Flow-S: A Field-Deployable Device with Minimal Hands-On Effort to Concentrate and Quantify Schistosoma Circulating Anodic Antigen (CAA) from Large Urine Volumes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Cohort and Clinical Samples
2.2. Standard UCP-LF CAA Tests
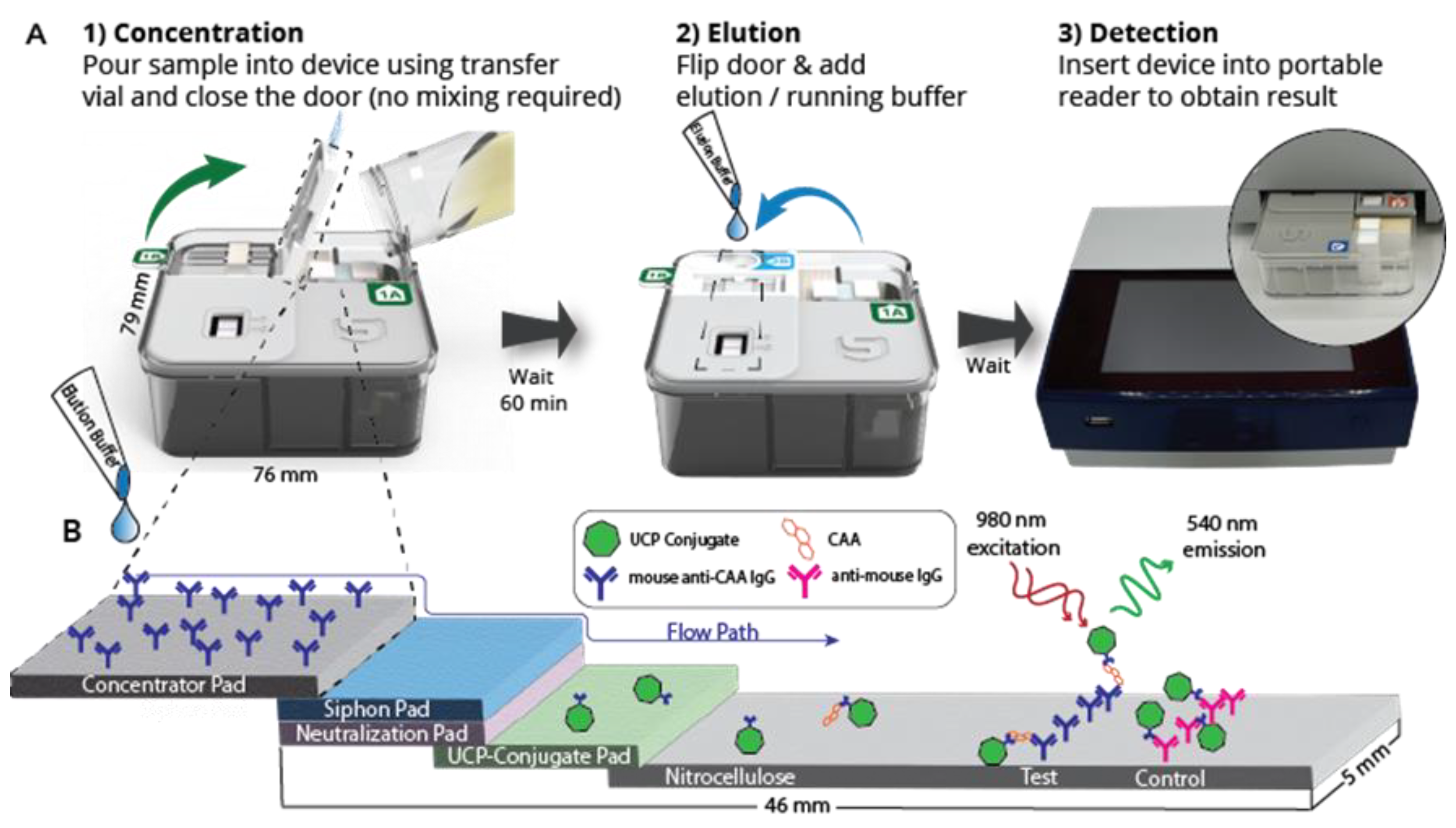
2.3. UCP-LF CAA Test in the Flow-S Device
2.4. CAA Quantification and LF Strip Analysis
3. Results
3.1. Description of the Flow-S Device
3.2. Targeted Sensitivity of the Flow-S Device
3.3. Evaluation of the Flow-S Device with Banked Urine Samples
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Schistosomiasis [Internet]. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 15 December 2023).
- Knopp, S.; Person, B.; Ame, S.M.; Ali, S.M.; Hattendorf, J.; Juma, S.; Muhsin, J.; Khamis, I.S.; Mohammed, K.A.; Utzinger, J.; et al. Evaluation of integrated interventions layered on mass drug administration for urogenital schistosomiasis elimination: A cluster-randomised trial. Lancet Glob. Health 2019, 7, e1118-29. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, H.; Poggensee, G. Diagnostic techniques in schistosomiasis control. A review. Acta Trop. 1993, 52, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Doehring, E.; Feldmeier, H.; Daffalla, A.A. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann. Trop. Med. Parasitol. 1983, 77, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.A.M.; de Dood, C.J.; Knopp, S.; Clements, M.N.; Ortu, G.; Umulisa, I.; Ruberanziza, E.; Wittmann, U.; Kariuki, T.; LoVerde, P.; et al. Circulating anodic antigen (CAA): A highly sensitive diagnostic biomarker to detect active Schistosoma infections—Improvement and use during SCORE. Am. J. Trop. Med. Hyg. 2020, 103 (Suppl. S1), 50–57. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, M.C.C.; Hoogerwerf, M.-A.; Koopman, J.P.R.; Janse, J.J.; Oosterhoud, J.K.-V.; Feijt, C.; Jochems, S.P.; de Dood, C.J.; van Schuijlenburg, R.; Ozir-Fazalalikhan, A.; et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat. Med. 2020, 26, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.; Nyakundi, R.K.; De Dood, C.J.; Kariuki, T.M.; Ochola, E.A.; Karanja, D.M.; Mwinzi, P.N.; Van Dam, G.J. Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasites Vectors 2015, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.A.M.; Hoekstra, P.T.; de Dood, C.J.; van Dam, G.J. Utilizing the ultrasensitive Schistosoma up-converting phosphor lateral flow circulating anodic antigen (UCP-LF CAA) assay for sample pooling-strategies. Infect. Dis. Poverty 2017, 6, 127. [Google Scholar] [CrossRef]
- Sousa, M.S.; van Dam, G.J.; Pinheiro, M.C.C.; de Dood, C.J.; Peralta, J.M.; Peralta, R.H.S.; Daher, E.d.F.; Corstjens, P.L.A.M.; Bezerra, F.S.M. Performance of an Ultra-Sensitive Assay Targeting the Circulating Anodic Antigen (CAA) for Detection of Schistosoma mansoni Infection in a Low Endemic Area in Brazil. Front. Immunol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, P.T.; Schwarz, N.G.; Adegnika, A.A.; Andrianarivelo, M.R.; Corstjens, P.L.; Rakotoarivelo, R.A.; Rakotozandrindrainy, R.; Sicuri, E.; Kreidenweiss, A.; van Dam, G.J. Fast and reliable easy-to-use diagnostics for eliminating bilharzia in young children and mothers: An introduction to the freeBILy project. Acta Trop. 2020, 211, 105631. [Google Scholar] [CrossRef]
- Honkpehedji, Y.J.; Adegnika, A.A.; Dejon-Agobe, J.C.; Zinsou, J.F.; Mba, R.B.; Gerstenberg, J.; Rakotozandrindrainy, R.; Rakotoarivelo, R.A.; Rasamoelina, T.; Sicuri, E.; et al. Prospective, observational study to assess the performance of CAA measurement as a diagnostic tool for the detection of Schistosoma haematobium infections in pregnant women and their child in Lambaréné, Gabon: Study protocol of the freeBILy clinical trial in Gabon. BMC Infect. Dis. 2020, 20, 718. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Molehin, A.J.; Zhang, W.; Ganapathy, P.K.; Kim, E.; Rojo, J.U.; Redman, W.K.; Sennoune, S.R.; Sudduth, J.; Freeborn, J.; et al. Sm-p80-based vaccine trial in baboons: Efficacy when mimicking natural conditions of chronic disease, praziquantel therapy, immunization, and Schistosoma mansoni re-encounter. Ann. N. Y. Acad. Sci. 2018, 1425, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.P.R.; Houlder, E.L.; Janse, J.J.; Casacuberta-Partal, M.; Lamers, O.A.; Sijtsma, J.C.; de Dood, C.; Hilt, S.T.; Ozir-Fazalalikhan, A.; Kuiper, V.P.; et al. Safety and infectivity of female cercariae in Schistosoma-naïve, healthy participants: A controlled human Schistosoma mansoni infection study. eBioMedicine 2023, 97, 104832. [Google Scholar] [CrossRef] [PubMed]
- van Dam, G.J.; de Dood, C.J.; Lewis, M.; Deelder, A.M.; van Lieshout, L.; Tanke, H.J.; van Rooyen, L.H.; Corstjens, P.L. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp. Parasitol. 2013, 135, 274–282. [Google Scholar] [CrossRef] [PubMed]
- WHO. Basic Laboratory Methods in Medical Parasitology [Internet]. 1991. Available online: https://iris.who.int/bitstream/handle/10665/40793/9241544104_%28part1%29.pdf?sequence=1&isAllowed=y (accessed on 15 December 2023).
- Dupnik, K.M.; Reust, M.J.; Vick, K.M.; Yao, B.; Miyaye, D.; Lyimo, E.; Mukerebe, C.; Mngara, J.; Kalluvya, S.E.; de Dood, C.J.; et al. Gene expression differences in host response to Schistosoma haematobium infection. Infect. Immun. 2019, 87, e00291-18. [Google Scholar] [CrossRef] [PubMed]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple, device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo 1972, 14, 397–400. [Google Scholar] [PubMed]
- Corstjens, P.; Li, S.; Zuiderwijk, M.; Kardos, K.; Abrams, W.; Niedbala, R.; Tanke, H. Infrared up-converting phosphors for bioassays. IEE Proc.-Nanobiotechnol. 2005, 152, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.; de Dood, C.J.; Priest, J.W.; Tanke, H.J.; Handali, S. Cysticercosis Working Group in Peru. Feasibility of a lateral flow test for neurocysticercosis using novel up-converting nanomaterials and a lightweight strip analyzer. PLoS Negl. Trop. Dis. 2014, 8, e2944. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.A.M.; van Lieshout, L.; Zuiderwijk, M.; Kornelis, D.; Tanke, H.J.; Deelder, A.M.; van Dam, G.J. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J. Clin. Microbiol. 2008, 46, 171–176. [Google Scholar] [CrossRef] [PubMed]
- United States Patent Application Publication US2021/0349084A1, Sample Concentration and Detection Systems and Methods (11/11 2021). Salus Discovery LLC: Madison, WI, USA.
- Downs, J.A.; van Dam, G.J.; Changalucha, J.M.; Andreasen, A.; Johnson, W.D.; Kalluvya, S.E.; Fitzgerald, D.W.; de Dood, C.J.; Bang, H.; Corstjens, P.L.A.M.; et al. Association of schistosomiasis and HIV infection in Tanzania. Am. J. Trop. Med. Hyg. 2012, 87, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Colombe, S.; Paul, N.; Mlingi, J.; Tosiri, I.; Aristide, C.; Gao, J.; Kashangaki, P.; Nagai, H.; Kalluvya, S.E.; et al. Insufficiency of annual praziquantel treatment to control Schistosoma manson infections in adult women: A longitudinal cohort study in rural Tanzania. PLoS Negl. Trop. Dis. 2019, 13, e0007844. [Google Scholar] [CrossRef] [PubMed]
- Bullington, B.W.; Lee, M.H.; Mlingi, J.; Paul, N.; Aristide, C.; Fontana, E.; Littmann, E.R.; Mukerebe, C.; Shigella, P.; Kashangaki, P.; et al. Cervicovaginal bacterial communities in reproductive-aged Tanzania women with Schistosoma mansoni, Schistosoma haematobium, or without schistosome infection. ISME J. 2021, 15, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Mtalitinya, G.S.; Aristide, C.; Airewele, E.A.; Nyakaru, D.K.; McMahon, P.; Mulaki, G.M.; Corstjens, P.L.; Dood, C.J.; van Dam, G.J.; et al. Effects of Schistosoma mansoni and praziquantel treatment on the lower gastrointestinal mucosa: A cohort study in Tanzania. Acta Trop. 2023, 238, 106752. [Google Scholar] [CrossRef] [PubMed]
- Tanke, H.J.; Zuiderwijk, M.; Wiesmeijer, K.C.; Breedveld, R.N.; Abrams, W.R.; de Dood, C.J.; Fat, E.M.T.K.; Corstjens, P.L.A.M. The use of upconverting phosphors in point-of-care (POC) testing. Proc. SPIE 2014, 89470, 89470P-1. [Google Scholar] [CrossRef]
- Parks, D.R.; Roederer, M.; Moore, W.A. A new “Logicle” display method avoids deceptive effects of logarithmic scaling for low signals and compensated data. Cytom. A 2006, 69, 541–551. [Google Scholar] [CrossRef] [PubMed]
- de Dood, C.J.; Hoekstra, P.T.; Mngara, J.; Kalluvya, S.E.; van Dam, G.J.; Downs, J.A.; Corstjens, P.L.A.M. Refining Diagnosis of Schistosoma haematobium infections: Antigen and antibody detection in urine. Front. Immunol. 2018, 9, 2635. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diagnostic Target Product Profiles for Monitoring, Evaluation and Surveillance of Schistosomisasis Control Programmes [Internet]. 2021. Available online: https://www.who.int/publications/i/item/9789240031104 (accessed on 15 December 2023).
- Pierneef, L.; van Hooij, A.; de Jong, D.; Fat, E.M.T.K.; van Meijgaarden, K.E.; Petruccioli, E.; Vanini, V.; Roukens, A.H.; Goletti, D.; Corstjens, P.L.; et al. Host biomarker-based quantitative rapid tests for detection and treatment monitoring of tuberculosis and COVID-19. iScience 2023, 26, 105873. [Google Scholar] [CrossRef] [PubMed]
- van Dam, G.J.; Bogitsh, B.J.; van Zeyl, R.J.M.; Rotmans, J.P.; Deelder, A.M. Schistosoma mansoni: In vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J. Parasitol. 1996, 82, 557–564. [Google Scholar] [CrossRef]
- Markwalter, C.F.; Corstjens, P.L.; Mammoser, C.M.; Camps, G.; van Dam, G.J.; Wright, D.W. Poly(amidoamine)-coated magnetic particles for enhanced detection of Schistosoma circulating anodic antigen in endemic urine samples. Analyst 2019, 144, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Nathavitharana, R.R.; Garcia-Basteiro, A.L.; Ruhwald, M.; Cobelens, F.; Theron, G. Reimagining the status quo: How close are we to rapid sputum-free tuberculosis diagnostics for all? eBioMedicine 2022, 78, 103939. [Google Scholar] [CrossRef] [PubMed]
- Saetun, P.; Semangoen, T.; Thongboonkerd, V. Characterizations of urinary sediments precipitated after freezing and their effects on urinary protein and chemical analyses. Am. J. Physiol. Renal Physiol. 2009, 296, F1346–F1354. [Google Scholar] [CrossRef]
- Casacuberta-Partal, M.; van Lieshout, L.; van Diepen, A.; Sijtsma, J.C.; Ozir-Fazalalikhan, A.; Koopman, J.P.R.; de Dood, C.J.; Corstjens, P.L.; van Dam, G.J.; Hokke, C.H.; et al. Excretion patterns of Schistosoma mansoni antigens CCA and CAA by adult male and female worms, using a mouse model and ex vivo parasite cultures. Parasitology 2022, 149, 306–313. [Google Scholar] [CrossRef]



| Assay Format * | Amicon Device † | Threshold (pg/mL) § Dry vs. Wet |
|---|---|---|
| SCAA20 | None | 30 vs. 10 |
| SCAA500 | 0.5 mL | 3 vs. 1 |
| UCAAhT17 | None | 20 vs. 6 |
| UCAAhT417 | 0.5 mL | 2 vs. 0.6 |
| UCAAhT417-YF | 0.5 mL | 2 vs. 0.6 |
 |  |  |  | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Class | SCAA20 pg/mL | Eggs 10 mL | Ratio T/C | Conc pg/mL | SCAA/ UCAA Factor | Ratio T/C | Conc pg/mL | Precip. | SCAA/Flow Factor | UCAA/Flow Factor |
| 1 | Positive | 37,750 | 13 | 3.560 | 845.6 | 44.6 | 1.164 | 158.3 | S | 238.5 | 5.3 |
| 2 | Positive | 9915 | 0 | 3.300 | 682.5 | 14.5 | 0.886 | 104.5 | S | 94.9 | 6.5 |
| 3 | Positive | 7733 | 0 | 2.864 | 482.0 | 16.0 | 1.101 | 144.8 | M | 53.4 | 3.3 |
| 4 | Positive | 4848 | 69 | 1.225 | 110.1 | 44.0 | invalid | invalid | S | na | na |
| 5 | Positive | 4767 | 0 | 1.421 | 137.2 | 34.8 | 0.833 | 95.8 | 49.8 | 1.4 | |
| 6 | Positive | 1872 | 0 | 0.539 | 36.5 | 51.3 | 0.654 | 69.5 | 26.9 | 0.5 | |
| 7 | Positive | 1168 | 0 | 0.467 | 30.4 | 38.4 | 0.074 | 6.2 | 188.8 | 4.9 | |
| 8 | Positive | 686 | 75 | 3.676 | 933.3 | 0.7 | 1.394 | 216.6 | S | 3.2 | 4.3 |
| 9 | Positive | 642 | 24 | 1.112 | 95.9 | 6.7 | 0.375 | 35.5 | 18.1 | 2.7 | |
| 10 | Positive | 295 | 0 | 0.408 | 25.6 | 11.5 | 0.015 | 1.1 | M | 267.0 | 23.2 |
| 11 | Positive | 276 | 0 | 0.357 | 21.6 | 12.7 | 0.123 | 10.5 | 26.2 | 2.1 | |
| 12 | Positive | 200 | 0 | 0.214 | 11.4 | 17.6 | <0.014 | <1 | L | na | na |
| 13 | Positive | 92 | 0 | 0.206 | 10.8 | 8.5 | 0.052 | 4.2 | 21.8 | 2.6 | |
| 14 | Positive | 86 | 0 | <0.061 | <2 | na | <0.014 | <1 | S | na | na |
| 15 | Positive | 72 | 0 | 0.262 | 14.7 | 4.9 | 0.154 | 13.3 | 5.4 | 1.7 | |
| 16 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | S | na | na |
| 17 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | na | na | |
| 18 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | S | na | na |
| 19 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | na | na | |
| 20 | Negative | <30 | 0 | <0.061 | <2 | na | 0.019 | 1.5 | na | na | |
| 21 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | na | na | |
| 22 | Negative | <30 | 0 | 0.082 | 3.2 | na | 0.014 | 1.0 | S | na | 3.2 |
| 23 | Negative | <30 | 0 | 0.103 | 4.4 | na | 0.022 | 1.7 | na | 2.6 | |
| 24 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | na | na | |
| 25 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | S | na | na |
| 26 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | S | na | na |
| 27 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | S | na | na |
| 28 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | na | na | |
| 29 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | na | na | |
| 30 | Negative | <30 | 0 | <0.061 | <2 | na | <0.014 | <1 | M | na | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jong, D.; Carrell, C.; Maganga, J.K.; Mhango, L.; Shigella, P.S.; Gill, M.; Shogren, R.; Mullins, B.; Warrick, J.W.; Changalucha, J.M.; et al. Flow-S: A Field-Deployable Device with Minimal Hands-On Effort to Concentrate and Quantify Schistosoma Circulating Anodic Antigen (CAA) from Large Urine Volumes. Diagnostics 2024, 14, 820. https://doi.org/10.3390/diagnostics14080820
de Jong D, Carrell C, Maganga JK, Mhango L, Shigella PS, Gill M, Shogren R, Mullins B, Warrick JW, Changalucha JM, et al. Flow-S: A Field-Deployable Device with Minimal Hands-On Effort to Concentrate and Quantify Schistosoma Circulating Anodic Antigen (CAA) from Large Urine Volumes. Diagnostics. 2024; 14(8):820. https://doi.org/10.3390/diagnostics14080820
Chicago/Turabian Stylede Jong, Daniëlle, Cody Carrell, Jane K. Maganga, Loyce Mhango, Peter S. Shigella, Maddy Gill, Ryan Shogren, Brianna Mullins, Jay W. Warrick, John M. Changalucha, and et al. 2024. "Flow-S: A Field-Deployable Device with Minimal Hands-On Effort to Concentrate and Quantify Schistosoma Circulating Anodic Antigen (CAA) from Large Urine Volumes" Diagnostics 14, no. 8: 820. https://doi.org/10.3390/diagnostics14080820





