The Role of MRI in Groin Pain Syndrome in Athletes
Abstract
1. Introduction
- GPS from traumatic origin: the onset of pain follows an acute trauma, documented by medical history, clinical examination and imaging;
- GPS from functional overload: the onset may be insidious and unaccompanied by acute trauma or it may be attributed to a known cause;
- Long-standing GPS (LSGPS) or chronic GPS: the patient experiences a cohort of symptoms over a period of more than 12 weeks that does not respond to conservative therapy.
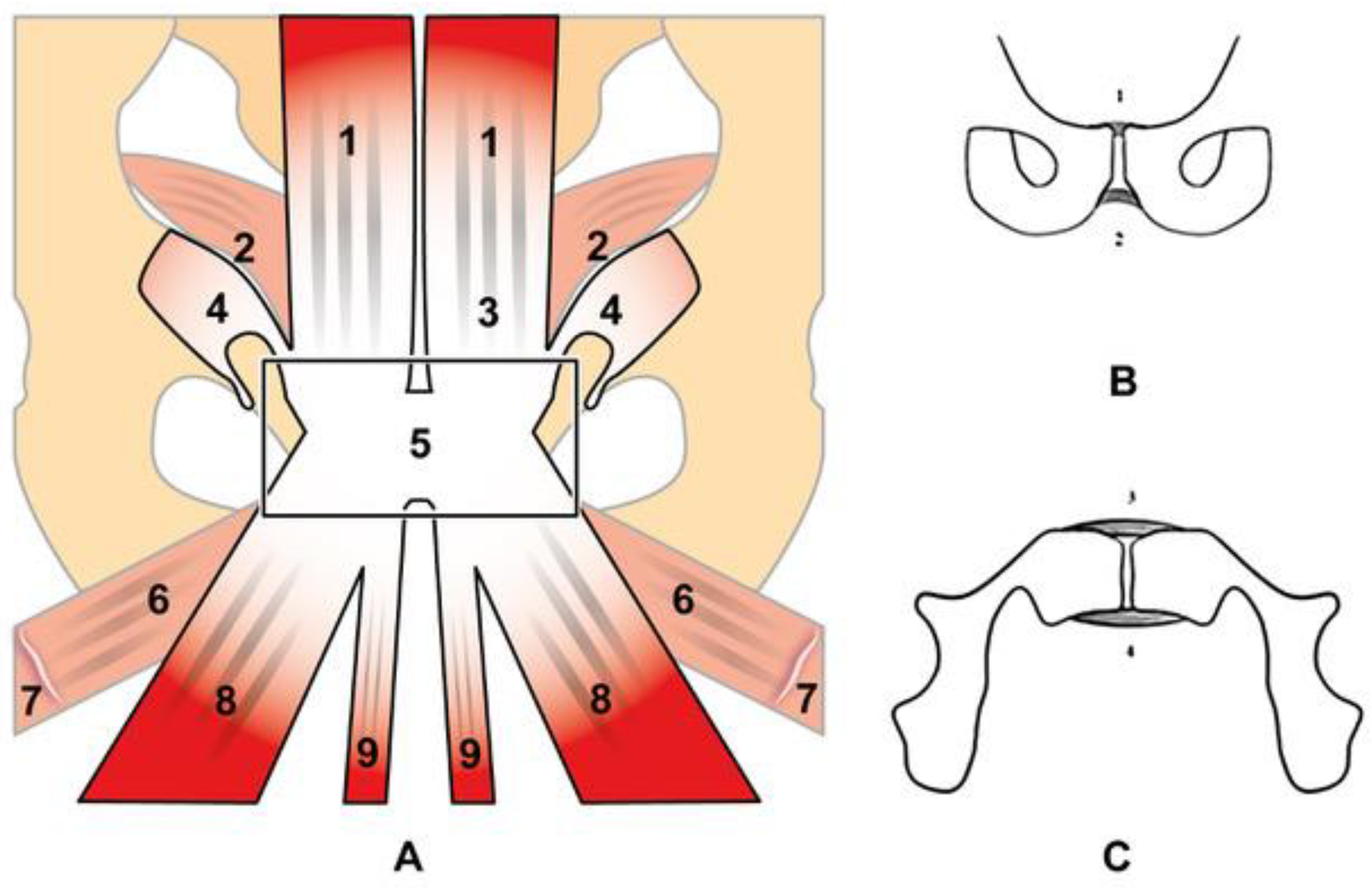
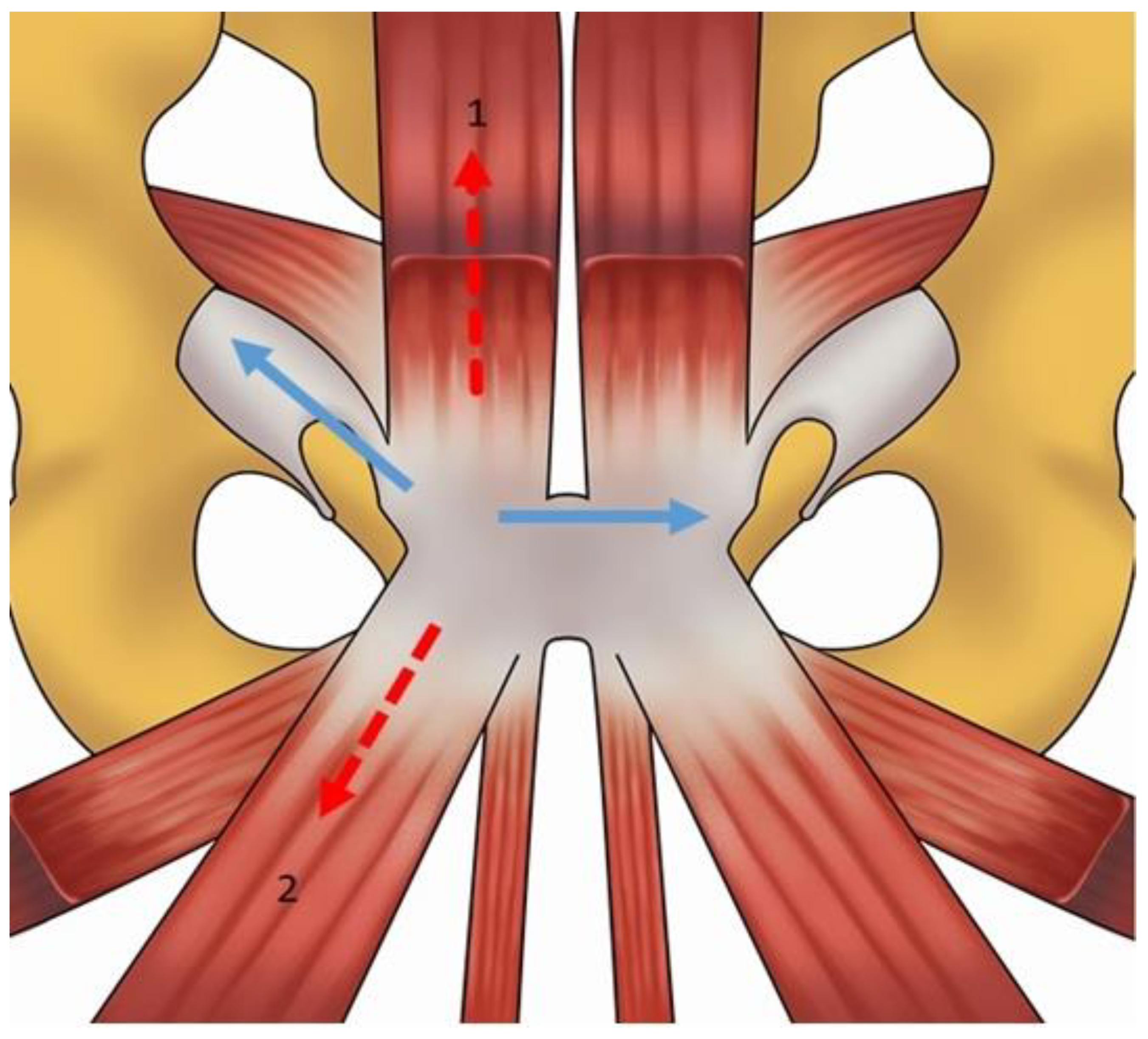
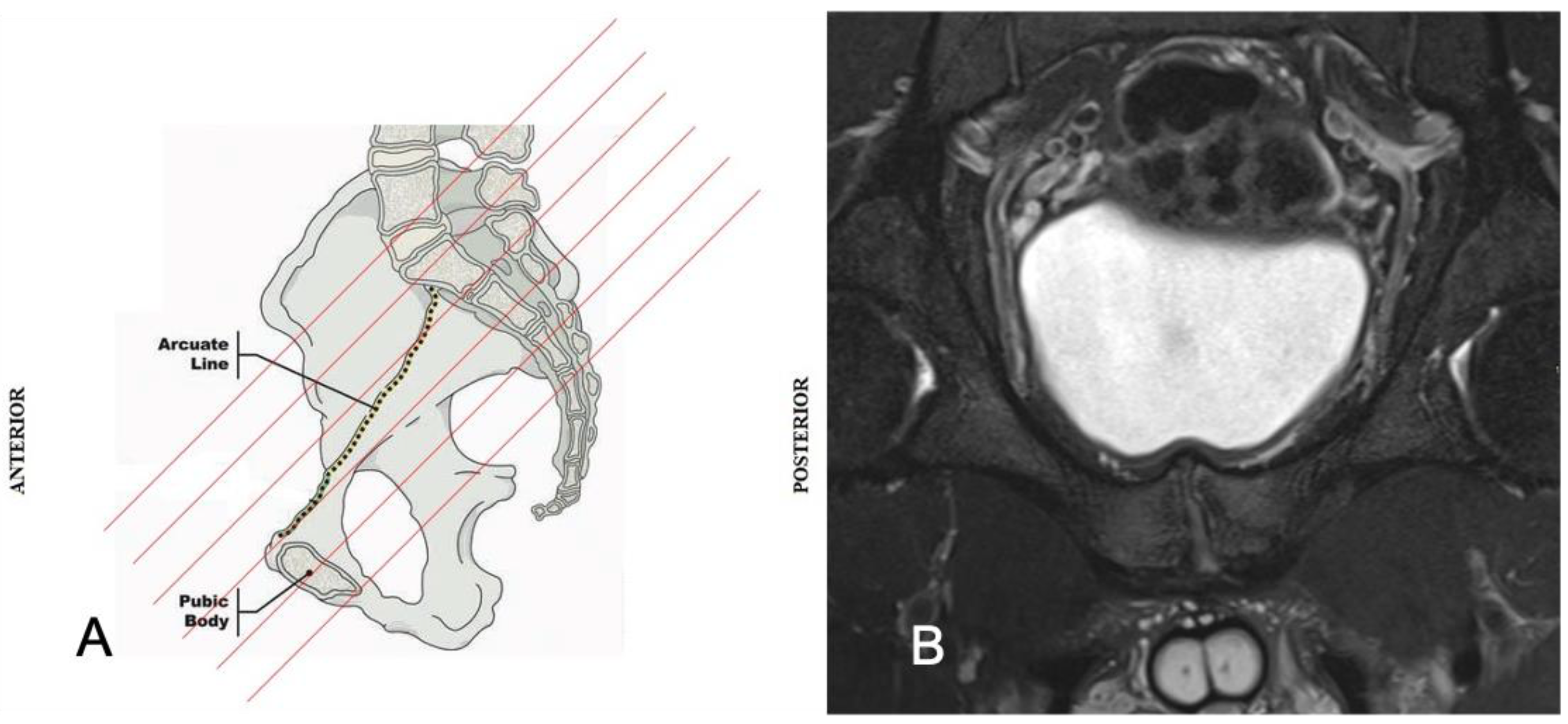
2. Anatomy and Biomechanics of the Pubic Symphisis
3. MRI Techniques
4. GPS MRI Assessment
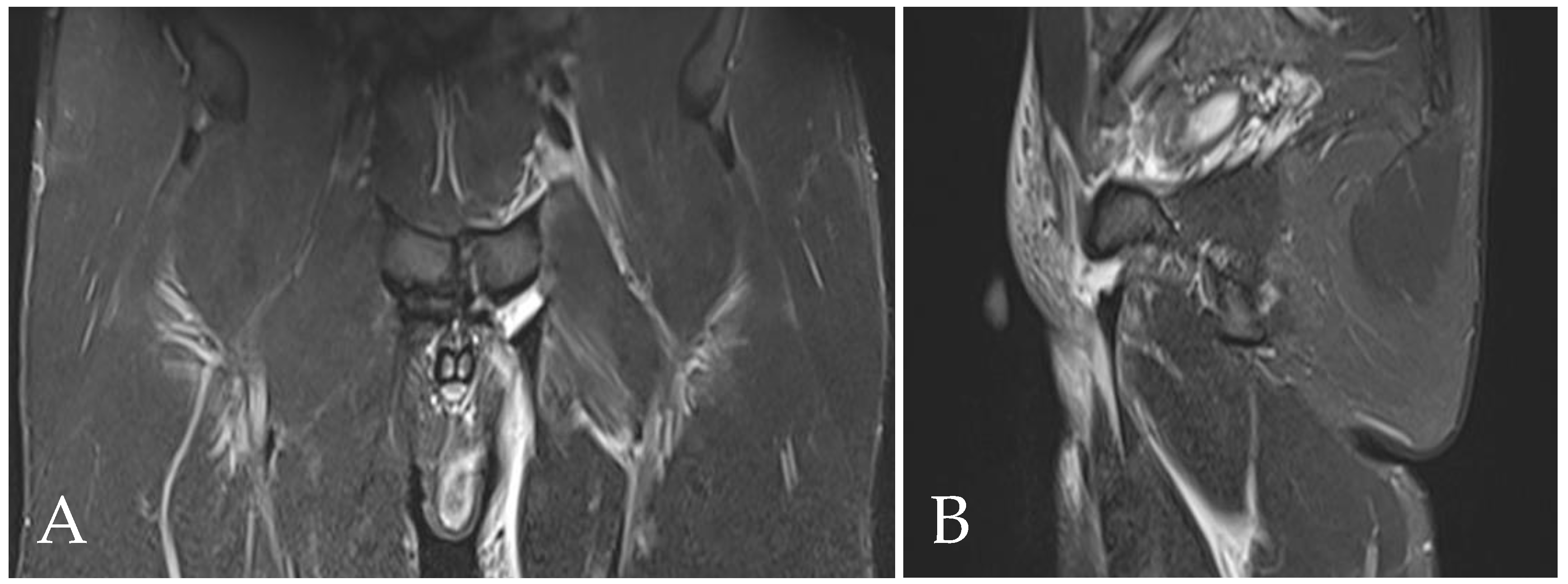
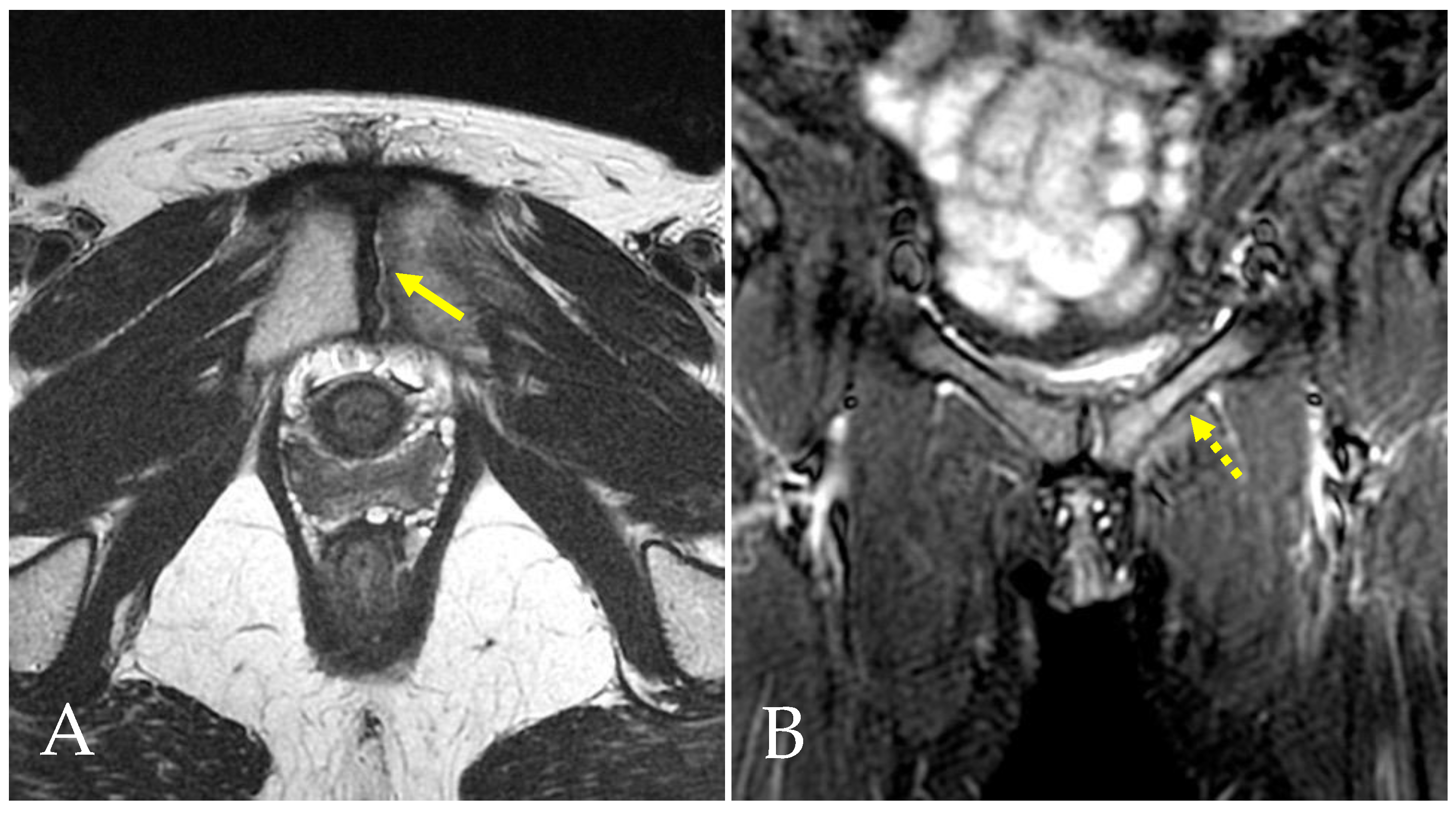
4.1. Prepubic Aponeurotic Complex Injuries
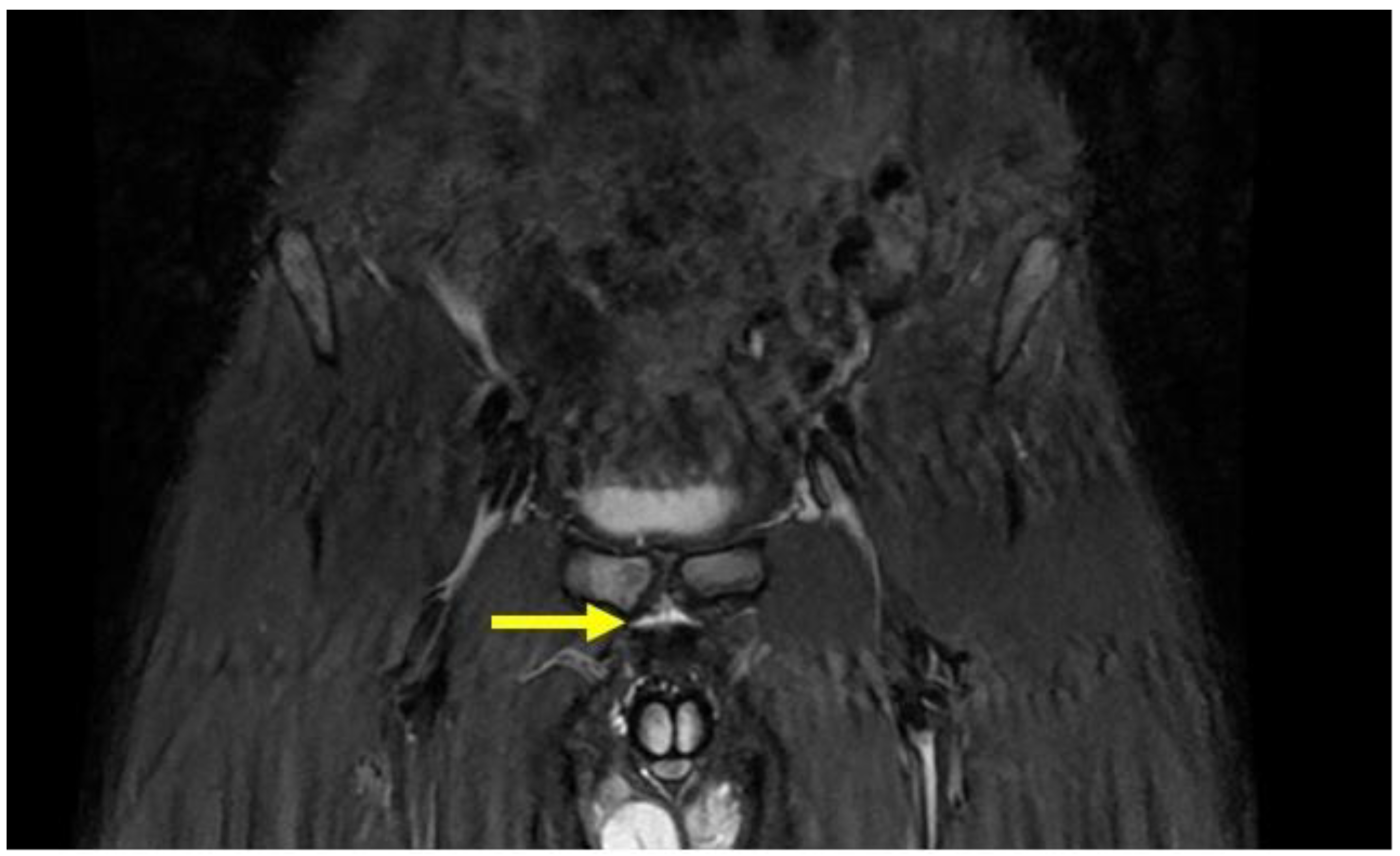
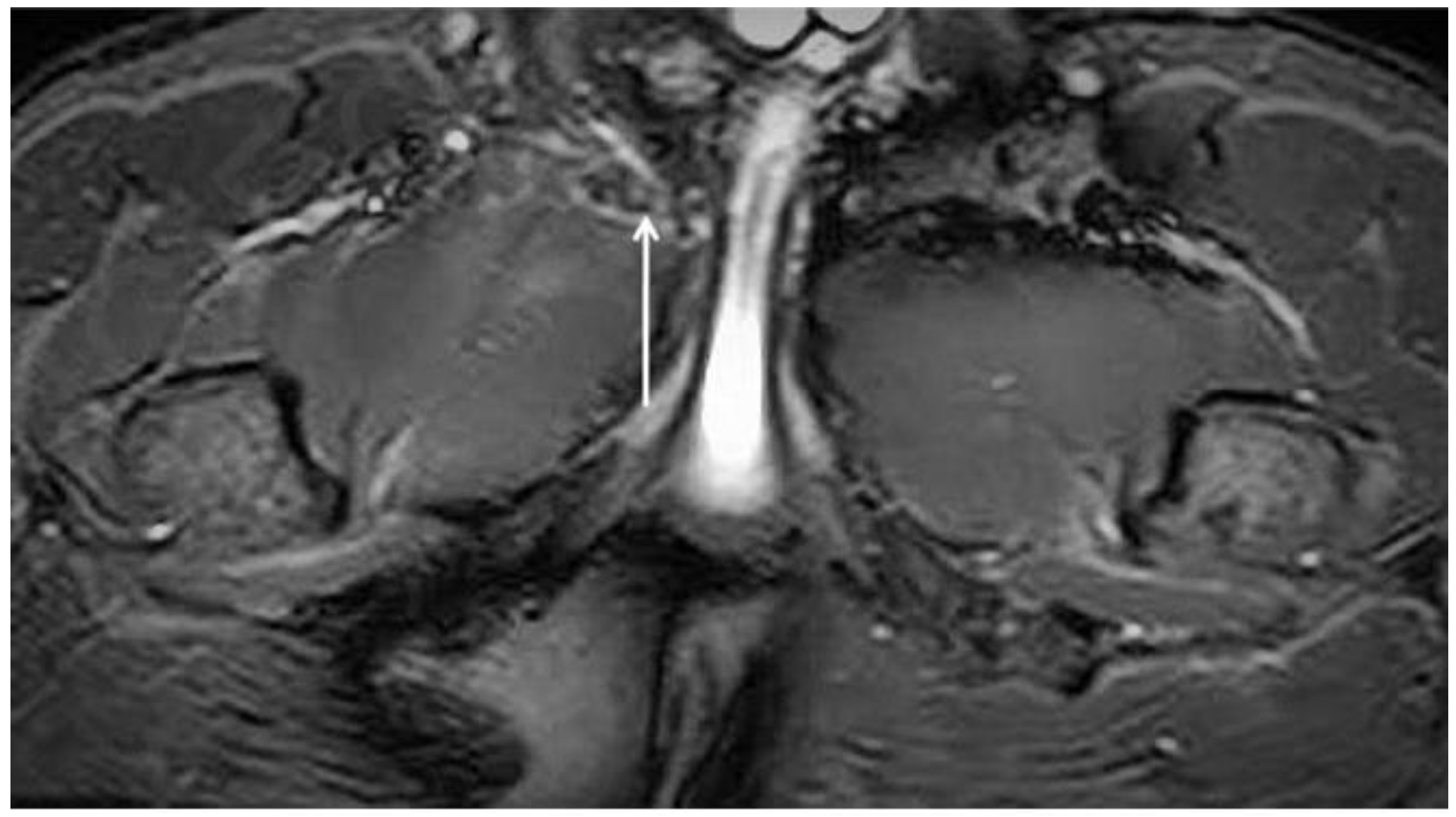
4.2. Pubic Osteopathy
- Bone marrow oedema of the pubic branches;
- Signs of bone reabsorption and sclerosis of the pubic branches;
- Symphysis irregularity and/or signs of bone erosion;
- Subchondral cysts and/or osteophyte formations;
- Central disc protrusion.
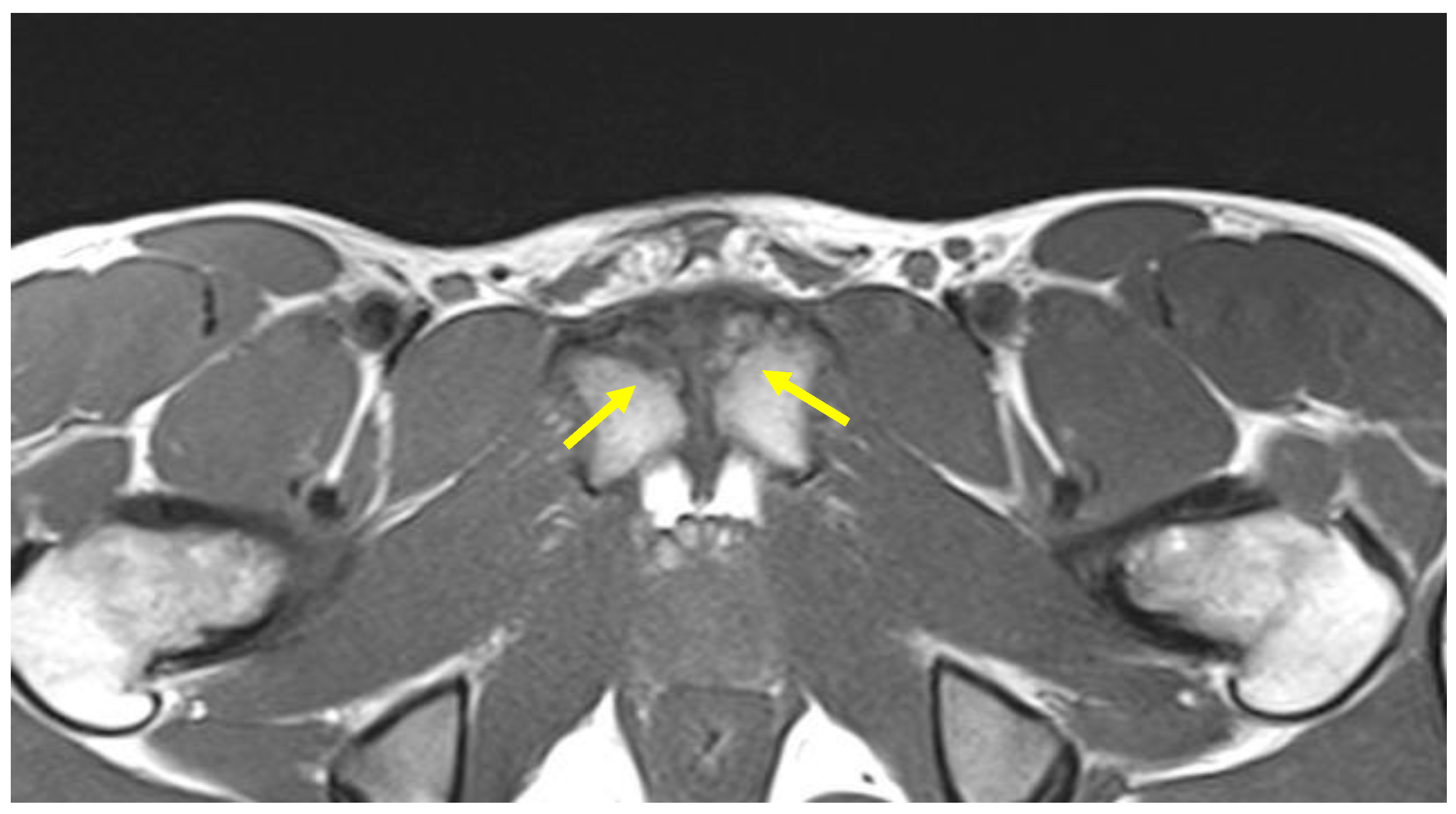
4.3. Adductor Muscle Injuries
4.4. Adductor Tendinopathy
- Oblique axial T1;
- Oblique axial PD FS; T2 FS and T1;
- Coronal T1.
4.5. Rectus Abdominis Injuries
4.6. Rectus Abdominis Tendinopathy
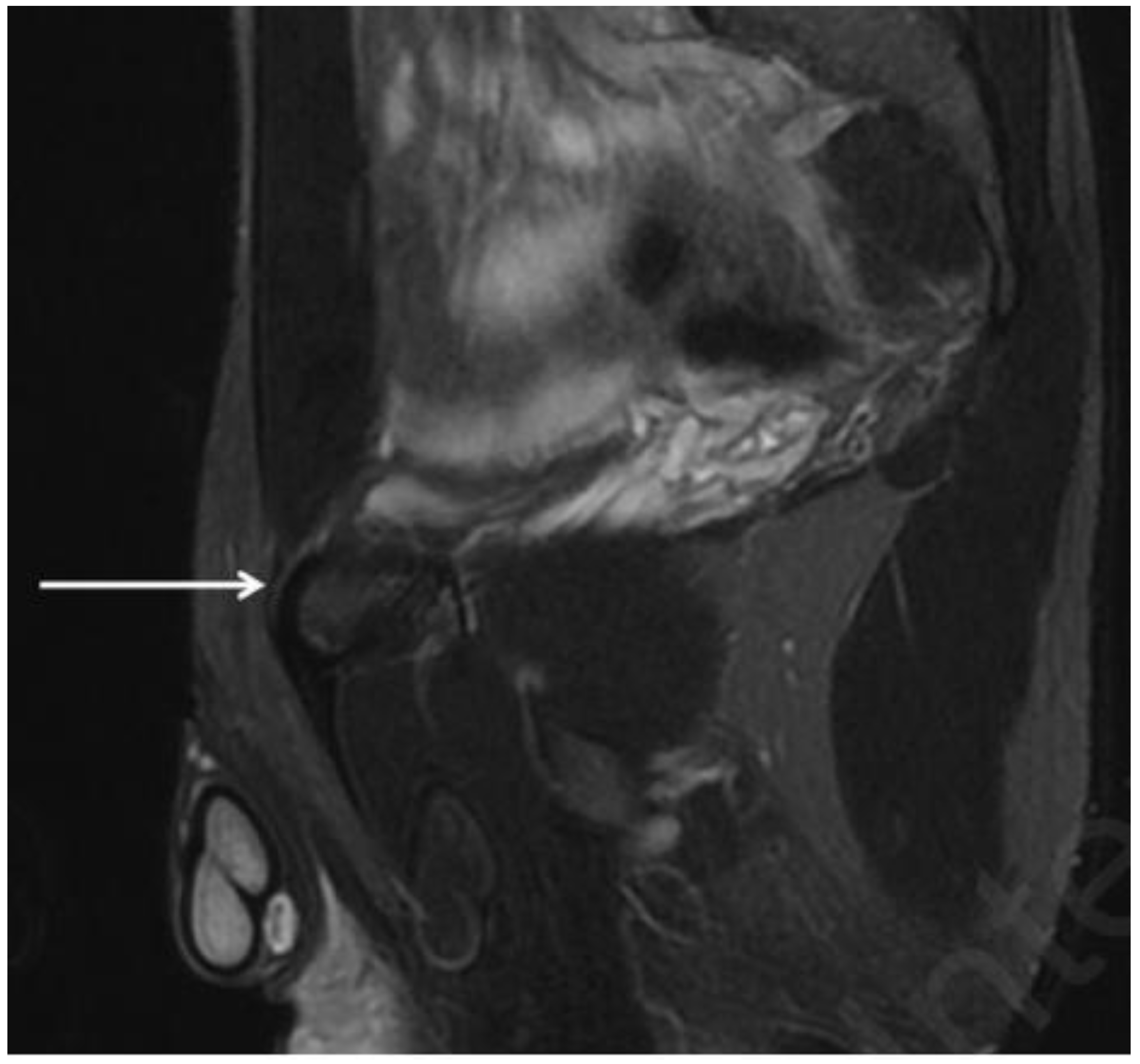
4.7. Inguinal Hernia
- Type I: the hernia contains only preperitoneal connective tissue and fat.
- Type II: the hernia progresses in the obturator canal and invaginates the peritoneal sac.
- Type III: the hernia presents further herniation of pelvic or peritoneal viscera such as bowel, bladder or ovary.
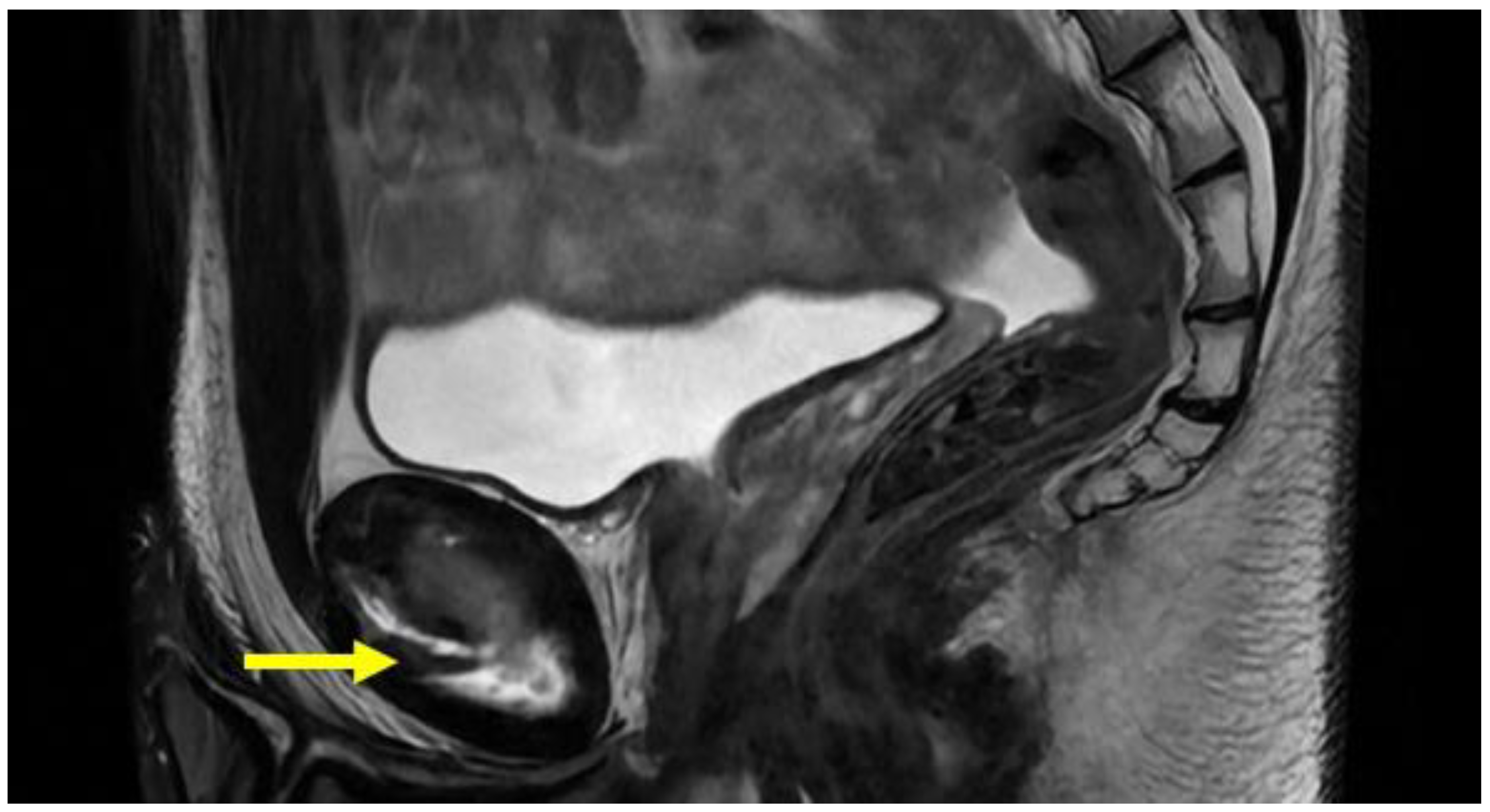
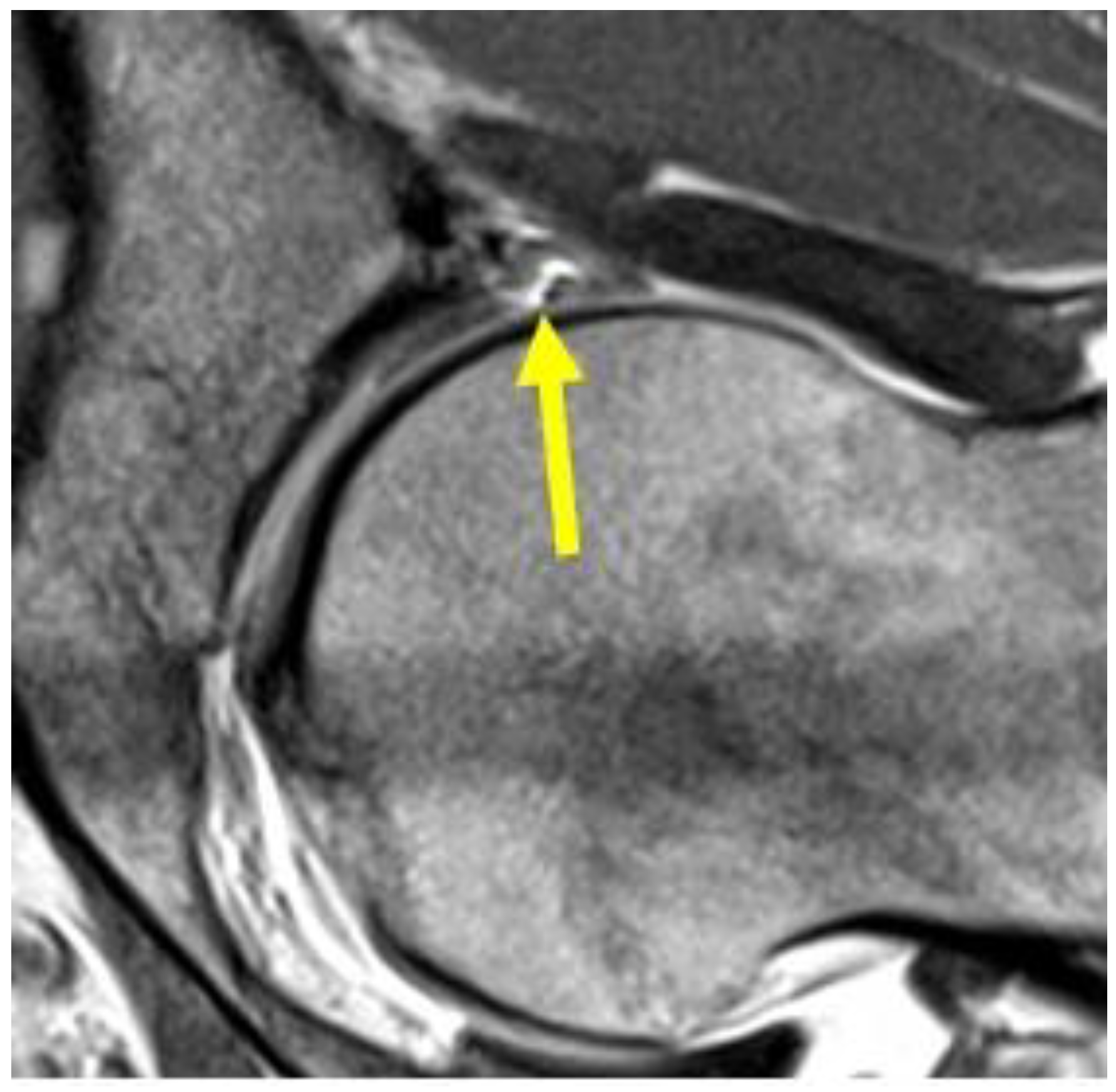
4.8. Hip Pathologies
- Coronal STIR (FOV 30–40 cm);
- Coronal PD or intermediate FS (FOV 16 cm),
- Sagittal or intermediate FS (FOV 16 cm),
- Radiant T1 or T1 FS.
4.9. Stress Fractures
4.10. Symphyseal Apophysitis
4.11. Bone Marrow Edema
5. Discussion
Limitations of the Study
6. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bisciotti, G.N.; Volpi, P.; Zini, R.; Auci, A.; Aprato, A.; Belli, A.; Bellistri, G.; Benelli, P.; Bona, S.; Bonaiuti, D.; et al. Groin Pain Syndrome Italian Consensus Conference on terminology, clinical evaluation and imaging assessment in groin pain in athlete. BMJ Open Sport Exerc. Med. 2016, 2, e000142, Erratum in: BMJ Open Sport Exerc. Med. 2017, 2, e000142corr1. [Google Scholar] [CrossRef] [PubMed]
- Mosler, A.B.; Weir, A.; Eirale, C.; Farooq, A.; Thorborg, K.; Whiteley, R.J.; Hölmich, P.; Crossley, K.M. Epidemiology of time loss groin injuries in a men’s professional football league: A 2-year prospective study of 17 clubs and 606 players. Br. J. Sports Med. 2018, 52, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Hölmich, P. Long-standing groin pain in sportspeople falls into three primary patterns, a “clinical entity” approach: A prospective study of 207 patients. Br. J. Sports Med. 2007, 41, 247–252, discussion 252. [Google Scholar] [CrossRef] [PubMed]
- Waldén, M.; Hägglund, M.; Ekstrand, J. The epidemiology of groin injury in senior football: A systematic review of prospective studies. Br. J. Sports Med. 2015, 49, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Junge, A.; Dvorak, J. Soccer injuries: A review on incidence and prevention. Sports Med. 2004, 34, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Junge, A.; Derman, W.; Schwellnus, M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br. J. Sports Med. 2011, 45, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Bjørneboe, J.; Bahr, R.; Andersen, T.E. Gradual increase in the risk of match injury in Norwegian male professional football: A 6-year prospective study. Scand. J. Med. Sci. Sports 2014, 24, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Noya Salces, J.; Gómez-Carmona, P.M.; Gracia-Marco, L.; Moliner-Urdiales, D.; Sillero-Quintana, M. Epidemiology of injuries in First Division Spanish football. J. Sports Sci. 2014, 32, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Harøy, J.; Andersen, T.E.; Bahr, R. Groin Problems in Male Soccer Players Are More Common Than Previously Reported: Response. Am. J. Sports Med. 2017, 45, NP32–NP33. [Google Scholar] [CrossRef] [PubMed]
- Bahr, R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br. J. Sports Med. 2009, 43, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Hägglund, M.; Ekstrand, J.; Waldén, M. Hip and groin time-loss injuries decreased slightly but injury burden remained constant in men’s professional football: The 15-year prospective UEFA Elite Club Injury Study. Br. J. Sports Med. 2019, 53, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.N.; Zini, R.; Aluigi, M.; Aprato, A.; Auci, A.; Bellinzona, E.; Benelli, P.; Bigoni, M.; Bisciotti, A.; Bisciotti, A.; et al. Groin Pain Syndrome Italian Consensus Conference update 2023. J. Sports Med. Phys. Fit. 2023, 64, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Omar, I.M.; Zoga, A.C.; Kavanagh, E.C.; Koulouris, G.; Bergin, D.; Gopez, A.G.; Morrison, W.B.; Meyers, W.C. Athletic pubalgia and “sports hernia”: Optimal MR imaging technique and findings. Radiographics 2008, 28, 1415–1438. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.; Auci, A.; Cena, E.; Corsini, A.; Bisciotti, A.; Eirale, C.; Parra, F.; Gassaghi, G.; Di Marzo, F.; Vuckvovic, Z.; et al. Potential MRI findings associated with inguinal hernia and inguinal canal posterior wall weakness in athletes. MLTJ 2018, 8, 290–304. [Google Scholar] [CrossRef]
- Becker, I.; Woodley, S.J.; Stringer, M.D. The adult human pubic symphysis: A systematic review. J. Anat. 2010, 217, 475–487. [Google Scholar] [CrossRef] [PubMed]
- McMinn, R.M. Last’s Anatomy. Regional and Applied, 9th ed.; Churchill Livingstone: Edinburgh, UK, 1994; p. 414. [Google Scholar]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th ed.; Churchill Livingstone Elsevier: New York, NY, USA, 2008; p. 1365. [Google Scholar]
- Rosse, C.; Gaddum-Rosse, P. Hollinshead’s Textbook of Anatomy, 5th ed.; Lippincott-Raven: New York, NY, USA, 1997; p. 313. [Google Scholar]
- Gray, H. Anatomy: Descriptive and Surgical; John W Parker and Son: London, UK, 1858; pp. 155–156. [Google Scholar]
- Testut, J.; Latarjet, A. Traite d’Anatomie Humaine, 8th ed.; Gaston Doin & C: Paris, France, 1928; pp. 663–669. [Google Scholar]
- Gamble, J.G.; Simmons, S.C.; Freedman, M. The symphysis pubis. Anatomic and pathologic considerations. Clin. Orthop. Relat. Res. 1986, 261–272. [Google Scholar] [CrossRef]
- Schilders, E.; Mitchell, A.W.M.; Johnson, R.; Dimitrakopoulou, A.; Kartsonaki, C.; Lee, J.C. Proximal adductor avulsions are rarely isolated but usually involve injury to the PLAC and pectineus: Descriptive MRI findings in 145 athletes. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, A.; Bisciotti, G.N.; Eirale, C.; Bisciotti, A.; Auci, A.; Bona, S.; Zini, R. Prepubic aponeurotic complex injuries: A structured narrative review. J. Sports Med. Phys. Fit. 2022, 62, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Pieroh, P.; Li, Z.L.; Kawata, S.; Ogawa, Y.; Josten, C.; Steinke, H.; Dehghani, F.; Itoh, M. The topography and morphometrics of the pubic ligaments. Ann. Anat. 2021, 236, 151698. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, T.; Van Glabbeek, F.; Van Nassauw, L.; Van Den Plas, K.; Denteneer, L.; Stassijns, G. New insights into the musculotendinous and ligamentous attachments at the pubic symphysis: A systematic review. Ann. Anat. 2022, 244, 151959. [Google Scholar] [CrossRef]
- Meissner, A.; Fell, M.; Wilk, R.; Boenick, U.; Rahmanzadeh, R. Zur Biomechanik der Symphyse. Welche Kräfte führen zur Mobilität der Symphyse unter physiologischen Bedingungen? [Biomechanics of the pubic symphysis. Which forces lead to mobility of the symphysis in physiological conditions?]. Unfallchirurg 1996, 99, 415–421. (In German) [Google Scholar] [PubMed]
- Birmingham, P.M.; Kelly, B.T.; Jacobs, R.; McGrady, L.; Wang, M. The effect of dynamic femoroacetabular impingement on pubic symphysis motion: A cadaveric study. Am. J. Sports Med. 2012, 40, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.N.; Di Marzo, F.; Auci, A.; Parra, F.; Cassaghi, G.; Corsini, A.; Petrera, M.; Volpi, P.; Vuckovic, Z.; Panascì, M.; et al. Cam morphology and inguinal pathologies: Is there a possible connection? J. Orthop. Traumatol. 2017, 18, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Radin, E.L.; Sheldon, R.S. Practical Biomechanics for the Orthopedic Surgeon, 2nd ed.; Churchill Livingstone: London, UK, 1979. [Google Scholar]
- Tuma, F.; Lopez, R.A.; Varacallo, M. Anatomy, Abdomen and Pelvis: Inguinal Region (Inguinal Canal). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lytle, W.J. Inguinal anatomy. J. Anat. 1979, 128 Pt 3, 581–594. [Google Scholar] [PubMed]
- HerniaSurge Group. International guidelines for groin hernia management. Hernia 2018, 22, 1–165. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, K.; Daruge, P.; Bordalo-Rodrigues, M.; Pedrinelli, A.; Busetto, A.M. Imaging Assessment of the Pubis in Soccer Players. Rev. Bras. Ortop. 2019, 54, 118–127. [Google Scholar] [CrossRef]
- Aguirre, D.A.; Santosa, A.C.; Casola, G.; Sirlin, C.B. Abdominal wall hernias: Imaging features, complications, and diagnostic pitfalls at multi-detector row CT. Radiographics 2005, 25, 1501–1520. [Google Scholar] [CrossRef] [PubMed]
- Garvey, J.F.; Read, J.W.; Turner, A. Sportsman hernia: What can we do? Hernia 2010, 14, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mercouris, P. Sports hernia: A pictorial review. SA J. Radiol. 2014, 18, 1–4. [Google Scholar] [CrossRef]
- Matsuda, D.K.; Matsuda, N.A.; Head, R.; Tivorsak, T. Endoscopic Rectus Abdominis and Prepubic Aponeurosis Repairs for Treatment of Athletic Pubalgia. Arthrosc. Tech. 2017, 6, e183–e188. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.; O’connell, M.J.; Ryan, M.; Cunningham, P.; Taylor, D.; Cronin, C.; O’neill, P.; Eustace, S. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology 2005, 235, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.N.; Auci, A.; Bona, S.; Bisciotti, A.; Bisciotti, A.; Cassaghi, G.; Di Marzo, F.; Di Pietto, F.; Eirale, C.; Panascì, M.; et al. A multidisciplinary assessment of 320 athletes with long-standing groin pain syndrome in keeping with the Italian consensus agreement: The high incidence and the multiple causes of inguinal and hip pathologies and pubic osteopathy. J. Sports Med. Phys. Fit. 2021, 61, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Zoga, A.C.; Meyers, W.C. Magnetic resonance imaging of athletic pubalgia and the sports hernia: Current understanding and practice. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Palisch, A.; Zoga, A.C.; Meyers, W.C. Imaging of athletic pubalgia and core muscle injuries: Clinical and therapeutic correlations. Clin. Sports Med. 2013, 32, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.M.; Brennan, D.; O‘Connell, M.; MacMahon, P.; O‘Neill, P.; Eustace, S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: Observations at MRI. AJR Am. J. Roentgenol. 2007, 188, W291–W296. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, M.B.; Romero, A.A.; Silvers, H.J.; Harris, D.J.; Watanabe, D.; Mandelbaum, B.R. The prevalence of radiographic hip abnormalities in elite soccer players. Am. J. Sports Med. 2012, 40, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Verrall, G.M.; Hamilton, I.A.; Slavotinek, J.P.; Oakeshott, R.D.; Spriggins, A.J.; Barnes, P.G.; Fon, G.T. Hip joint range of motion reduction in sports-related chronic groin injury diagnosed as pubic bone stress injury. J. Sci. Med. Sport 2005, 8, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Vuckovic, Z.; Mosler, A.; Agricola, R.; Otten, R.; Jacobsen, P.; Holmich, P.; Weir, A. Multidisciplinary Assessment of 100 Athletes With Groin Pain Using the Doha Agreement: High Prevalence of Adductor-Related Groin Pain in Conjunction With Multiple Causes. Clin. J. Sport. Med. 2018, 28, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zini, R.; Panascì, M.; Santori, N.; Potestio, D.; Di Pietto, F.; Bisciotti, G.N. The Italian Consensus Conference on FAI Syndrome in Athletes (Cotignola Agreement). MLTJ 2023, 13, 46–60. [Google Scholar] [CrossRef]
- Serner, A.; Tol, J.L.; Jomaah, N.; Weir, A.; Whiteley, R.; Thorborg, K.; Robinson, M.; Hölmich, P. Diagnosis of Acute Groin Injuries: A Prospective Study of 110 Athletes. Am. J. Sports Med. 2015, 43, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Serner, A.; Weir, A.; Tol, J.L.; Thorborg, K.; Roemer, F.; Guermazi, A.; Yamashiro, E.; Hölmich, P. Characteristics of acute groin injuries in the adductor muscles: A detailed MRI study in athletes. Scand. J. Med. Sci. Sports 2018, 28, 667–676. [Google Scholar] [CrossRef] [PubMed]
- De Maeseneer, M.; Forsyth, R.; Provyn, S.; Milants, A.; Lenchik, L.; De Smet, A.; Marcelis, S.; Shahabpour, M. MR imaging-anatomical-histological evaluation of the abdominal muscles, aponeurosis, and adductor tendon insertions on the pubic symphysis: A cadaver study. Eur. J. Radiol. 2019, 118, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.N.; Volpi, P.; Alberti, G.; Aprato, A.; Artina, M.; Auci, A.; Bait, C.; Belli, A.; Bellistri, G.; Bettinsoli, P.; et al. Italian consensus statement (2020) on return to play after lower limb muscle injury in football (soccer). BMJ Open Sport. Exerc. Med. 2019, 5, e000505. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Canonico, R.; De Luca, G.; Bisciotti, G.N.; Rusconi, G.; Barillaro, A.; Di Pietto, F. Magnetic resonance imaging predicts the days lost from training and competition: Evaluation of 56 indirect muscle injuries in professional football players. J. Sports Med. Phys. Fit. 2024, 64, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Branci, S.; Thorborg, K.; Nielsen, M.B.; Hölmich, P. Radiological findings in symphyseal and adductor-related groin pain in athletes: A critical review of the literature. Br. J. Sports Med. 2013, 47, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Branci, S.; Thorborg, K.; Bech, B.H.; Boesen, M.; Nielsen, M.B.; Hölmich, P. MRI findings in soccer players with long-standing adductor-related groin pain and asymptomatic controls. Br. J. Sports Med. 2015, 49, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Paajanen, H.; Hermunen, H.; Ristolainen, L.; Branci, S. Long-standing groin pain in contact sports: A prospective case-control and MRI study. BMJ Open Sport. Exerc. Med. 2019, 5, e000507. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Hägglund, M.; Waldén, M.; Ekstrand, J. UEFA injury study: A prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br. J. Sports Med. 2009, 43, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.N.; Chamari, K.; Cena, E.; Garcia, G.R.; Vuckovic, Z.; Bisciotti, A.; Bisciotti, A.; Zini, R.; Corsini, A.; Volpi, P. The conservative treatment of longstanding adductor-related groin pain syndrome: A critical and systematic review. Biol. Sport. 2021, 38, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.N.; Auci, A.; Bona, S.; Bisciotti, A.; Bisciotti, A.; Cassaghi, G.; Di Marzo, F.; Di Pietto, F.; Eirale, C.; Panascì, M.; et al. Long-standing groin pain syndrome in athletic women: A multidisciplinary assessment in keeping with the Italian Consensus Agreement. J. Sports Med. Phys. Fit. 2022, 62, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Droukas, D.D.; Zoland, M.P.; Klein, D.A. Radiographic and surgical findings of type I obturator hernias in patients with refractory groin pain. Clin. Imaging 2019, 55, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mercado, M.; Diab, J.; Loi, K. A delayed diagnosis of obturator hernia hoodwinked by previous laparoscopic inguinal hernia repair. J. Surg. Case Rep. 2021, 2021, rjab407. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, R.J., Jr.; Forse, R.A. Clinical practice. Groin hernias in adults. N. Engl. J. Med. 2015, 372, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Hesper, T.; Neugroda, C.; Schleich, C.; Antoch, G.; Hosalkar, H.; Krauspe, R.; Zilkens, C.; Bittersohl, B. T2*-Mapping of Acetabular Cartilage in Patients With Femoroacetabular Impingement at 3 Tesla: Comparative Analysis with Arthroscopic Findings. Cartilage 2018, 9, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Blankenbaker, D.G.; De Smet, A.A. Hip injuries in athletes. Radiol. Clin. N. Am. 2010, 48, 1155–1178. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.; Zubler, V.; Hoffmann, A.; Mamisch-Saupe, N.; Dora, C.; Kalberer, F.; Zanetti, M.; Hodler, J.; Pfirrmann, C.W. Hip MRI: How useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am. J. Roentgenol. 2014, 202, 160–169. [Google Scholar] [CrossRef]
- Llopis, E.; Fernandez, E.; Cerezal, L. MR and CT arthrography of the hip. Semin. Musculoskelet. Radiol. 2012, 16, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Naraghi, A.; White, L.M. MRI of Labral and Chondral Lesions of the Hip. AJR Am. J. Roentgenol. 2015, 205, 479–490. [Google Scholar] [CrossRef]
- Weishuhn, L.J.; Seidman, A. Hip Arthrogram. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bernstein, E.M.; Kelsey, T.J.; Cochran, G.K.; Deafenbaugh, B.K.; Kuhn, K.M. Femoral Neck Stress Fractures: An Updated Review. J. Am. Acad. Orthop. Surg. 2022, 30, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, A.; Schilders, E. Current concepts of inguinal-related and adductor-related groin pain. Hip Int. 2016, 26 (Suppl. S1), 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kijowski, R.; Tuite, M.J. Pediatric throwing injuries of the elbow. Semin. Musculoskelet. Radiol. 2010, 14, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.D. Imaging in athletic groin pain. In Sports Hernia and Athletic Pubalgia; Diduch, D.R., Brunt, L.M., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Nakayama, K.; Utsunomiya, H.; Murata, Y.; Takada, S.; Tsukamoto, M.; Sakai, A.; Uchida, S. Cleft Sign and Bone Marrow Edema of the Pubic Symphysis Are Associated With Sports and Bony Morphology in Patients with Femoroacetabular Impingement and Labral Tears. Orthop. J. Sports Med. 2022, 10, 23259671211068477. [Google Scholar] [CrossRef] [PubMed]
- Verrall, G.M.; Slavotinek, J.P.; Fon, G.T. Incidence of pubic bone marrow oedema in Australian rules football players: Relation to groin pain. Br. J. Sports Med. 2001, 35, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zoga, A.C.; Mullens, F.E.; Meyers, W.C. The spectrum of MR imaging in athletic pubalgia. Radiol. Clin. N. Am. 2010, 48, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Riff, A.J.; Movassaghi, K.; Beck, E.C.; Neal, W.H.; Inoue, N.; Coleman, S.H.; Nho, S.J. Surface Mapping of the Musculotendinous Attachments at the Pubic Symphysis in Cadaveric Specimens: Implications for the Treatment of Core Muscle Injury. Arthroscopy 2019, 35, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Hölmich, P.; Uhrskou, P.; Ulnits, L.; Kanstrup, I.L.; Nielsen, M.B.; Bjerg, A.M.; Krogsgaard, K. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: Randomised trial. Lancet 1999, 353, 439–443. [Google Scholar] [CrossRef] [PubMed]
- DeLang, M.D.; Garrison, J.C.; Hannon, J.P.; McGovern, R.P.; Sheedy, P.J.; Christoforetti, J.J.; Thorborg, K. Midseason Screening for Groin Pain, Severity, and Disability in 101 Elite American Youth Soccer Players: A Cross-Sectional Study. Clin. J. Sport. Med. 2022, 32, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Bordalo, M.; Arnaiz, J.; Yamashiro, E.; Al-Naimi, M.R. Imaging of Muscle Injuries: MR Imaging-Ultrasound Correlation. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 163–179. [Google Scholar] [CrossRef]
- Lee, J.H.; Houck, D.A.; Gruizinga, B.A.; Garabekyan, T.; Jesse, M.K.; Kraeutler, M.J.; Mei-Dan, O. Correlation of Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) Value With Hip Arthroscopy Intraoperative Findings and Midterm Periacetabular Osteotomy Outcomes. Orthop. J. Sports Med. 2022, 10, 23259671221117606. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Yamasaki, T.; Izumi, S.; Sawa, M.; Akiyama, Y.; Yasunaga, Y.; Adachi, N. Evaluation of articular cartilage following rotational acetabular osteotomy for hip dysplasia using T2 mapping MRI. Skelet. Radiol. 2018, 47, 1467–1474. [Google Scholar] [CrossRef]





| Acquisition Plan | Sequences | Slice (Max) | FOV (Max) |
|---|---|---|---|
| Entire pelvis coronal | STIR | 5 mm | 32–40 cm |
| Coronal | T1 | 3 mm | 14–18 cm |
| Axial | T2 | 3 mm | 14–18 cm |
| Oblique axial | PD FS or Intermediate FS | 3 mm | 14–18 cm |
| Oblique coronal | PD FS or Intermediate FS | 3 mm | 14–18 cm |
| (1) Articular causes |
| Acetabular labrum tear |
| Femoroacetabular impingement (FAI) |
| Hip osteoarthritis |
| Intra-articular loose bodies |
| Hip instability |
| Adhesive capsulitis |
| Legg–Calvé–Perthes disease and its outcomes |
| Dysplasia and its outcomes |
| Epiphysiolysis and its outcomes |
| Avascular necrosis of the femoral head |
| Sacroiliac joint disorders |
| Lumbar spine disorders |
| Synovitis |
| (2) Extra-articular causes |
| Anterior inferior iliac spine impingement |
| Hip antero-superior labral tear with avulsion of rectus femoris |
| Ischiofemoral impingement syndrome |
| (3) Visceral causes |
| Inguinal hernia |
| Other types of abdominal hernia |
| Intestinal diseases |
| (4) Bone causes |
| Fractures and their outcomes |
| Stress fractures |
| Avulsion fractures |
| Iliac crest contusion (hip pointers) |
| (5) Musculotendinous causes |
| Rectus abdominis injuries and/or tendinopathy |
| Adductors muscles injuries and/or tendinopathy |
| Rectus abdominis–adductor longus common aponeurosis injuries |
| Iliopsoas injuries and/or tendinopathy |
| Prepubic aponeurotic complex (PPAC) injuries |
| Other indirect muscle injuries and their outcomes |
| Direct muscle injuries |
| Iliopsoas impingement (I) |
| Snapping internal or external hip |
| Bursitis |
| Weakness of the inguinal canal posterior wall |
| (6) Pubic symphysis-related causes |
| Osteitis pubis |
| Symphysis instability |
| Symphysis degenerative arthropathy |
| (7) Neurological causes |
| Nerve entrapment syndrome |
| Anterior cutaneous nerve entrapment syndrome |
| (8) Developmental causes |
| Apophysitis |
| Growth plate at pubic level |
| (9) Genitourinary disease-related causes (inflammatory and non-inflammatory) |
| Prostatitis |
| Epididymitis |
| Corditis |
| Orchitis |
| Varicocele |
| Hydrocele |
| Urethritis |
| Other infections of the urinary tract |
| Cystitis |
| Ovarian cysts |
| Endometriosis |
| Ectopic pregnancy |
| Round ligament entrapment |
| Testicular/ovarian torsion |
| Ureteral lithiasis |
| (10) Neoplastic causes |
| Testicular carcinoma |
| Osteoid osteoma |
| Other carcinomas |
| (11) Infectious causes |
| Osteomyelitis |
| Septic arthritis |
| (12) Systemic causes |
| Inguinal lymphadenopathy |
| Rheumatic diseases |
| Pathologies | RM Sequences | RM Findings |
|---|---|---|
| PPAC injuries | T2, STIR, PD FS and intermediate FS sequences in axial, coronal and sagittal plans | Signal hyperintensity in fluid-sensitive sequences. |
| Adductor muscle injuries | Axial oblique PD FS and T2 FS. Coronal STIR | Signal hyperintensity in fluid-sensitive sequences. |
| Adductor tendinopathy | Axial oblique T1; axial oblique PD FS; T2 FS and T1; coronal T1 | Increased signal intensity at the tendon level and/or at its enthesis level in the fluid-sensitive sequences. Tendon swelling and/or changes in enthesis morphology. |
| Rectus abdominis injuries | Sagittal STIR and axial oblique PD FS | Signal hyperintensity in fluid-sensitive sequences. |
| Rectus abdominis tendinopathy | Sagittal STIR and axial oblique PD FS | Increased signal intensity in the fluid-sensitive sequence at rectus abdominis muscle–tendon junction level and/or an increased rectus abdominis tendon volume. |
| Obturator hernia | Coronal and axial T1- and PD-weighted sequences | Protrusion of fat through the foramen between the pectineus and obturator externus muscles. Very important evaluation of the comparison for symmetry with the contralateral canal. |
| Acetabular labrum lesion | MRI arthrography: coronal STIR (FOV 30–40 cm); coronal PD or intermediate FS (FOV 16 cm); sagittal or intermediate FS (FOV 16 cm); radiant T1 or T1 FS. | Spreading of the contrast medium into the labral defect. |
| Stress fractures | T1, T2 and STIR in coronal, sagittal and axial view | Signal hyperintensity in the fluid-sensitive sequences and signal hypointensity in T1 sequences. |
| Symphyseal apophysitis | Coronal T1; axial T1 | Signal hypointensity corresponding to the anteromedial ossification nucleus. |
| Bone marrow oedema | Coronal T1; coronal T2 FS; axial oblique T2 FS; axial oblique PD FS | Signal hyperintensity in the fluid-sensitive sequences. Signal hypointensity in T1 sequences. Grade 1: BMO ≤ 1 cm; Grade 2: BMO ≥ 1 cm and ≤2 cm; Grade 3: BMO ≥ 2 cm. |
| Subchondral cyst | Coronal STIR. Axial oblique T2 | Presence of subchondral cyst (hyperintense subchondral cystic element in fluid-sensitive sequences). |
| Central disc protrusion | Coronal T1. Axial oblique T1 | Protrusion of the central symphyseal fibrous disc. In coronal images, the central disc protrudes cranially with respect to the margins of the symphyseal joint. In oblique axial sequences, it protrudes posteriorly. |
| Secondary inferior cleft sign | Coronal STIR. Axial oblique PD FS | High signal intensity line extending laterally and inferiorly to the lower part of the symphysis, which appears to be in communication with the symphyseal joint space. |
| Secondary superior cleft sign | Coronal STIR. Axial oblique PD FS | High signal intensity line in fluid-sensitive sequences extending parallel to the inferior border of the superior pubic ramus shows connection with the symphyseal joint space. |
| Sclerosis of the symphysis | Coronal T1; axial oblique T1 | Presence of bone sclerosis along the articular margins of the symphysis. The sclerotic area appears as hypointense bone formation (increased thickness) along the articular margins of the symphysis. |
| Fatty infiltration | Coronal T1; coronal STIR; axial oblique T2 FS; axial oblique PD FS | Areas of high signal intensity at the level of the symphysis in T1-weighted sequences and areas of low signal intensity in fat-saturated sequences |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisciotti, G.N.; Di Pietto, F.; Rusconi, G.; Bisciotti, A.; Auci, A.; Zappia, M.; Romano, S. The Role of MRI in Groin Pain Syndrome in Athletes. Diagnostics 2024, 14, 814. https://doi.org/10.3390/diagnostics14080814
Bisciotti GN, Di Pietto F, Rusconi G, Bisciotti A, Auci A, Zappia M, Romano S. The Role of MRI in Groin Pain Syndrome in Athletes. Diagnostics. 2024; 14(8):814. https://doi.org/10.3390/diagnostics14080814
Chicago/Turabian StyleBisciotti, Gian Nicola, Francesco Di Pietto, Giovanni Rusconi, Andrea Bisciotti, Alessio Auci, Marcello Zappia, and Stefania Romano. 2024. "The Role of MRI in Groin Pain Syndrome in Athletes" Diagnostics 14, no. 8: 814. https://doi.org/10.3390/diagnostics14080814
APA StyleBisciotti, G. N., Di Pietto, F., Rusconi, G., Bisciotti, A., Auci, A., Zappia, M., & Romano, S. (2024). The Role of MRI in Groin Pain Syndrome in Athletes. Diagnostics, 14(8), 814. https://doi.org/10.3390/diagnostics14080814






