A Machine Learning System to Indicate Diagnosis of Idiopathic Pulmonary Fibrosis Non-Invasively in Challenging Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. The Fibresolve Classifier: Development and Initial Validation
2.2. Clinical Panel Reviews
2.3. Fibresolve Classifier Results
2.4. Statistical Plan
3. Results
3.1. Baseline Characteristics
3.2. Final Ground Truth Diagnosis
3.3. Performance Assessments
3.4. Analysis of Error Cases
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikolasch, T.A.; Garthwaite, H.S.; Porter, J.C. Update in diagnosis and management of interstitial lung disease. Clin. Med. 2017, 17, 146–153. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Thomson, C.C.; Remy-Jardin, M.; Richeldi, L.; Martinez, F.J.; Kolb, M.; Raghu, G. Idiopathic pulmonary fibrosis: State of the art for 2023. Eur. Respir. J. 2023, 61, 2200957. [Google Scholar] [CrossRef]
- He, M.; Yang, T.; Zhou, J.; Wang, R.; Li, X. A real-world study of antifibrotic drugs-related adverse events based on the United States food and drug administration adverse event reporting system and VigiAccess databases. Front. Pharmacol. 2024, 15, 1310286. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.P.; Fogarty, A.W.; McKeever, T.M.; Hubbard, R.B. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am. J. Respir. Crit. Care Med. 2016, 193, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Maher, T.M.; Kolb, M.; Poletti, V.; Nusser, R.; Richeldi, L.; Vancheri, C.; Wilsher, M.L.; Antoniou, K.M.; Behr, J.; et al. Diagnostic accuracy of a clinical diagnosis of idiopathic pulmonary fibrosis: An international case-cohort study. Eur. Respir. J. 2017, 50, 1700936. [Google Scholar] [CrossRef] [PubMed]
- Lamas, D.J.; Kawut, S.M.; Bagiella, E.; Philip, N.; Arcasoy, S.M.; Lederer, D.J. Delayed access and survival in idiopathic pulmonary fibrosis: A cohort study. Am. J. Respir. Crit. Care Med. 2011, 184, 842–847. [Google Scholar] [CrossRef]
- Lamas, D.; Kawut, S.M.; Peterson, E.; Philip, N.; Patel, N.; Arcasoy, S.M.; Lederer, D.J. Publicly-insured patients with idiopathic pulmonary fibrosis have longer delays in accessing subspecialty care. Am. J. Respir. Crit. Care Med. 2012, 185, A4581. [Google Scholar]
- Chung, J.H.; Chawla, A.; Peljto, A.L.; Cool, C.D.; Groshong, S.D.; Talbert, J.L.; McKean, D.F.; Brown, K.K.; Fingerlin, T.E.; Schwarz, M.I.; et al. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest 2015, 147, 450–459. [Google Scholar] [CrossRef]
- Brownell, R.; Moua, T.; Henry, T.S.; Elicker, B.M.; White, D.; Vittinghoff, E.; Jones, K.D.; Urisman, A.; Aravena, C.; Johannson, K.A.; et al. The use of pretest probability increases the value of high-resolution CT in diagnosing usual interstitial pneumonia. Thorax 2017, 72, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Calandriello, L.; Sverzellati, N.; Wells, A.U.; Hansell, D.M. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax 2016, 71, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Lynch, D.; Godwin, J.D.; Webb, R.; Colby, T.V.; Leslie, K.O.; Behr, J.; Brown, K.K.; Egan, J.J.; Flaherty, K.R.; et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: Secondary analysis of a randomised, controlled trial. Lancet Respir. Med. 2014, 2, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Fukihara, J.; Kondoh, Y.; Brown, K.K.; Kimura, T.; Kataoka, K.; Matsuda, T.; Yamano, Y.; Suzuki, A.; Furukawa, T.; Sumikawa, H.; et al. Probable usual interstitial pneumonia pattern on chest CT: Is it sufficient for a diagnosis of idiopathic pulmonary fibrosis? Eur. Respir. J. 2020, 55, 1802465. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Flaherty, K.R.; Lederer, D.J.; Lynch, D.A.; Colby, T.V.; Myers, J.L.; Groshong, S.D.; Larsen, B.T.; Chung, J.H.; Steele, M.P.; et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: A prospective validation study. Lancet Respir. Med. 2019, 7, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Sheth, J.S.; Belperio, J.A.; Fishbein, M.C.; Kazerooni, E.A.; Lagstein, A.; Murray, S.; Myers, J.L.; Simon, R.H.; Sisson, T.H.; Sundaram, B.; et al. Utility of Transbronchial vs Surgical Lung Biopsy in the Diagnosis of Suspected Fibrotic Interstitial Lung Disease. Chest 2017, 151, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, G.P.; Bianchi, P.; Danese, S.; Lederer, D.J. Barriers to timely diagnosis of interstitial lung disease in the real world: The INTENSITY survey. BMC Pulm. Med. 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Mlodzinski, E.; Stone, D.J.; Celi, L.A. Machine Learning for Pulmonary and Critical Care Medicine: A Narrative Review. Pulm. Ther. 2020, 6, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Karwoski, R.A.; Gierada, D.S.; Bartholmai, B.J.; Koo, C.W. Quantitative CT Analysis of Diffuse Lung Disease. Radiographics 2020, 40, 28–43. [Google Scholar] [CrossRef]
- Walsh, S.L.F.; Calandriello, L.; Silva, M.; Sverzellati, N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: A case-cohort study. Lancet Respir. Med. 2018, 6, 837–845. [Google Scholar] [CrossRef]
- Shaish, H.; Ahmed, F.S.; Lederer, D.; D’Souza, B.; Armenta, P.; Salvatore, M.; Saqi, A.; Huang, S.; Jambawalikar, S.; Mutasa, S. Deep learning of computed tomography virtual wedge resection for prediction of histologic usual interstitial pneumonitis. Ann. Am. Thorac. Soc. 2021, 18, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Mackintosh, J.A.; Calandriello, L.; Silva, M.; Sverzellati, N.; Larici, A.R.; Humphries, S.M.; Lynch, D.A.; Jo, H.E.; Glaspole, I.; et al. Deep Learning-based Outcome Prediction in Progressive Fibrotic Lung Disease Using High-resolution Computed Tomography. American journal of respiratory and critical care medicine. Am. J. Respir. Crit. Care Med. 2022, 206, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Maddali, M.V.; Kalra, A.; Muelly, M.; Reicher, J.J. Development and validation of a CT-based deep learning algorithm to augment non-invasive diagnosis of idiopathic pulmonary fibrosis. Respir. Med. 2023, 219, 107428. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Nyberg, F.; Morgan, G. The epidemiology of interstitial lung disease and its association with lung cancer. Br. J. Cancer 2004, 91 (Suppl. 2), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Nayfeh, A.S.; Chippa, V.; Moore, D.R. Nonspecific Interstitial Pneumonitis; Updated 28 March 2021; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK518974/ (accessed on 9 April 2024).
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, I.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef]



| - | Full Dataset—% (n) | 50-Case Subset—% (n) |
|---|---|---|
| Age | - | - |
| ≤40 | 5.0 (15) | 2.0 (1) |
| 41–50 | 10.0 (30) | 12.0 (6) |
| 51–60 | 28.0 (84) | 28.0 (14) |
| 61–70 | 41.3 (124) | 42.0 (21) |
| >70 | 15.7 (47) | 6.0 (6) |
| Sex | - | - |
| Female | 49.7 (149) | 50.0 (25) |
| Male | 50.3 (151) | 50.0 (25) |
| Race | - | - |
| American Indian or Alaskan Native | 1.0 (3) | 2.0 (1) |
| Asian | 0.3 (1) | 0.0 (0) |
| Black or African American | 10.3 (31) | 10.0 (5) |
| Multi-race | 0.7 (2) | 0.0 (0) |
| Unknown | 2.0 (6) | 4.0 (2) |
| White | 85.7 (257) | 84.0 42 |
| Ethnicity | - | - |
| Hispanic or Latino | 9.0 (27) | 8.0 (4) |
| Not Hispanic or Latino | 88.7 (266) | 90.0 (45) |
| Chooses not to disclose | 2.3 (7) | 2.0 (1) |
| Pathology | - | - |
| 1 (Surgical Tissue Recorded) | 94.7 (284) | 96.0 (48) |
| 0 (None Recorded) | 5.3 (16) | 4.0 (2) |
| Smoking History | - | - |
| Yes | 69.0 (207) | 74.0 (37) |
| No | 31.0 (93) | 26.0 (13) |
| Follow-Up Time | - | - |
| 0–150 Days | 28.3 (85) | 36.0 (18) |
| 151–300 Days | 40.3 (121) | 30.0 (15) |
| 300+ Days | 31.3 (94) | 34.0 (17) |
| Lung Function | - | - |
| ppFVC * < 50% | 19.7 (59) | 16.0 (8) |
| ppFVC 50–75% | 36.0 (108) | 40.0 (20) |
| ppFVC > 75% | 34.7 (104) | 36.0 (18) |
| Mortality | - | - |
| Alive at last time point | 32.0 (96) | 26.0 (13) |
| Dead at last time point | 16.7 (50) | 18.0 (9) |
| Unknown | 51.3 (154) | 56.0 (28) |
| - | Full Dataset—% (n) | 50-Case Subset—% (n) |
|---|---|---|
| Clinical Sites | - | - |
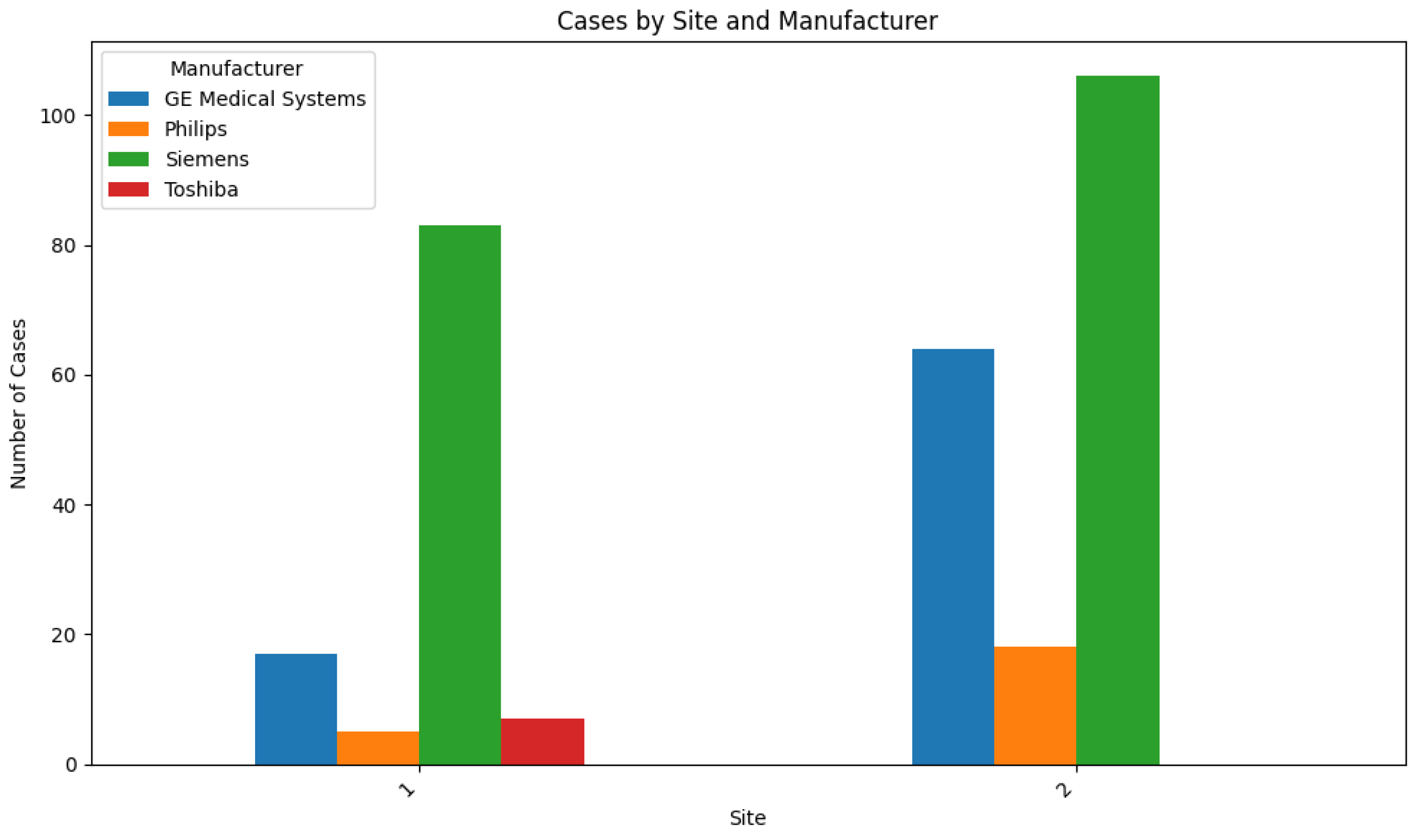
| Site #1 | 37.3 (112) | 36.0 (8) |
| Site #2 | 62.7 (188) | 64.0 (1) |
| Manufacturer | - | - |
| GE Medical Systems | 27.0 (81) | 26.0 (13) |
| Philips | 7.7 (23) | 12.0 (6) |
| Siemens | 63.0 (189) | 60.0 (30) |
| Toshiba | 2.3 (7) | 2.0 (1) |
| Slice Thickness | - | - |
| 1 mm | 14.7 (44) | 16.0 (8) |
| 1.25 mm | 3.7 (11) | 2.0 (1) |
| 1.5 mm | 12.7 (38) | 6.0 (3) |
| 2.0 mm | 1.3 (4) | 6.0 (3) |
| 2.5 mm | 1.3 (4) | 2.0 (1) |
| 3.0 mm | 13.3 (40) | 16.0 (8) |
| 3.75 mm | 0.7 (2) | 0.0 (0) |
| 4.0 mm | 0.3 (1) | 0.0 (0) |
| 5.0 mm | 53.3 (160) | 52.0 (26) |
| - | Full Dataset—% (n) | 50-Case Subset—% (n) |
|---|---|---|
| IPF | 27.7 (83) | 28.0 (14) |
| Not IPF | 72.3 (217) | 72.0 (36) |
| - UILD | 18.0 (54) | 24.0 (12) |
| - CHP | 16.7 (50) | 12.0 (6) |
| - NSIP | 10.7 (32) | 8.0 (4) |
| - COP | 7.7 (23) | 8.0 (4) |
| - CTD-ILD | 4.3 (13) | 6.0 (3) |
| - DIP | 3.0 (9) | 4.0 (2) |
| - EGPA | 3.0 (9) | 0.0 (0) |
| - Sarcoid | 2.7 (8) | 4.0 (2) |
| - No ILD | 1.3 (4) | 2.0 (1) |
| - Berylliosis | 1.3 (4) | 2.0 (1) |
| - RB–ILD | 1.0 (3) | 0.0 (0) |
| - CEP | 0.3 (1) | 0.0 (0) |
| - LIP | 0.3 (1) | 2.0 (1) |
| - | Expert Clinical Panel in All Cases | Fibresolve in All Cases | Fibresolve in Key Subgroup * |
|---|---|---|---|
| Cases | 300 | 300 | 124 |
| Sensitivity | 13.3% [CI: 6.8–22.5] | 41.0% [CI: 30.3–52.3] ** | 53.1% [CI: 41.3–64.9] |
| Specificity | 96.3% [CI: 92.9–98.4] | 86.6% [CI: 81.4–90.9] *** | 85.9% [CI: 76.7–92.6%] |
| - | Sensitivity | Specificity |
|---|---|---|
| Fibresolve | 57.1% | 88.9% |
| Expert Clinical Panel | 0% | 94.4% |
| Clinical Panel #1 | 0% | 97.2% |
| Clinical Panel #2 | 28.6% | 91.7% |
| Clinical Panel #3 | 14.3% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, Y.; Mooney, J.; Allen, I.E.; Seaman, J.; Kalra, A.; Muelly, M.; Reicher, J. A Machine Learning System to Indicate Diagnosis of Idiopathic Pulmonary Fibrosis Non-Invasively in Challenging Cases. Diagnostics 2024, 14, 830. https://doi.org/10.3390/diagnostics14080830
Ahmad Y, Mooney J, Allen IE, Seaman J, Kalra A, Muelly M, Reicher J. A Machine Learning System to Indicate Diagnosis of Idiopathic Pulmonary Fibrosis Non-Invasively in Challenging Cases. Diagnostics. 2024; 14(8):830. https://doi.org/10.3390/diagnostics14080830
Chicago/Turabian StyleAhmad, Yousef, Joshua Mooney, Isabel E. Allen, Julia Seaman, Angad Kalra, Michael Muelly, and Joshua Reicher. 2024. "A Machine Learning System to Indicate Diagnosis of Idiopathic Pulmonary Fibrosis Non-Invasively in Challenging Cases" Diagnostics 14, no. 8: 830. https://doi.org/10.3390/diagnostics14080830
APA StyleAhmad, Y., Mooney, J., Allen, I. E., Seaman, J., Kalra, A., Muelly, M., & Reicher, J. (2024). A Machine Learning System to Indicate Diagnosis of Idiopathic Pulmonary Fibrosis Non-Invasively in Challenging Cases. Diagnostics, 14(8), 830. https://doi.org/10.3390/diagnostics14080830





