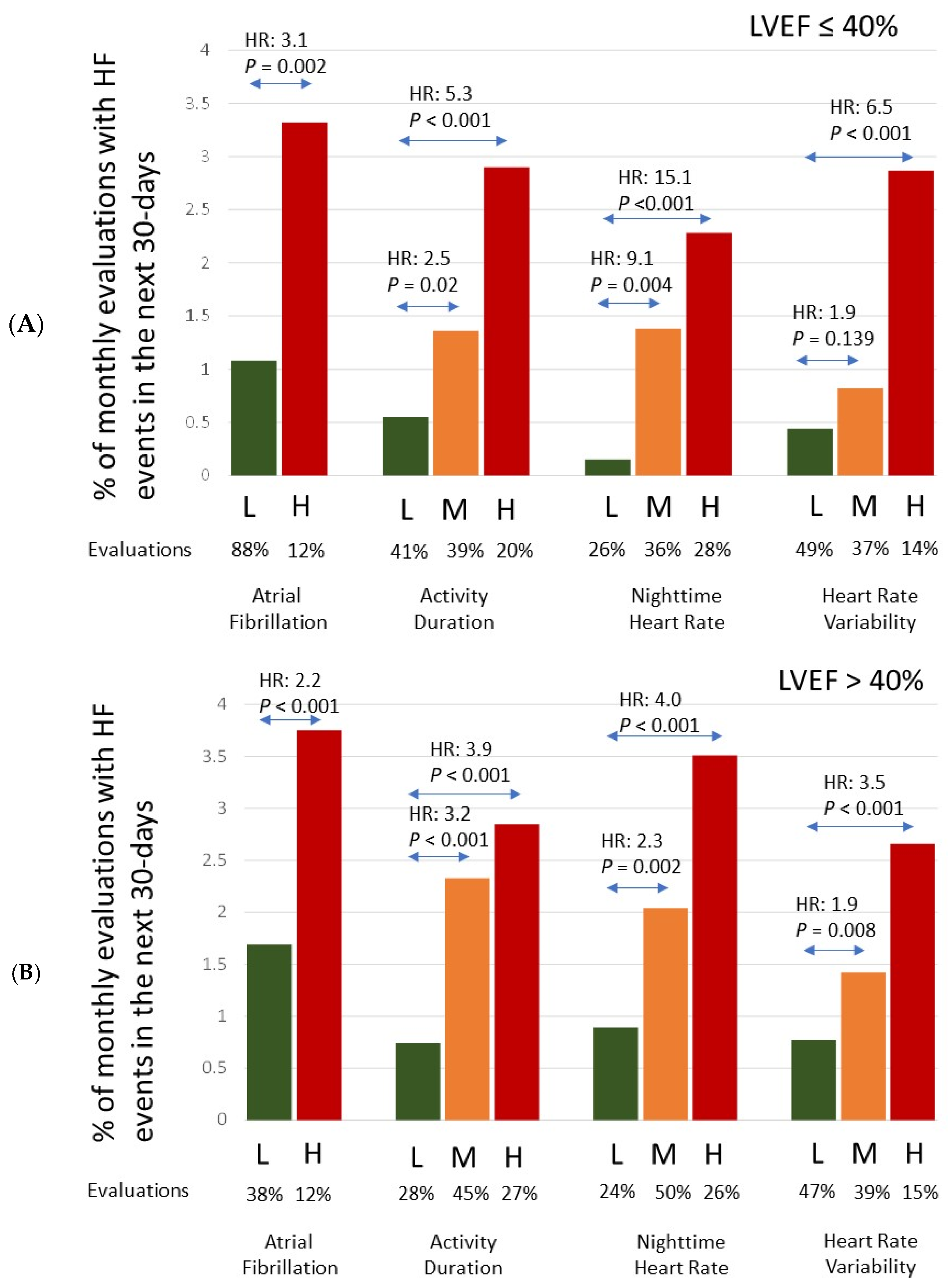
| Diagnostic Parameter/Risk State | Number of Evaluations (%) | Number of HF Events (% of Evals) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| AF | <0.001 | |||
| Low | 15,716 (88%) | 216 (1.37%) | Reference | |
| High | 2135 (12%) | 67 (3.14%) | 2.30 (1.59–3.33) | |
| Activity | <0.001 | |||
| Low | 5618 (31%) | 34 (0.61%) | Reference | |
| Medium | 8124 (44%) | 150 (1.85%) | 3.07 (2.05–4.58) | <0.001 |
| High | 4641 (25%) | 117 (2.52%) | 4.20 (2.68–6.58) | <0.001 |
| NHR | <0.001 | |||
| Low | 4746 (25%) | 28 (0.59%) | Reference | |
| Medium | 9321 (49%) | 151 (1.62%) | 2.76 (1.70–4.46) | <0.001 |
| High | 5060 (26%) | 148 (2.92%) | 5.00 (3.13–8.00) | <0.001 |
| HRV | <0.001 | |||
| Low | 8406 (48%) | 48 (0.57%) | Reference | |
| Medium | 6545 (37%) | 75 (1.15%) | 2.01 (1.35–2.99) | 0.001 |
| High | 2532 (14%) | 65 (2.57%) | 4.53 (2.83–7.25) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.; Koehler, J.; Vasudevan, N. Ambulatory Risk Stratification for Worsening Heart Failure in Patients with Reduced and Preserved Ejection Fraction Using Diagnostic Parameters Available in Implantable Cardiac Monitors. Diagnostics 2024, 14, 771. https://doi.org/10.3390/diagnostics14070771
Sarkar S, Koehler J, Vasudevan N. Ambulatory Risk Stratification for Worsening Heart Failure in Patients with Reduced and Preserved Ejection Fraction Using Diagnostic Parameters Available in Implantable Cardiac Monitors. Diagnostics. 2024; 14(7):771. https://doi.org/10.3390/diagnostics14070771
Chicago/Turabian StyleSarkar, Shantanu, Jodi Koehler, and Neethu Vasudevan. 2024. "Ambulatory Risk Stratification for Worsening Heart Failure in Patients with Reduced and Preserved Ejection Fraction Using Diagnostic Parameters Available in Implantable Cardiac Monitors" Diagnostics 14, no. 7: 771. https://doi.org/10.3390/diagnostics14070771
APA StyleSarkar, S., Koehler, J., & Vasudevan, N. (2024). Ambulatory Risk Stratification for Worsening Heart Failure in Patients with Reduced and Preserved Ejection Fraction Using Diagnostic Parameters Available in Implantable Cardiac Monitors. Diagnostics, 14(7), 771. https://doi.org/10.3390/diagnostics14070771







