Microbial Profiles in Oral Lichen Planus: Comparisons with Healthy Controls and Erosive vs. Non-Erosive Subtypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Extraction of Genomic DNA and Next-Generation Sequencing
2.3. Bioinformatic Analysis, Statistical Analysis, and Visualization
2.4. Data Availability Statement
2.5. Ethics Statement
3. Results
3.1. Patient Characterization
3.2. Diversity and Abundance of Microbiome HC, E-OLP, and NE-OLP Group
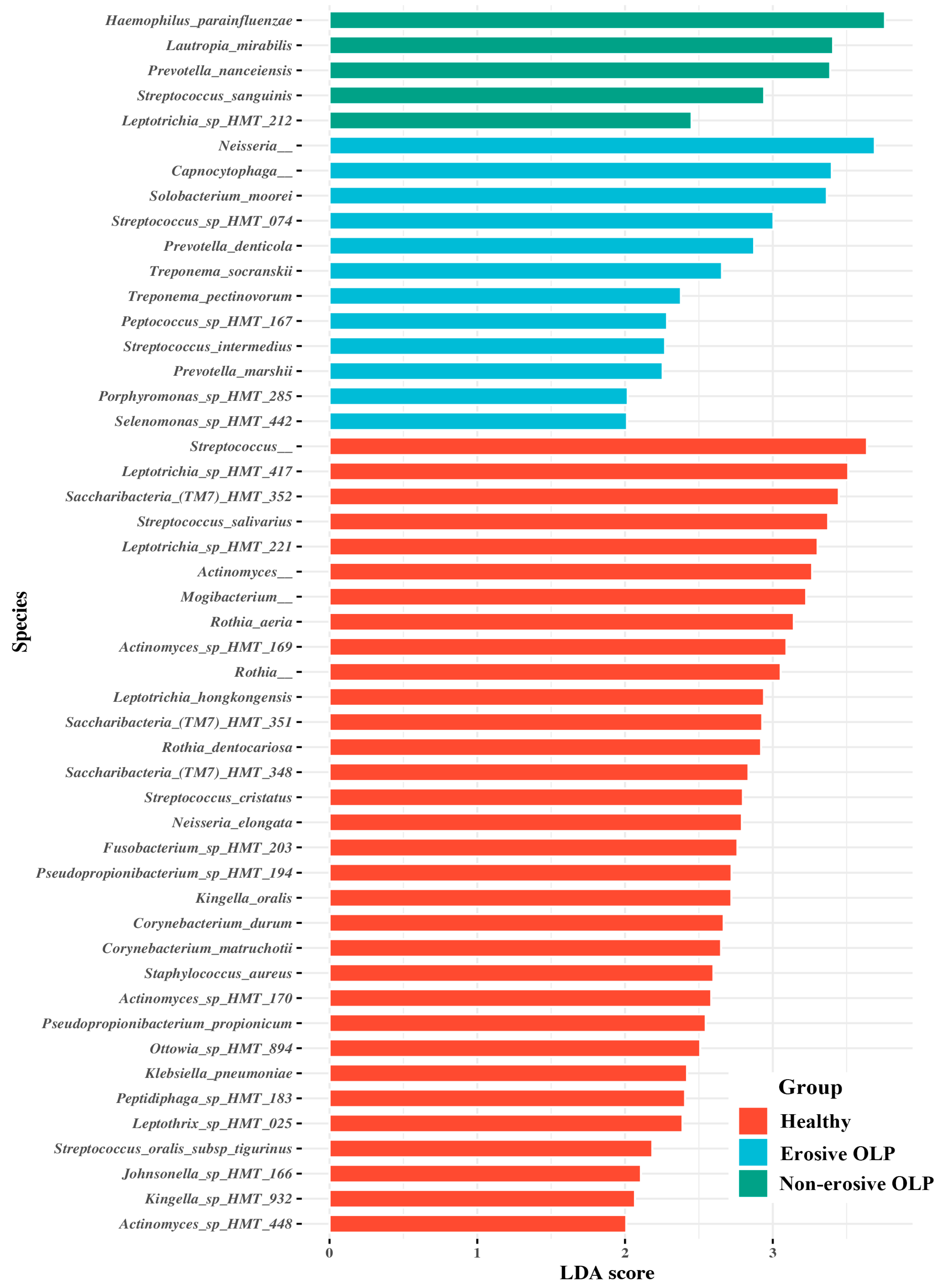
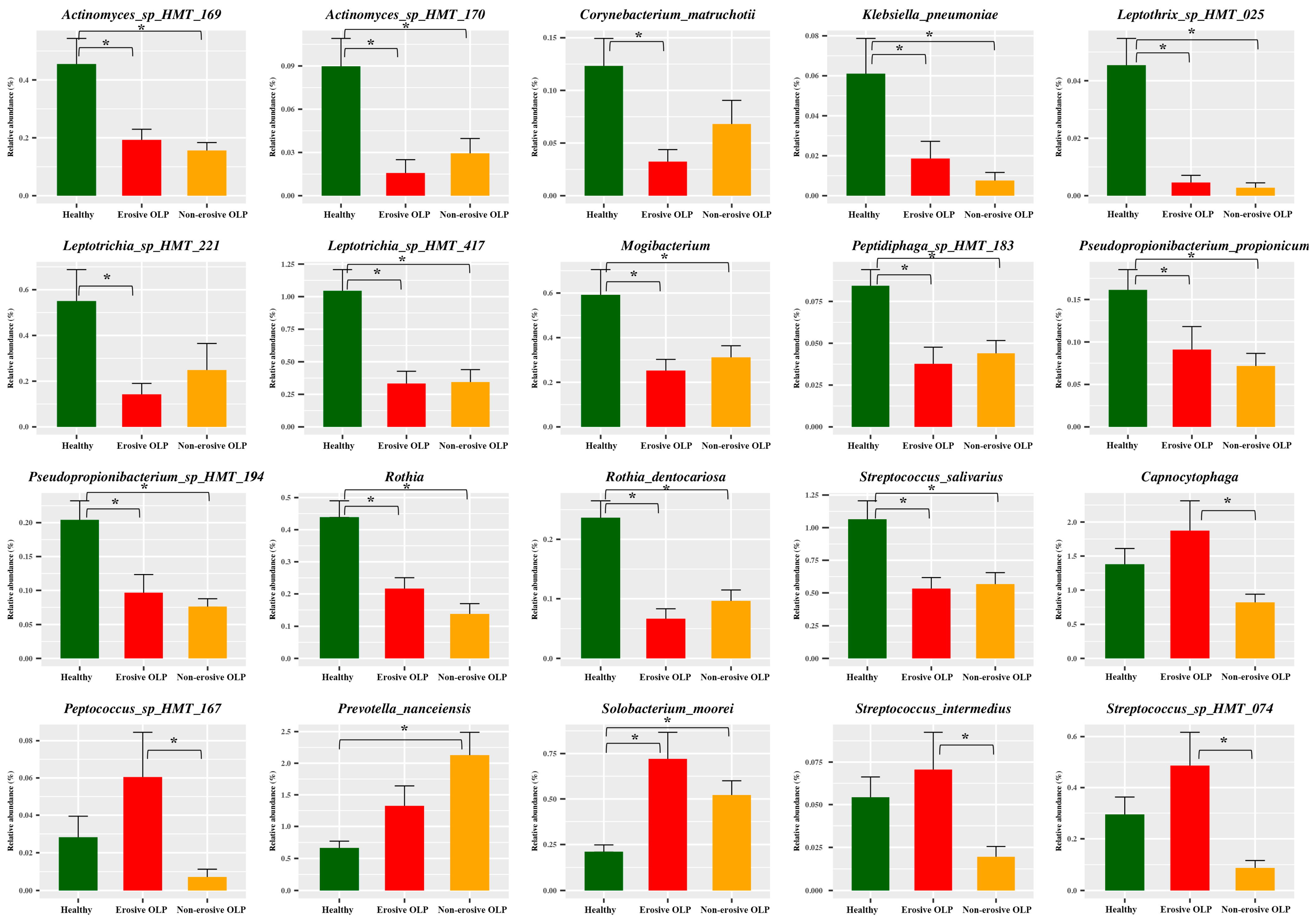
3.3. Species Taxa Comparison
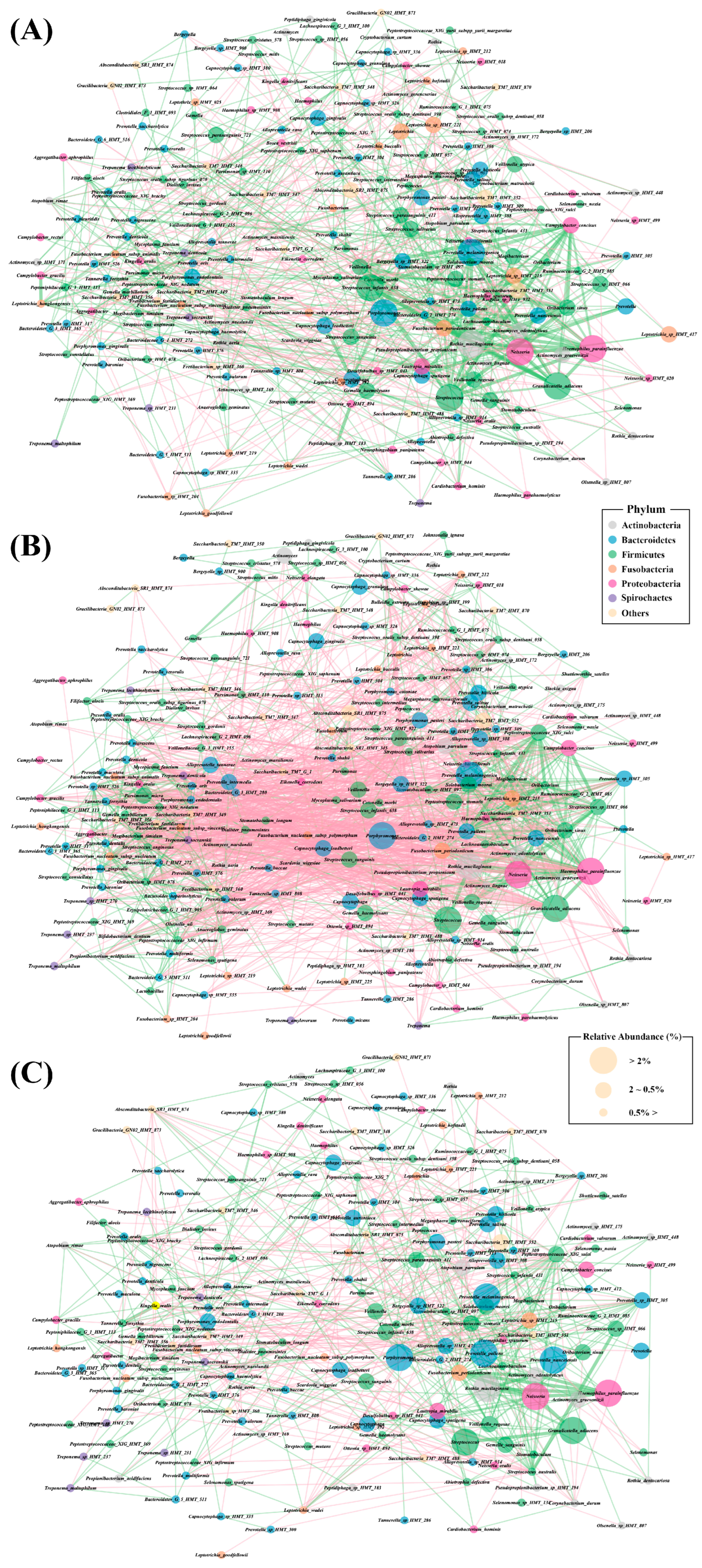
3.4. Bacterial Interaction Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Meulen, T.A.; Harmsen, H.J.; Bootsma, H.; Spijkervet, F.K.; Kroese, F.G.; Vissink, A. The microbiome–systemic disease connection. Oral Dis. 2016, 22, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Hwang, B.-O.; Lim, M.; Ok, S.-H.; Lee, S.-K.; Chun, K.-S.; Park, K.-K.; Hu, Y.; Chung, W.-Y.; Song, N.-Y. Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Kong, E.F.; Rizk, A.M.; Jabra-Rizk, M.A. The oral microbiome: A Lesson in coexistence. PLoS Pathog. 2018, 14, e1006719. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. The saliva microbiome profiles are minimally affected by collection method or DNA extraction protocols. Sci. Rep. 2017, 7, 8523. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Chan, Y.; Kheur, S.; Jin, L.J.; Watt, R.M.; Mattheos, N. Salivary microbiome in non-oral disease: A summary of evidence and commentary. Arch. Oral Biol. 2017, 83, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chung, S.W.; Auh, Q.-S.; Hong, S.-J.; Lee, Y.-A.; Jung, J.; Lee, G.-J.; Park, H.J.; Shin, S.-I.; Hong, J.-Y. Progress in oral microbiome related to oral and systemic diseases: An update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Fei, G.; Lundmark, A.; Benchimol, D.; Lee, L.; Hu, Y.O.; Kats, A.; Saevarsdottir, S.; Catrina, A.I.; Klinge, B. Periodontal health and oral microbiota in patients with rheumatoid arthritis. J. Clin. Med. 2019, 8, 630. [Google Scholar] [CrossRef]
- Corrêa, J.D.; Calderaro, D.C.; Ferreira, G.A.; Mendonça, S.M.S.; Fernandes, G.R.; Xiao, E.; Teixeira, A.L.; Leys, E.J.; Graves, D.T.; Silva, T.A. Subgingival microbiota dysbiosis in systemic lupus erythematosus: Association with periodontal status. Microbiome 2017, 5, 34. [Google Scholar] [CrossRef]
- Fabbri, C.; Fuller, R.; Bonfá, E.; Guedes, L.K.; D’Alleva, P.S.R.; Borba, E.F. Periodontitis treatment improves systemic lupus erythematosus response to immunosuppressive therapy. Clin. Rheumatol. 2014, 33, 505–509. [Google Scholar] [CrossRef]
- Sugerman, P.B.; Sabage, N. Oral lichen planus: Causes, diagnosis and management. Aust. Dent. J. 2002, 47, 290–297. [Google Scholar] [CrossRef]
- Roopashree, M.; Gondhalekar, R.V.; Shashikanth, M.; George, J.; Thippeswamy, S.; Shukla, A. Pathogenesis of oral lichen planus–A review. J. Oral Pathol. Med. 2010, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jawanda, M.K. Oral lichen planus: An update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J. Dermatol. 2015, 60, 222. [Google Scholar] [CrossRef]
- Jung, W.; Jang, S. Oral microbiome research on Oral lichen planus: Current findings and perspectives. Biology 2022, 11, 723. [Google Scholar] [CrossRef]
- Brant, J.; Aguiar, M.C.F.; Grandinetti, H.A.; Rodrigues, L.V.; Vasconcelos, A.C. A comparative study of apoptosis in reticular and erosive oral lichen planus. Braz. Dent. J. 2012, 23, 564–569. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.; Luo, Z.; Fu, J.; Wang, H.; Sun, Z. Overexpression and varied clinical significance of Th9 versus Th17 cells in distinct subtypes of oral lichen planus. Arch. Oral Biol. 2017, 80, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Shiva, A.; Zamanian, A.; Arab, S.; Boloki, M. Immunohistochemical study of p53 expression in patients with erosive and non-erosive oral lichen planus. J. Dent. 2018, 19, 118. [Google Scholar]
- Jana, A.; Thomas, J.; Ghosh, P. Erosive oral lichen planus inflicts higher cellular stress than reticular type. J. Oral Maxillofac. Pathol. 2021, 25, 279. [Google Scholar]
- Rodríguez-Núñez, I.; Blanco-Carrión, A.; García, A.G.; Rey, J.G. Peripheral T-cell subsets in patients with reticular and atrophic-erosive oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 91, 180–188. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Lombardelli, L.; Logiodice, F.; Tesi, D.; Kullolli, O.; Biagiotti, R.; Giudizi, M.; Romagnani, S.; Maggi, E.; Ficarra, G. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2014, 20, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Hurwitz, S.; Woo, S.-B. Oral lichen planus: REU scoring system correlates with pain. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Na, H.S.; Song, Y.; Yu, Y.; Chung, J. Comparative Analysis of Primers Used for 16S rRNA Gene Sequencing in Oral Microbiome Studies. Methods Protoc. 2023, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2. Methods Mol. Biol. 2018, 1849, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Z.; Tang, N.; Zhao, Y.; Xu, J.; Li, L.; Qian, L.; Zhang, J.; Fan, Y. Microbial community analysis of saliva and biopsies in patients with oral lichen planus. Front. Microbiol. 2020, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Y.; Wang, Q.Q.; Li, M.; Cheng, Y.-H.; Cheng, Y.-S.L.; Zhou, Y.; Yang, X.; Zhang, F.; Ge, X.; Zhao, B. Dysbiosis of saliva microbiome in patients with oral lichen planus. BMC Microbiol. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Keijser, B.; Zaura, E.; Huse, S.; Van Der Vossen, J.; Schuren, F.; Montijn, R.; Ten Cate, J.; Crielaard, W. Pyrosequencing analysis of the oral microflora of healthy adults. J. Dent. Res. 2008, 87, 1016–1020. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy. BMC Microbiol. 2009, 9, 259. [Google Scholar]
- Wang, K.; Lu, W.; Tu, Q.; Ge, Y.; He, J.; Zhou, Y.; Gou, Y.; Van Nostrand, J.D.; Qin, Y.; Li, J. Preliminary analysis of salivary microbiome and their potential roles in oral lichen planus. Sci. Rep. 2016, 6, 22943. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, Y.; Yoon, H.-J.; Baek, K.J.; Alam, J.; Park, H.K.; Choi, Y. The presence of bacteria within tissue provides insights into the pathogenesis of oral lichen planus. Sci. Rep. 2016, 6, 29186. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.; Maruyama, F.; Michi, Y.; Nakagawa, I.; Harada, K. Analysis of the factors affecting the formation of the microbiome associated with chronic osteomyelitis of the jaw. Clin. Microbiol. Infect. 2014, 20, O309–O317. [Google Scholar] [CrossRef] [PubMed]
- Garvey, G. Current concepts of bacterial infections of the central nervous system: Bacterial meningitis and bacterial brain abscess. J. Neurosurg. 1983, 59, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, M.; Bhat, S.; Lakshmi, K.N.B. Prevotella intermedia-An overview and its role in periodontitis. J. Adv. Clin. Res. Insights 2021, 8, 79–82. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, R.; Laine, M.; Aarden, L.; Van Winkelhoff, A. Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol. Immunol. 2007, 22, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, A.; Elborn, S.J.; Schock, B.C. Toll like Receptor signalling by Prevotella histicola activates alternative NF-κB signalling in Cystic Fibrosis bronchial epithelial cells compared to P. aeruginosa. PLoS ONE 2020, 15, e0235803. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Mustafa, R.; Listyarifah, D.; Al-Samadi, A.; Barreto, G.; Nordström, D.; Eklund, K.K. Altered expression of toll-like receptors in human oral epithelium in oral lichenoid reactions. Am. J. Dermatopathol. 2017, 39, 811–818. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, M.; Zhang, S.; Wang, Z.; Jiang, L.; Shen, J.; Bai, J.; Gao, F.; Zhou, M.; Chen, Q. NF-κB-dependent cytokines in saliva and serum from patients with oral lichen planus: A study in an ethnic Chinese population. Cytokine 2008, 41, 144–149. [Google Scholar] [CrossRef]
- Gu, G.M.; Martin, M.D.; Darveau, R.P.; Truelove, E.; Epstein, J. Oral and serum IL-6 levels in oral lichen planus patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Wang, J.; Chia, J.; Chiang, C. Serum interleukin-8 level is a more sensitive marker than serum interleukin-6 level in monitoring the disease activity of oral lichen planus. Br. J. Dermatol. 2005, 152, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Umeda, M.; Sakamoto, M.; Benno, Y.; Huang, Y.; Ishikawa, I. Treponema socranskii, Treponema denticola, and Porphyromonas gingivalis are associated with severity of periodontal tissue destruction. J. Periodontol. 2001, 72, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Rezaee, M. Comparison of periodontal status in gingival oral lichen planus patients and healthy subjects. Dermatol. Res. Pract. 2012, 2012, 561232. [Google Scholar] [CrossRef]
- Ramon-Fluixa, C.; Bagán-Sebastián, J.; Milián-Masanet, M.; Scully, C. Periodontal status in patients with oral lichen planus: A study of 90 cases. Oral Dis. 1999, 5, 303–306. [Google Scholar] [CrossRef]
- Rai, N.P.; Kumar, P.; Mustafa, S.M.; Divakar, D.D.; Kheraif, A.A.; Ramakrishnaiah, R.; Vellapally, S.; Dalati, M.; Parine, N.R.; Anil, S. Relation between periodontal status and pre-cancerous condition (oral lichen planus): A pilot study. Adv. Clin. Exp. Med. 2016, 25, 763–766. [Google Scholar]
- Salgado, D.S.; Jeremias, F.; Capela, M.V.; Onofre, M.A.; Massucato, E.M.S.; Orrico, S.R. Plaque control improves the painful symptoms of oral lichen planus gingival lesions. A short-term study. J. Oral Pathol. Med. 2013, 42, 728–732. [Google Scholar] [CrossRef]
- Jolivet-Gougeon, A.; Bonnaure-Mallet, M. Screening for prevalence and abundance of Capnocytophaga spp by analyzing NGS data: A scoping review. Oral Dis. 2021, 27, 1621–1630. [Google Scholar] [CrossRef]
- Seerangaiyan, K.; van Winkelhoff, A.J.; Harmsen, H.J.; Rossen, J.W.; Winkel, E.G. The tongue microbiome in healthy subjects and patients with intra-oral halitosis. J. Breath Res. 2017, 11, 036010. [Google Scholar] [CrossRef]


| HC (n = 30) | E-OLP (n = 25) | NE-OLP (n = 35) | p-Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 55.13 ± 10.75 | 58.44 ± 10.17 | 55.66 ± 12.75 | 0.525 |
| Male/female | 3/27 | 9/16 | 9/26 | 0.069 |
| NRS | 4.66 ± 2.52 | 3.54 ± 2.09 | 0.067 | |
| No. of teeth (mean ± SD) | 25.29 ± 4.45 | 23.48 ± 7.17 | 26.17 ± 4.53 | 0.170 |
| REU score/No. of gingival erosive lesion | 9.82 ± 5.18/12 | 2.34 ± 1.45/13 | ||
| Cigarette smoking | 0 | 1 | 2 | |
| Alcohol drinking | 0 | 0 | 0 | |
| Diabetes | 1 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, H.-M.; Ahn, Y.-W.; Ok, S.-M.; Jeong, S.-H.; Na, H.-S.; Chung, J. Microbial Profiles in Oral Lichen Planus: Comparisons with Healthy Controls and Erosive vs. Non-Erosive Subtypes. Diagnostics 2024, 14, 828. https://doi.org/10.3390/diagnostics14080828
Ju H-M, Ahn Y-W, Ok S-M, Jeong S-H, Na H-S, Chung J. Microbial Profiles in Oral Lichen Planus: Comparisons with Healthy Controls and Erosive vs. Non-Erosive Subtypes. Diagnostics. 2024; 14(8):828. https://doi.org/10.3390/diagnostics14080828
Chicago/Turabian StyleJu, Hye-Min, Yong-Woo Ahn, Soo-Min Ok, Sung-Hee Jeong, Hee-Sam Na, and Jin Chung. 2024. "Microbial Profiles in Oral Lichen Planus: Comparisons with Healthy Controls and Erosive vs. Non-Erosive Subtypes" Diagnostics 14, no. 8: 828. https://doi.org/10.3390/diagnostics14080828






