CAR-T Cell Therapy in Ovarian Cancer: Where Are We Now?
Abstract
:1. Introduction
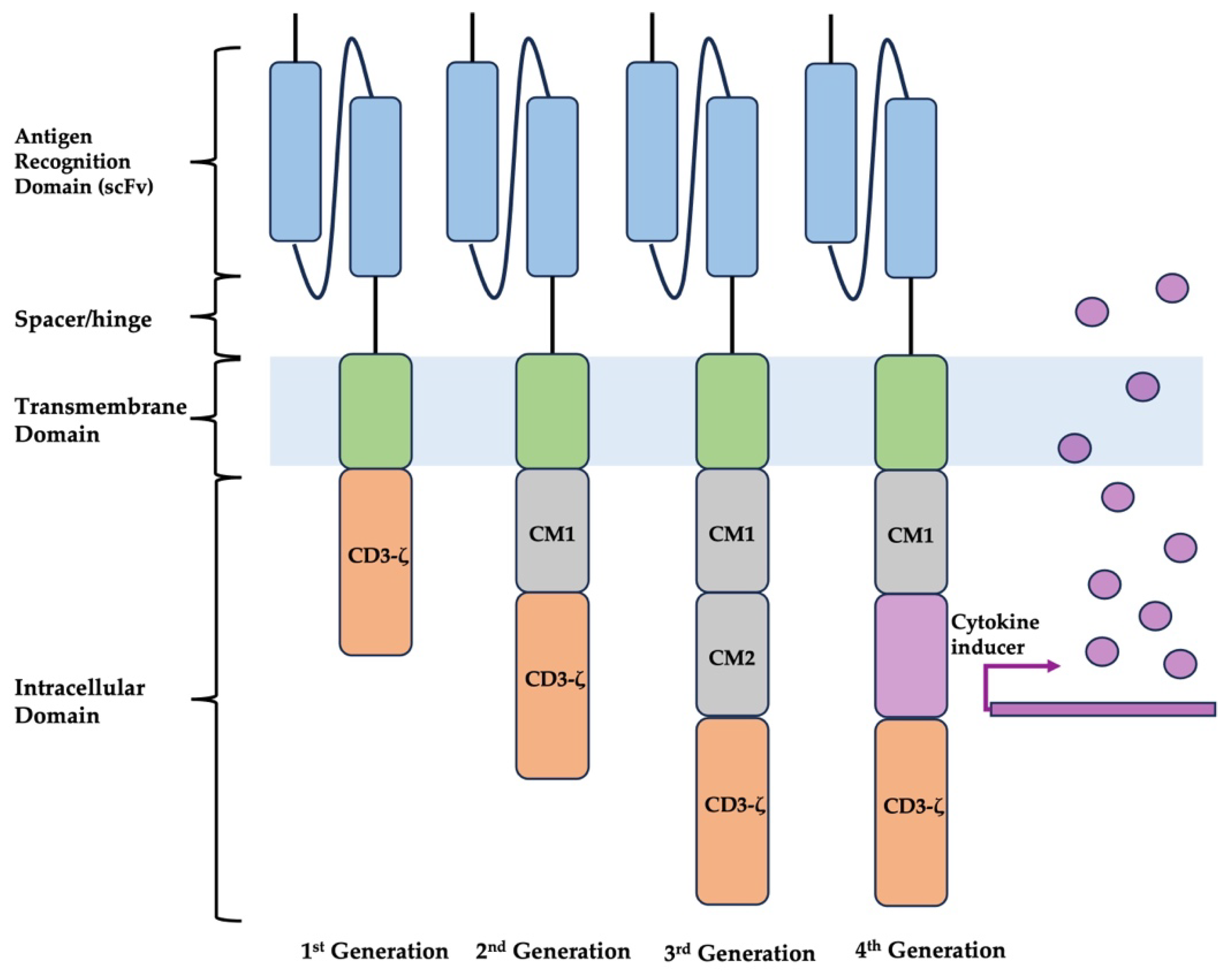
2. CAR-T Design
3. Target Antigens
3.1. Mesothelin
3.2. Folate Receptor Alpha
3.3. Mucin 16
3.4. Human Epidermal Growth Factor Receptor 2
3.5. Other
| Antigen | Expression in Normal Tissues | |
|---|---|---|
| Clinical Trials | ALPP | placenta [46] |
| B7H3 | epithelial cells, pleural effusion, anterior pituitary progenitor cells, and human serum [47] | |
| c-MET | epithelial cells [48] | |
| CD70 | antigen-activated T and B cells [49] | |
| Claudin 18 | gastric mucosa [50] | |
| FRα | lung, kidneys, choroid plexus, intestines, heart, and placental tissue [25] | |
| HER2 | nervous system, epithelial cells, or the mammary gland [51] | |
| HLA-G | embryonic tissues, immune-privileged organs (thymus, cornea, pancreatic islets), hematopoietic lineage (erythroblasts, macrophages, antigen-presenting cells, and dendritic cells) [52] | |
| Mesothelin | pancreatic, ovarian, mesothelioma cells, and squamous epithelium [20] | |
| MUC 1 | mammary gland, esophagus, stomach, duodenum, pancreas, uterus, prostate lungs, and hematopoietic cells [53] | |
| MUC 16 | Bronchial, endometrial, ovarian, and corneal epithelial cells [30] | |
| NKG2D | NK cells, CD8+ T cells, γδ T cells, NK1.1+ T cells, and some myeloid cells [54] | |
| TAG72 | secretory endometrial tissue, duodenal goblet cells [55] | |
| Preclinical Studies | 5T4 Oncofetal antigen | placenta [42] |
| AMHR2 | gonads, endometrium, liver parenchyma, epithelial lining of the lung, small intestine mucosa, adrenal, pancreas, and kidney [56] | |
| ANXA2 | endothelial cells, macrophages, and mononuclear cells [57] | |
| Claudin 6 | fetal stomach, fetal kidney, fetal pancreas, and fetal lung [32,33] | |
| EPCAM | epithelial cells (excluding squamous epithelial, epidermal keratinocytes, gastric parietal cells, myoepithelial cells, thymic cortical epithelium, and hepatocytes), embryonic and hepatic stem cells [58] | |
| NGcGM3 | not present [44] | |
| PDL-1 | antigen-presenting cells, mesenchymal stem cells, and bone marrow-derived mast cells [59] | |
| PTK7 | embryonic tissues, immature CD4+ recent thymic emigrants (RTEs), and plasmacytoid dendritic cells (pDCs) [34,35] | |
| SSEA-4 | embryonic tissues, germ cells [60] | |
| TM4SF1 | endothelium, skin, lung, and germ cells [61] |
4. Clinical Trials and Outcomes
4.1. Mesothelin
4.2. Other
| Target | Phase | N | Treatment | Route | Premedication Regimen | Clinical Outcome | Toxicities | Location | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MESO | I | 15 (5 Ovarian) | lentiviral transduced CART-meso cells | IV | +/− cyclophosphamide | SD 11/15 at 28 days, 3/8 at 2–3 months | Grade 3: ascites (n = 4), fatigue, abdominal pain, abdominal distension, AKI, bacteremia, hepatic failure, hepatitis, dyspnea, DIC, lymphopenia, increased alkaline phosphate, increased ALT, increased AST, increased bilirubin Grade 4: sepsis, anemia | USA | [63] |
| MESO | – | 3 Ovarian | anti-MESO CAR-T cells (LD013) | IV | – | SD 2/3 (PFS 4.6, 5.6 months) | Grade 3/4: none | China | [66] |
| FRα | I | 14 Ovarian | anti-alpha folate receptor CAR-T | IV | – | PD 14/14 | Grade 3/4: hypotension (n = 4), dyspnea (n = 2), fatigue, leukopenia, anemia, thrombocytopenia, rigors, sinus tachycardia, diarrhea | USA | [72] |
| Target | Phase | Treatment | Route | Premedication Regimen | Patient Population | N | Status | Study Completion | Location | Clinical Trial Identifier |
|---|---|---|---|---|---|---|---|---|---|---|
| ALPP | I | retroviral vector-transducted autologous T cells to express anti-ALPP CARs | IV | cyclophosphamide, fludarabine | ALPP-positive metastatic ovarian and endometrial cancer | 20 | Not Yet Recruiting | 12/31/2023 (estimated) | China | NCT04627740 |
| B7H3 | I, II | fhB7H3.CAR-Ts | IP | cyclophosphamide, fludarabine | ovarian cancer, especially patients with refractory ascites, with expression of B7H3 antigen in tumor tissue | 15 | Recruiting | 8/31/2026 (estimated) | China | NCT05211557 |
| B7H3 | I | CAR.B7-H3 T cells | IP | cyclophosphamide, fludarabine | recurrent epithelial ovarian cancer | 21 | Recruiting | 2/1/2030 (estimated) | USA | NCT04670068 |
| c-Met | I, II | CAR-T cell immunotherapy (anti-c-MET in ovarian cancer patients) | IV | cyclophosphamide, fludarabine | malignancy with positive expression of one of 10 different antigens (c-MET for ovarian cancer patients) | 73 | Unknown | 3/1/2023 (estimated) | China | NCT03638206 |
| CD70 | I | CD70 CAR T cells | IV or IP | cyclophosphamide, fludarabine | CD70-positive advanced/metastatic solid tumors | 36 | Recruiting | 5/30/2025 (estimated) | China | NCT05518253 |
| CD70 | I | CD70 CAR T cells | IV or IP | cyclophosphamide, fludarabine | CD70-positive advanced/metastatic solid tumors | 36 | Recruiting | 12/31/2024 (estimated) | China | NCT05420545 |
| CD70 | I | CD70 CAR T cells | IV or IP | cyclophosphamide, fludarabine | CD70-positive advanced/metastatic solid tumors | 48 | Recruiting | 7/17/2024 (estimated) | China | NCT05468190 |
| CD70 | I | CD70 CAR T cells | IV or IP | cyclophosphamide, fludarabine | CD70-positive advanced/metastatic solid tumors | 48 | Recruiting | 9/30/2026 (estimated) | China | NCT06010875 |
| CD70 | I, II | anti-hCD70 CAR T cells | IV | cyclophosphamide, fludarabine, aldesleukin | metastatic or unresectable CD70-expressing cancer | 124 | Recruiting | 1/1/2028 (estimated) | USA | NCT02830724 |
| Claudin 18 | I | claudin 18.2 CAR-T | IV | yes, not specified | advanced solid tumors with positive CLDN18.2 expression | 30 | Recruiting | 12/1/2024 (estimated) | China | NCT05472857 |
| FRα | I | lentiviral transduced MOv19-BBz CAR T cells | IP | with and without cyclophosphamide, fludarabine | advanced persistent or recurrent high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer-expressing aFR | 18 | Recruiting | 10/2038 (estimated) | USA | NCT03585764 |
| HER2 | I | CCT303-406 CAR-modified autologous T cells | IV | cyclophosphamide, fludarabine | relapsed or refractory stage IV HER2-positive cancers | 15 | Recruiting | 3/29/2025 (estimated) | China | NCT04511871 |
| HLA-G | I, II | anti-HLA-G CAR-T cells IVS-3001 | IV | cyclophosphamide, fludarabine | previously treated, locally advanced, or metastatic HLA-G-positive solid tumors | 117 | Recruiting | 12/29/2029 (estimated) | USA | NCT05672459 |
| MESO | I | MESO CAR-T cells | IV | cyclophosphamide, fludarabine | relapsed and refractory ovarian cancer | 20 | Unknown | 4/1/2022 (estimated) | China | NCT03799913 |
| MESO | I, II | anti-MESO CAR-T cells | IV | cyclophosphamide, fludarabine | MESO-positive relapsed and refractory epithelial ovarian cancer | 20 | Unknown | 4/20/2023 (estimated) | China | NCT03916679 |
| MESO | I | MSLN-CAR T cells secreting PD-1 nanobodies | IV | cyclophosphamide | advanced solid tumor, positive for MSLN and PDL1 expression | 10 | Unknown | 6/1/2022 (estimated) | China | NCT04503980 |
| MESO | I | LCAR-M23 cells | IV | cyclophosphamide, fludarabine | relapsed and refractory epithelial ovarian cancer | 15 (actual) | Terminated | 6/7/2022 (actual) | China | NCT04562298 |
| MESO | I | MESO CAR-T cells | IV | cyclophosphamide, fludarabine | mesothelin-positive refractory-relapsed ovarian cancer | 10 | Unknown | 1/1/2023 (estimated) | China | NCT03814447 |
| MESO | I | lentiviral transduced huCART-meso cells | IV or IP | with and without cyclophosphamide | mesothelin-expressing advanced solid tumors | 65 (actual) | Active, Not Recruiting | 3/1/2025 (estimated) | USA | NCT03054298 |
| MESO | I | anti-mesothelin CAR-T cells | IV | cyclophosphamide, fludarabine, aldesleukin | mesothelin-expressing advanced solid tumors | 15 (actual) | Terminated due to slow/insufficient accrual | 12/17/2018 (actual) | USA | NCT01583686 |
| MESO | I, II | TCR-like CAR-T | IV | – | mesothelin-expressing advanced ovarian cancer | 10 | Recruiting | 5/16/2025 (estimated) | China | NCT05963100 |
| MESO | I | anti-MESO CAR-T cells | IV | – | relapsed or refractor mesothelin-positive tumors | 20 | Unknown | 11/2018 (estimated) | China | NCT02580747 |
| MESO | I | SynKIR-110 | IV | – | recurrent or relapsed advanced ovarian, primary peritoneal, fallopian tube, cholangiocarcinoma, or epithelial mesothelioma-expressing mesothelin | 42 | Recruiting | 3/30/2026 (estimated) | USA | NCT05568680 |
| MESO | I | lentiviral transduced huCART-meso cells in combination with VCN-01 | IV | – | persistent or recurrent serous epithelial ovarian carcinoma or unresectable or metastatic pancreatic adenocarcinoma | 12 | Recruiting | 9/2023 (estimated) | USA | NCT05057715 |
| MUC 16 | I | PRGN-3005 T cells targetting MUC16 | IV or IP | none | recurrent, advanced, platinum-resistant ovarian, fallopian tube, and primary peritoneal cancers | 71 | Recruiting | 11/15/2028 (estimated) | USA | NCT03907527 |
| MUC 16 | I, II | A2B694 (an autologous Logic-gated Tmod CART) | IV | yes, not specified | recurrent, unresectable, locally advanced, or metastatic solid tumors that express mesothelin and have lost HLA-A*02 expression | 230 | Not Yet Recruiting | 6/1/2019 (estimated) | USA | NCT06051695 |
| MUC 16 | I | 4H11-28z/fIL-12/EGFRt+ genetically modified T cells secreting IL-12 | IV followed by IP | cyclophosphamide +/− fludarabine | recurrent high-grade serous ovarian, primary peritoneal, or fallopian tube carcinoma-expressing MUC16ecto+ | 18 (actual) | Active, Not Recruiting | 8/2024 (estimated) | USA | NCT02498912 |
| MUC1 | I | P-MUC1C-ALLO1 | IV | yes, not specified | advanced or metastatic epithelial-derived solid tumors | 100 | Recruiting | 4/2039 (estimated) | USA | NCT05239143 |
| Tn-MUC1 | I | CAR-TnMUC1 | IV | cyclophosphamide, fludarabine | advanced TnMUC1-positive solid tumors and multiple myeloma | 16 (actual) | Terminated due to unfavorable risk/benefit analysis | 12/2/2022 (estimated) | USA | NCT04025216 |
| NKG2D | I, II | NKR-2 cells | IV | – | metastatic cancer (multiple) | 146 | Unknown | 8/2021 (estimated) | Belgium, USA | NCT03018405 |
| TAG72 | I | TAG72-CAR-T | IP | cyclophosphamide, fludarabine | platinum-resistant epithelial ovarian cancer | 33 | Recruiting | 4/5/2027 (estimated) | USA | NCT05225363 |
5. Adverse Events
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schepisi, G.; Casadei, C.; Toma, I.; Poti, G.; Iaia, M.L.; Farolfi, A.; Conteduca, V.; Lolli, C.; Ravaglia, G.; Brighi, N.; et al. Immunotherapy and Its Development for Gynecological (Ovarian, Endometrial and Cervical) Tumors: From Immune Checkpoint Inhibitors to Chimeric Antigen Receptor (CAR)-T Cell Therapy. Cancers 2021, 13, 840. [Google Scholar] [CrossRef] [PubMed]
- Siminiak, N.; Czepczynski, R.; Zaborowski, M.P.; Izycki, D. Immunotherapy in Ovarian Cancer. Arch. Immunol. Ther. Exp. 2022, 70, 19. [Google Scholar] [CrossRef] [PubMed]
- Sarivalasis, A.; Morotti, M.; Mulvey, A.; Imbimbo, M.; Coukos, G. Cell therapies in ovarian cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211008399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Abila, B.; Mostafa Kamel, Y. CAR-T: What Is Next? Cancers 2023, 15, 663. [Google Scholar] [CrossRef] [PubMed]
- Sengsayadeth, S.; Savani, B.N.; Oluwole, O.; Dholaria, B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. EJHaem 2022, 3 (Suppl. 1), 6–10. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Riddell, S.R.; Schumacher, T.N. Adoptive cellular therapy: A race to the finish line. Sci. Transl. Med. 2015, 7, 280ps287. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.V.; June, C.H. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin. Cancer Res. 2016, 22, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Chailyan, A.; Marcatili, P.; Tramontano, A. The association of heavy and light chain variable domains in antibodies: Implications for antigen specificity. FEBS J. 2011, 278, 2858–2866. [Google Scholar] [CrossRef]
- Dwivedi, A.; Karulkar, A.; Ghosh, S.; Rafiq, A.; Purwar, R. Lymphocytes in Cellular Therapy: Functional Regulation of CAR T Cells. Front. Immunol. 2018, 9, 3180. [Google Scholar] [CrossRef]
- Guedan, S.; Calderon, H.; Posey, A.D., Jr.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2019, 12, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, J.; Zhang, F.; Xu, Y.; Yu, Y.; Liang, W.; Li, Q. The challenge of selecting tumor antigens for chimeric antigen receptor T-cell therapy in ovarian cancer. Med. Oncol. 2022, 39, 232. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Zhang, X.W.; Wu, Y.S.; Xu, T.M.; Cui, M.H. CAR-T Cells in the Treatment of Ovarian Cancer: A Promising Cell Therapy. Biomolecules 2023, 13, 465. [Google Scholar] [CrossRef]
- Flugel, C.L.; Majzner, R.G.; Krenciute, G.; Dotti, G.; Riddell, S.R.; Wagner, D.L.; Abou-El-Enein, M. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 2023, 20, 49–62. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- O’Hara, M.H.; Stashwick, C.; Plesa, G.; Tanyi, J.L. Overcoming barriers of car T-cell therapy in patients with mesothelin-expressing cancers. Immunotherapy 2017, 9, 767–780. [Google Scholar] [CrossRef]
- Moon, E.K.; Wang, L.C.; Dolfi, D.V.; Wilson, C.B.; Ranganathan, R.; Sun, J.; Kapoor, V.; Scholler, J.; Pure, E.; Milone, M.C.; et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 2014, 20, 4262–4273. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Morello, A.; Sadelain, M.; Adusumilli, P.S. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov. 2016, 6, 133–146. [Google Scholar] [CrossRef]
- Kaneko, O.; Gong, L.; Zhang, J.; Hansen, J.K.; Hassan, R.; Lee, B.; Ho, M. A binding domain on mesothelin for CA125/MUC16. J. Biol. Chem. 2009, 284, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Kreitman, R.J.; Pastan, I.; Willingham, M.C. Localization of mesothelin in epithelial ovarian cancer. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, D.B.; Shi, H. Application of chimeric antigen receptor-engineered T cells in ovarian cancer therapy. Immunotherapy 2017, 9, 851–861. [Google Scholar] [CrossRef]
- Kelemen, L.E. The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Cernigoi, C.; Russo, A.; Gallo, A.; Bagnoli, M.; Boiocchi, M. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 1997, 74, 193–198. [Google Scholar] [CrossRef]
- Siu, M.K.; Kong, D.S.; Chan, H.Y.; Wong, E.S.; Ip, P.P.; Jiang, L.; Ngan, H.Y.; Le, X.F.; Cheung, A.N. Paradoxical impact of two folate receptors, FRalpha and RFC, in ovarian cancer: Effect on cell proliferation, invasion and clinical outcome. PLoS ONE 2012, 7, e47201. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef]
- Chang, K.L.; Lee, M.Y.; Chao, W.R.; Han, C.P. The status of Her2 amplification and Kras mutations in mucinous ovarian carcinoma. Hum. Genom. 2016, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, K.; Rengstl, B.; Oehm, P.; Michel, K.; Billmeier, A.; Hayduk, N.; Klein, O.; Kuna, K.; Ouchan, Y.; Woll, S.; et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 2020, 367, 446–453. [Google Scholar] [CrossRef]
- Wang, L.; Jin, X.; Lin, D.; Liu, Z.; Zhang, X.; Lu, Y.; Liu, Y.; Wang, M.; Yang, M.; Li, J.; et al. Clinicopathologic significance of claudin-6, occludin, and matrix metalloproteinases -2 expression in ovarian carcinoma. Diagn. Pathol. 2013, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Liu, G.; Feng, L.; Li, Y.; E, M.; Wu, L.; Li, Y.; Rong, G.; Li, Y.; Wei, H.; et al. PTK7-Targeting CAR T-Cells for the Treatment of Lung Cancer and Other Malignancies. Front. Immunol. 2021, 12, 665970. [Google Scholar] [CrossRef]
- Xu, T.; Wang, C.; Wang, X.; Wang, E.; Wang, B.; Sun, M. A novel TREM1/DAP12-based multiple chain CAR-T cell targets PTK7 in ovarian cancer therapy. Med. Oncol. 2023, 40, 226. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Shang, Y.; Qian, Z.; Hou, J.; Yan, F.; Liu, G.; Dehua, L.; Tian, X. Chimeric Antigen receptor-T (CAR-T) cells targeting Epithelial cell adhesion molecule (EpCAM) can inhibit tumor growth in ovarian cancer mouse model. J. Vet. Med. Sci. 2021, 83, 241–247. [Google Scholar] [CrossRef]
- Leong, L.; Tan, H.L.; Cua, S.; Yong, K.S.M.; Chen, Q.; Choo, A. Preclinical Activity of Embryonic Annexin A2-Specific Chimeric Antigen Receptor T Cells Against Ovarian Cancer. Int. J. Mol. Sci. 2020, 21, 381. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Sharma, P.; Poussin, M.; Boesteanu, A.C.; Minutolo, N.G.; Gitto, S.B.; Omran, D.K.; Robinson, M.K.; Adams, G.P.; Simpkins, F.; et al. CAR T Cells Targeting MISIIR for the Treatment of Ovarian Cancer and Other Gynecologic Malignancies. Mol. Ther. 2020, 28, 548–560. [Google Scholar] [CrossRef]
- Ramos, C.A.; Rouce, R.; Robertson, C.S.; Reyna, A.; Narala, N.; Vyas, G.; Mehta, B.; Zhang, H.; Dakhova, O.; Carrum, G.; et al. In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin’s Lymphomas. Mol. Ther. 2018, 26, 2727–2737. [Google Scholar] [CrossRef]
- Murad, J.P.; Kozlowska, A.K.; Lee, H.J.; Ramamurthy, M.; Chang, W.C.; Yazaki, P.; Colcher, D.; Shively, J.; Cristea, M.; Forman, S.J.; et al. Effective Targeting of TAG72+ Peritoneal Ovarian Tumors via Regional Delivery of CAR-Engineered T Cells. Front. Immunol. 2018, 9, 2268. [Google Scholar] [CrossRef]
- Ma, Q.; He, X.; Zhang, B.; Guo, F.; Ou, X.; Yang, Q.; Shu, P.; Chen, Y.; Li, K.; Gao, G.; et al. A PD-L1-targeting chimeric switch receptor enhances efficacy of CAR-T cell for pleural and peritoneal metastasis. Signal Transduct. Target. Ther. 2022, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.L.; Sheard, V.E.; Kalaitsidou, M.; Blount, D.; Lad, Y.; Cheadle, E.J.; Edmondson, R.J.; Kooner, G.; Gilham, D.E.; Harrop, R. Preclinical Assessment of CAR T-Cell Therapy Targeting the Tumor Antigen 5T4 in Ovarian Cancer. J. Immunother. 2018, 41, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, G.; Zhang, Q.; Tian, X.; Ouyang, L.; Zhang, L. Construction of CAR-T cells targeting TM4SF1 and its anti-tumor capacity in ovarian cancer. Immunol. Lett. 2023, 255, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cribioli, E.; Giordano Attianese, G.M.P.; Coukos, G.; Irving, M. CAR T cells targeting the ganglioside NGcGM3 control ovarian tumors in the absence of toxicity against healthy tissues. Front. Immunol. 2022, 13, 951143. [Google Scholar] [CrossRef] [PubMed]
- Monzo, H.J.; Kalander, K.; Hyytiainen, M.M.; Elbasani, E.; Wall, J.; Moyano-Galceran, L.; Tanjore Ramanathan, J.; Jukonen, J.J.J.; Laakkonen, P.; Ristimaki, A.; et al. Efficacy and safety of glycosphingolipid SSEA-4 targeting CAR-T cells in an ovarian carcinoma model. Mol. Cancer Ther. 2023, 22, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Basiri, M.; Pahlavanneshan, S. Evaluation of Placental Alkaline Phosphatase Expression as A Potential Target of Solid Tumors Immunotherapy by Using Gene and Protein Expression Repositories. Cell J. 2021, 23, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, O.; Tan, M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.R.; Tsao, M.S. c-MET as a potential therapeutic target and biomarker in cancer. Ther. Adv. Med. Oncol. 2011, 3, S21–S35. [Google Scholar] [CrossRef]
- Flieswasser, T.; Van den Eynde, A.; Van Audenaerde, J.; De Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 axis in oncology: The new kids on the block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef]
- Coati, I.; Lotz, G.; Fanelli, G.N.; Brignola, S.; Lanza, C.; Cappellesso, R.; Pellino, A.; Pucciarelli, S.; Spolverato, G.; Guzzardo, V.; et al. Claudin-18 expression in oesophagogastric adenocarcinomas: A tissue microarray study of 523 molecularly profiled cases. Br. J. Cancer 2019, 121, 257–263. [Google Scholar] [CrossRef]
- Casalini, P.; Iorio, M.V.; Galmozzi, E.; Menard, S. Role of HER receptors family in development and differentiation. J. Cell. Physiol. 2004, 200, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, S.; Han, Y.; Wang, Y.; Sun, Y. Human leukocyte antigen-G expression and polymorphisms promote cancer development and guide cancer diagnosis/treatment. Oncol. Lett. 2018, 15, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Raulet, D.H. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003, 3, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Evtimov, V.J.; Hammett, M.V.; Nguyen, N.N.; Zhuang, J.; Hudson, P.J.; Howard, M.C.; Pupovac, A.; Trounson, A.O.; Boyd, R.L. Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer. Mol. Ther. Oncolytics 2021, 20, 325–341. [Google Scholar] [CrossRef]
- Bakkum-Gamez, J.N.; Aletti, G.; Lewis, K.A.; Keeney, G.L.; Thomas, B.M.; Navarro-Teulon, I.; Cliby, W.A. Mullerian inhibiting substance type II receptor (MISIIR): A novel, tissue-specific target expressed by gynecologic cancers. Gynecol. Oncol. 2008, 108, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Lin, C.F. Annexin A2: Its molecular regulation and cellular expression in cancer development. Dis. Markers 2014, 2014, 308976. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases (Review). Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, Y.C.; Li, J. PD-L1 expression levels on tumor cells affect their immunosuppressive activity. Oncol. Lett. 2019, 18, 5399–5407. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Harichandan, A.; Schilbach, K.; Mack, A.F.; Bedke, J.; Stenzl, A.; Kanz, L.; Niederfellner, G.; Buhring, H.J. Expression of stage-specific embryonic antigen-4 (SSEA-4) defines spontaneous loss of epithelial phenotype in human solid tumor cells. Glycobiology 2015, 25, 902–917. [Google Scholar] [CrossRef]
- Fu, F.; Yang, X.; Zheng, M.; Zhao, Q.; Zhang, K.; Li, Z.; Zhang, H.; Zhang, S. Role of Transmembrane 4 L Six Family 1 in the Development and Progression of Cancer. Front. Mol. Biosci. 2020, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Arita, M.; Takahashi, M.; Saida, Y.; Koya, T.; Kikuchi, T. Effect of Lymphodepletion on Donor T Cells and the Role of Recipient Cells Persisting after Cytotoxic Treatments in Cancer Immunotherapies. Crit. Rev. Immunol. 2017, 37, 59–73. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.R.; Golden, R.J.; Litzky, L.A.; Engels, B.; Zhao, L.; Xu, F.; Taraszka, J.A.; Ramones, M.; Granda, B.; Chang, W.J.; et al. Two cases of severe pulmonary toxicity from highly active mesothelin-directed CAR T cells. Mol. Ther. 2023, 31, 2309–2325. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.; Haas, A.; Aggarwal, C.; O’Hara, M.; Lacey, S.; Golden, R.; Hwang, W.-T.; Young, R.; Sheppard, N.; Albelda, S.; et al. Phase I study of autologous T cells bearing fully-humanized chimeric antigen receptors targeting mesothelin in mesothelin- expressing cancers (314). Gynecol. Oncol. 2022, 166, S164–S165. [Google Scholar] [CrossRef]
- Chen, J.; Hu, J.; Gu, L.; Ji, F.; Zhang, F.; Zhang, M.; Li, J.; Chen, Z.; Jiang, L.; Zhang, Y.; et al. Anti-mesothelin CAR-T immunotherapy in patients with ovarian cancer. Cancer Immunol. Immunother. 2023, 72, 409–425. [Google Scholar] [CrossRef]
- Fang, J.; Ding, N.; Guo, X.; Sun, Y.; Zhang, Z.; Xie, B.; Li, Z.; Wang, H.; Mao, W.; Lin, Z.; et al. alphaPD-1-mesoCAR-T cells partially inhibit the growth of advanced/refractory ovarian cancer in a patient along with daily apatinib. J. Immunother. Cancer 2021, 9, e001162. [Google Scholar] [CrossRef]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 2021, 14, 118. [Google Scholar] [CrossRef]
- Liao, J.B.; Stanton, S.E.; Chakiath, M.; Semnani, R.; Shah, R.R.; Tan, T.; Sabzevari, H.; Lankford, A.; Disis, M.L. Phase 1/1b study of PRGN-3005 autologous UltraCAR-T cells manufactured overnight for infusion next day to advanced stage platinum resistant ovarian cancer patients. J. Clin. Oncol. 2023, 41, 5590. [Google Scholar] [CrossRef]
- Castelletti, L.; Yeo, D.; van Zandwijk, N.; Rasko, J.E.J. Anti-Mesothelin CAR T cell therapy for malignant mesothelioma. Biomark. Res. 2021, 9, 11. [Google Scholar] [CrossRef]
- Annunziata, C.M.; Ghobadi, A.; Pennella, E.J.; Vanas, J.; Powell, C.; Pavelova, M.; Wagner, C.; Kuo, M.; Ullmann, C.D.; Hassan, R.; et al. Feasibility and preliminary safety and efficacy of first-in-human intraperitoneal delivery of MCY-M11, anti-human-mesothelin CAR mRNA transfected into peripheral blood mononuclear cells, for ovarian cancer and malignant peritoneal mesothelioma. J. Clin. Oncol. 2020, 38, 3014. [Google Scholar] [CrossRef]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006, 12 Pt 1, 6106–6115. [Google Scholar] [CrossRef]
- Gutierrez, R.; Shah, P.D.; Hamid, O.; Garfall, A.L.; Posey, A.; Bishop, M.R.; Blumenschein, G.R.; Johnson, M.L.; Lee, S.; Luke, J.J.; et al. Phase I experience with first in class TnMUC1 targeted chimeric antigen receptor T-cells in patients with advanced TnMUC1 positive solid tumors. J. Clin. Oncol. 2021, 39, e14513. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Adkins, S. CAR T-Cell Therapy: Adverse Events and Management. J. Adv. Pract. Oncol. 2019, 10 (Suppl. 3), 21–28. [Google Scholar] [CrossRef]
- Hines, M.R.; Knight, T.E.; McNerney, K.O.; Leick, M.B.; Jain, T.; Ahmed, S.; Frigault, M.J.; Hill, J.A.; Jain, M.D.; Johnson, W.T.; et al. Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome. Transplant. Cell. Ther. 2023, 29, 438.e1–438.e16. [Google Scholar] [CrossRef]
- Henter, J.I.; Horne, A.; Arico, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Major, A.; Collins, J.; Craney, C.; Heitman, A.K.; Bauer, E.; Zerante, E.; Stock, W.; Bishop, M.R.; Jasielec, J. Management of hemophagocytic lymphohistiocytosis (HLH) associated with chimeric antigen receptor T-cell (CAR-T) therapy using anti-cytokine therapy: An illustrative case and review of the literature. Leuk. Lymphoma 2021, 62, 1765–1769. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. Immune effector cell associated neurotoxicity syndrome in chimeric antigen receptor-T cell therapy. Front. Immunol. 2022, 13, 879608. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.; Kaminskiy, Y.; Ganeeva, I.; Zmievskaya, E.; Valiullina, A.; Rakhmatullina, A.; Petukhov, A.; Miftakhova, R.; Rizvanov, A.; Bulatov, E. Knowns and Unknowns about CAR-T Cell Dysfunction. Cancers 2022, 14, 1078. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Cherkassky, L.; Villena-Vargas, J.; Colovos, C.; Servais, E.; Plotkin, J.; Jones, D.R.; Sadelain, M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014, 6, 261ra151. [Google Scholar] [CrossRef] [PubMed]
- Al-Haideri, M.; Tondok, S.B.; Safa, S.H.; Maleki, A.H.; Rostami, S.; Jalil, A.T.; Al-Gazally, M.E.; Alsaikhan, F.; Rizaev, J.A.; Mohammad, T.A.M.; et al. CAR-T cell combination therapy: The next revolution in cancer treatment. Cancer Cell Int. 2022, 22, 365. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Rojas, J.J.; Gros, A.; Mercade, E.; Cascallo, M.; Alemany, R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010, 18, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.E.; Park, J.H.; Halton, E.F.; Diamonte, C.R.; Mead, E.; Lakhman, Y.; Kane, P.; Riviere, I.C.; Brentjens, R.J. A phase I clinical trial of autologous chimeric antigen receptor (CAR) T cells genetically engineered to secrete IL-12 and to target the MUC16ecto antigen in patients (pts) with MUC16ecto+ recurrent high-grade serous ovarian cancer (HGSOC). Gynecol. Oncol. 2020, 159, 42. [Google Scholar] [CrossRef]
- Wang, E.; Wang, L.C.; Tsai, C.Y.; Bhoj, V.; Gershenson, Z.; Moon, E.; Newick, K.; Sun, J.; Lo, A.; Baradet, T.; et al. Generation of Potent T-cell Immunotherapy for Cancer Using DAP12-Based, Multichain, Chimeric Immunoreceptors. Cancer Immunol. Res. 2015, 3, 815–826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutri-French, C.; Nasioudis, D.; George, E.; Tanyi, J.L. CAR-T Cell Therapy in Ovarian Cancer: Where Are We Now? Diagnostics 2024, 14, 819. https://doi.org/10.3390/diagnostics14080819
Cutri-French C, Nasioudis D, George E, Tanyi JL. CAR-T Cell Therapy in Ovarian Cancer: Where Are We Now? Diagnostics. 2024; 14(8):819. https://doi.org/10.3390/diagnostics14080819
Chicago/Turabian StyleCutri-French, Clare, Dimitrios Nasioudis, Erin George, and Janos L. Tanyi. 2024. "CAR-T Cell Therapy in Ovarian Cancer: Where Are We Now?" Diagnostics 14, no. 8: 819. https://doi.org/10.3390/diagnostics14080819
APA StyleCutri-French, C., Nasioudis, D., George, E., & Tanyi, J. L. (2024). CAR-T Cell Therapy in Ovarian Cancer: Where Are We Now? Diagnostics, 14(8), 819. https://doi.org/10.3390/diagnostics14080819






