Seeing and Sensing the Hepatorenal Syndrome (HRS): The Growing Role of Ultrasound-Based Techniques as Non-Invasive Tools for the Diagnosis of HRS
Abstract
:1. Introduction: The Spectrum of Acute Kidney Injuries in Cirrhosis
2. Hepatorenal Syndrome
2.1. General Approach
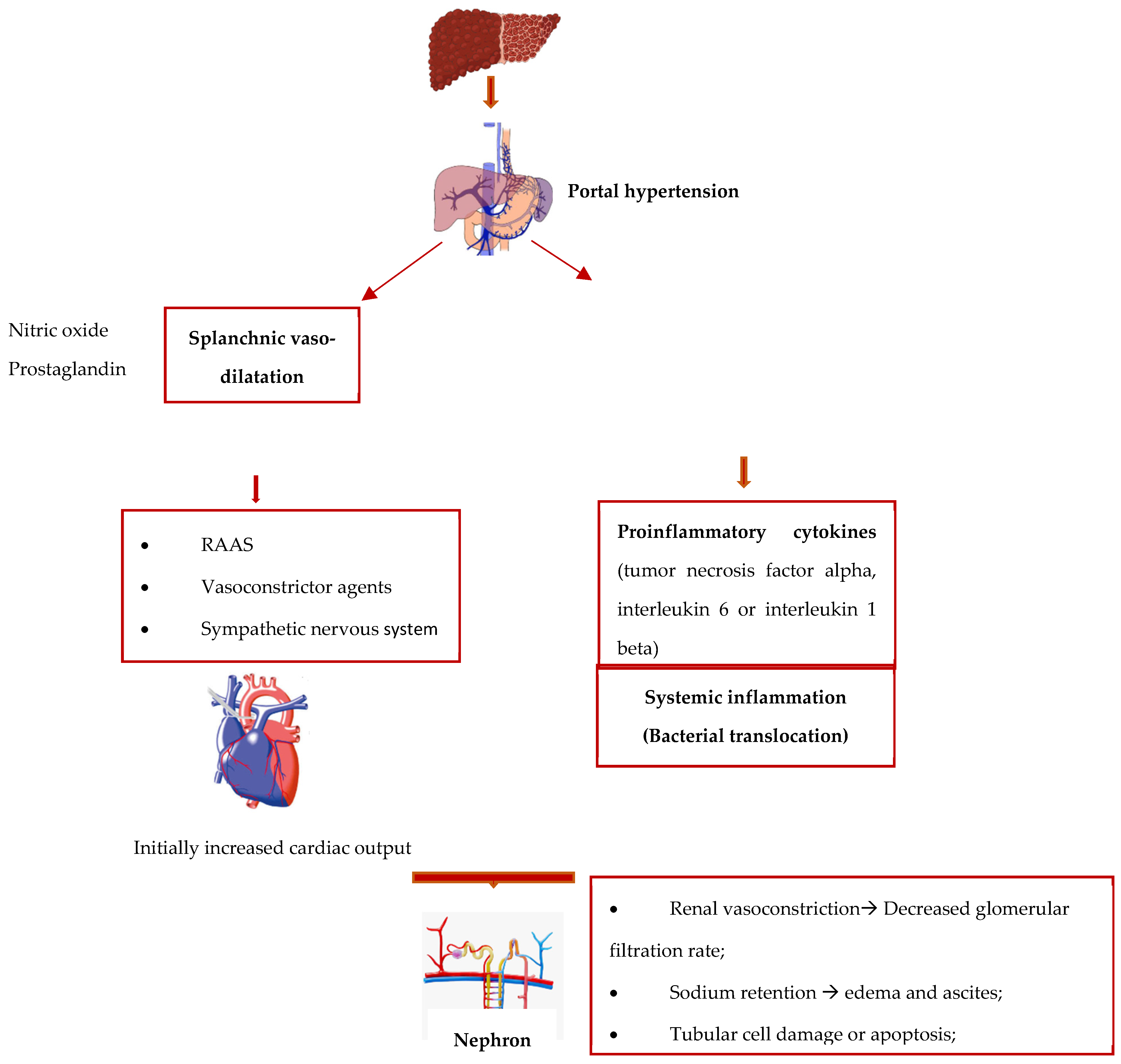
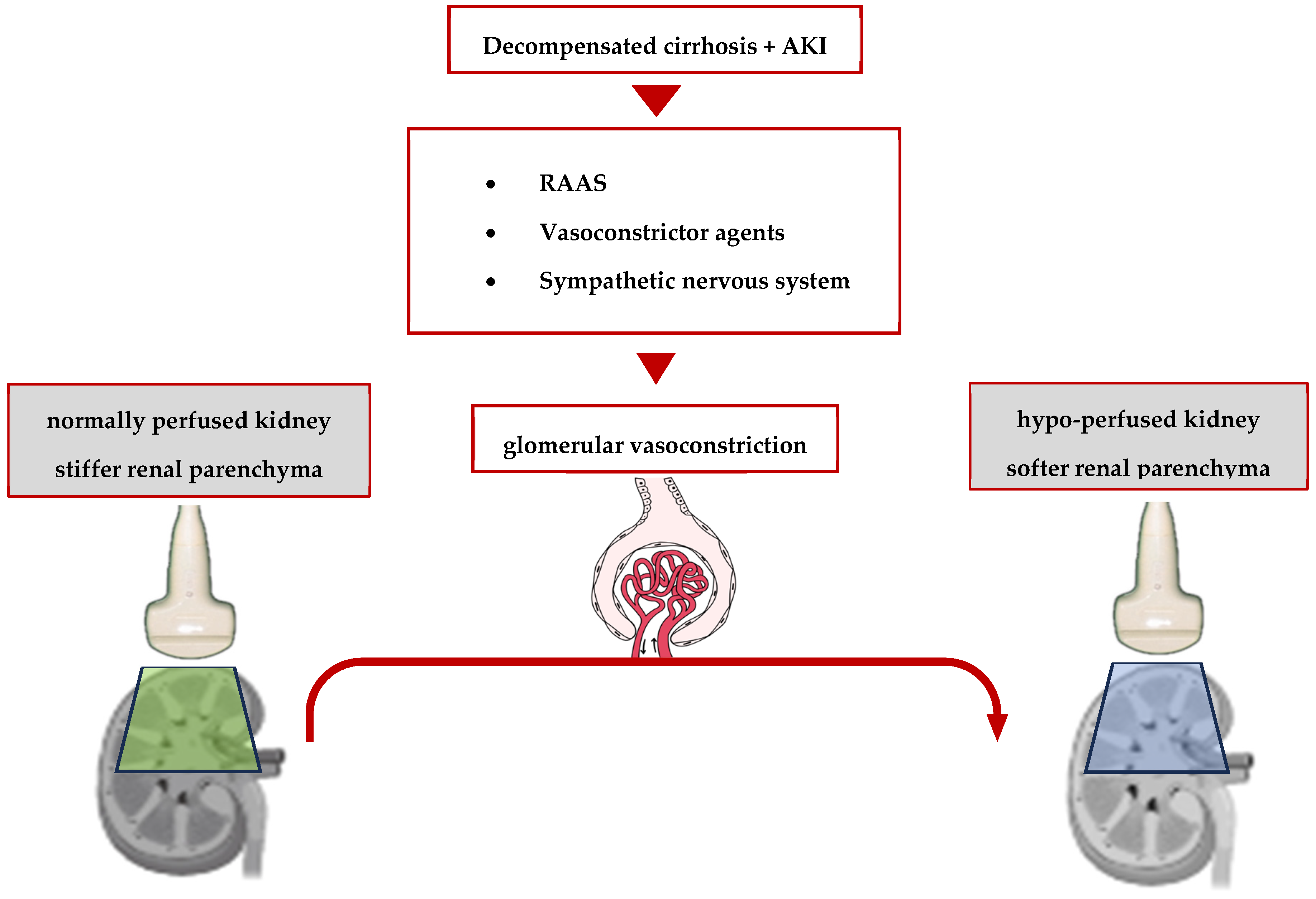
2.2. Pathophysiology of Hepatorenal Syndrome
3. Diagnosis Tools
3.1. Serum Biomarkers
3.2. Urinary Biomarkers
3.3. Imaging Studies
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V. Acute kidney injury (AKI) in cirrhosis: Should we change current definition and diagnostic criteria of renal failure in cirrhosis? J. Hepatol. 2013, 59, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.P.; Wright, G.A.K.; Banaji, M.; Mukhopadhya, A.; Mookerjee, R.; Moore, K.; Jalan, R. Relationship Between Activation of the Sympathetic Nervous System and Renal Blood Flow Autoregulation in Cirrhosis. Gastroenterology 2008, 134, 111–119.e2. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; James, M.T.; Abraldes, J.G.; Karvellas, C.J.; Ye, F.; Pannu, N. Relevance of new definitions to incidence and prognosis of acute kidney injury in hospitalized patients with cirrhosis: A retrospective population-based cohort study. PLoS ONE 2016, 11, e0160394. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.M.; Garcia-Tsao, G.; Sanyal, A.J.; Thiessen-Philbrook, H.; Peixoto, A.J.; Perazella, M.A.; Ansari, N.; Lim, J.; Coca, S.G.; Parikh, C.R. Urinary Biomarkers and Progression of AKI in Patients with Cirrhosis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Gines, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and Management of Acute Kidney Injury in Patients with Cirrhosis: Revised Consensus Recommendations of the International Club of Ascites. Available online: http://gut.bmj.com/ (accessed on 15 August 2015).
- Durand, F.; Graupera, I.; Ginès, P.; Olson, J.C.; Nadim, M.K. Pathogenesis of Hepatorenal Syndrome: Implications for Therapy. Am. J. Kidney Dis. 2016, 67, 318–328. [Google Scholar] [CrossRef]
- Cavallin, M.; Piano, S.; Romano, A.; Fasolato, S.; Chiara, A.; Benetti, G.; Gola, E.; Morando, F.; Stanco, M.; Rosi, S.; et al. Terlipressin Given by Continuous Intravenous Infusion Versus Intravenous Boluses in the Treatment of Hepatorenal Syndrome: A Randomized Controlled Study. Hepatology 2016, 63, 983–992. [Google Scholar] [CrossRef]
- Nadim, M.K.; Kellum, J.A.; Forni, L.; Francoz, C.; Asrani, S.K.; Ostermann, M.; Allegretti, A.S.; Neyra, J.A.; Olson, J.C.; Piano, S.; et al. Acute kidney injury in patients with Cirrhosis: Acute Disease Quality Initiative (ADQI) and international Club of ascites (ICA) joint multidisciplinary consensus meeting. J. Hepatol. 2024. [Google Scholar] [CrossRef]
- Nazar, A.; Pereira, G.H.; Guevara, M.; Martín-Llahi, M.; Pepin, M.N.; Marinelli, M.; Sola, E.; Baccaro, M.E.; Terra, C.; Arroyo, V.; et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology 2010, 51, 219–226. [Google Scholar] [CrossRef]
- Wong, F.; Pappas, S.C.; Boyer, T.D.; Sanyal, A.J.; Bajaj, J.S.; Escalante, S.; Jamil, K. Terlipressin Improves Renal Function and Reverses Hepatorenal Syndrome in Patients with Systemic Inflammatory Response Syndrome. Clin. Gastroenterol. Hepatol. 2017, 15, 266–272.e1. [Google Scholar] [CrossRef]
- Arab, J.P.; Claro, J.C.; Arancibia, J.P.; Contreras, J.; Gómez, F.; Muñoz, C.; Nazal, L.; Roessler, E.; Wolff, R.; Arrese, M.; et al. Therapeutic alternatives for the treatment of type 1 hepatorenal syndrome: A Delphi technique-based consensus. World J. Hepatol. 2016, 8, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Arroyo, V.; Bernardi, M.; Epstein, M.; Henriksen, J.H.; Rodes, J. Peripheral Arterial Vasodilation Hypothesis: A Proposal for the Initiation of Renal Sodium and Water Retention in Cirrhosis. Hepatology 1988, 8, 1151–1157. [Google Scholar] [CrossRef]
- Ruiz-Del-Arbol, L.; Monescillo, A.; Arocena, C.; Valer, P.; Ginès, P.; Moreira, V.; Milicua, J.M.; Jimenez, W.; Arroyo, V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005, 42, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Amoros, A.; Pitarch, C.; Titos, E.; Alcaraz-Quiles, J.; Schierwagen, R.; Deulofeu, C.; Fernandez-Gomez, J.; Piano, S.; Caraceni, C.; et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front. Immunol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Navasa, M.; Follo, A.; Filella, X.; Jiménez, W.; Francitorra, A.; Ramó, R.; Rimola, A.; Arroyo, V.; Rodes, J. Tumor Necrosis Factor and Interleukin-6 in Spontaneous Bacterial Peritonitis in Cirrhosis: Relationship with the Development of Renal Impairment and Mortality. Hepatology 1998, 27, 1227–1232. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Mohamed, F.E.; Jover-Cobos, M.; Macnaughtan, J.; Davies, N.; Moreau, R.; Paradis, V.; Moore, K.; Mookerjee, R.; Jalan, R. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 2013, 33, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Piano, S.; Schmidt, H.H.; Ariza, X.; Amoros, A.; Romano, A.; Hüsing-Kabar, A.; Solà, E.; Gerbes, A.; Bernardi, M.; Alessandria, C.; et al. Association Between Grade of Acute on Chronic Liver Failure and Response to Terlipressin and Albumin in Patients with Hepatorenal Syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 1792–1800.e3. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Gatta, A.; Caregaro, L.; Menon, F.; Sacerdoti, D.; Merkel, C.; Rondana, M.; De Toni, R.; Ruol, A. Tubular site of renal sodium retention in ascitic liver cirrhosis evaluated by lithium clearance. Eur. J. Clin. Investig. 1990, 20, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Nadim, M.K.; Kellum, J.A.; Salerno, F.; Bellomo, R.; Gerbes, A.; Angeli, P.; Moreau, R.; Davenport, A.; Jalan, R.; et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 2011, 60, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.S.; Fish, D.N.; Teitelbaum, I. Assessing renal function in cirrhotic patients: Problems and pitfalls. Am. J. Kidney Dis. 2003, 41, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K. Analytical reviews in clinical biochemistry: The estimation of creatinine. Ann. Clin. Biochem. 1986, 23, 1–25. [Google Scholar] [CrossRef]
- Caregaro, L.; Menon, F.; Angeli, P.; Amodio, P.; Merkel, C.; Bortoluzzi, A.; Alberino, F.; Gatta, A. Limitations of Serum Creatinine Level and Creatinine Clearance as Filtration Markers in Cirrhosis. Arch. Intern. Med. 1994, 154, 201–205. [Google Scholar] [CrossRef]
- Jaques, D.A.; Spahr, L.; Berra, G.; Poffet, V.; Lescuyer, P.; Gerstel, E.; Garina, N.; Martine, P.-Y.; Ponte, B. Biomarkers for acute kidney injury in decompensated cirrhosis: A prospective study. Nephrology 2019, 24, 170–180. [Google Scholar] [CrossRef]
- Gomaa, S.H.; Shamseya, M.M.; Madkour, M.A. Clinical utility of urinary neutrophil gelatinase-associated lipocalin and serum cystatin C in a cohort of liver cirrhosis patients with renal dysfunction: A challenge in the diagnosis of hepatorenal syndrome. Eur. J. Gastroenterol. Hepatol. 2019, 31, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Barreto, E.F.; Rule, A.D.; Murad, M.H.; Kashani, K.B.; Lieske, J.C.; Erwin, P.J.; Steckelberg, J.M.; Gajic, O.; Reid, J.M.; Kane-Gill, S.L. Prediction of the Renal Elimination of Drugs With Cystatin C vs. Creatinine: A Systematic Review. Mayo Clin. Proc. 2019, 94, 500–514. [Google Scholar] [CrossRef]
- Seo, Y.S.; Park, S.Y.; Kim, M.Y.; Kim, S.G.; Park, J.Y.; Yim, H.J.; Jang, B.K.; Park, S.H.; Kim, J.H.; Suk, K.T.; et al. Serum cystatin C level: An excellent predictor of mortality in patients with cirrhotic ascites. J. Gastroenterol. Hepatol. 2018, 33, 910–917. [Google Scholar] [CrossRef]
- Peres, L.A.; Cunha Júnior, A.D.; Schäfer, A.J.; Silva, A.L.; Gaspar, A.D.; Scarpari, D.F.; Alves, J.B.; Girelli Neto, R.; Oliveira, T.F. Biomarkers of acute kidney injury. J. Bras. Nefrol. 2013, 35, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Yin, W.J.; Zhou, L.Y.; Ma, R.R.; Liu, K.; Hu, C.; Zhoua, G.; Zuoa, X.C. Utility of cystatin C-based equations in patients undergoing dialysis. Clin. Chim. Acta 2018, 485, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.K.; Yang, J.; Hwang, S.M.; Lee, M.S.; Park, S.H. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci. Rep. 2019, 9, 14508. [Google Scholar] [CrossRef] [PubMed]
- Markwardt, D.; Holdt, L.; Steib, C.; Benesic, A.; Bendtsen, F.; Bernardi, M.; Moreau, R.; Teupser, D.; Wendon, J.; Nevens, F.; et al. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology 2017, 66, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Finkenstedt, A.; Dorn, L.; Edlinger, M.; Prokop, W.; Risch, L.; Griesmacher, A.; Graziadei, I.; Vogel, W.; Zoller, H. Cystatin C is a strong predictor of survival in patients with cirrhosis: Is a cystatin C-based MELD better? Liver Int. 2012, 32, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A. Original Investigations Pathogenesis and Treatment of Kidney Disease Accuracy of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Diagnosis and Prognosis in Acute Kidney Injury: A Systematic Review and Meta-Analysis. 2009. Available online: www.ajkd.org (accessed on 1 December 2009).
- Sharifian, R.; Okamura, D.M.; Denisenko, O.; Zager, R.A.; Johnson, A.; Gharib, S.A.; Bomsztyk, K. Distinct patterns of transcriptional and epigenetic alterations characterize acute and chronic kidney injury. Sci. Rep. 2018, 8, 17870. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, B.; Liu, Y.; Feng, J. The efficacy of biomarkers in the diagnosis of acute kidney injury secondary to liver cirrhosis. Medicine 2021, 100, E25411. [Google Scholar] [CrossRef]
- Fagundes, C.; Pépin, M.N.; Guevara, M.; Barreto, R.; Casals, G.; Solà, E.; Pereira, G.; Rodríguez, E.; Garcia, E.; Prado, V.; et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J. Hepatol. 2012, 57, 267–273. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transplant. 2009, 24, 3265–3268. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; Vaidya, V.S.; Waikar, S.S.; Collings, F.B.; Sunderland, K.E.; Gioules, C.J.; Bonventre, J.V. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010, 77, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Noiri, E.; Ono, Y.; Doi, K.; Negishi, K.; Kamijo, A.; Kimura, K.; Fujita, T.; Kinukawa, T.; Taniguchi, H.; et al. Renal L-type fatty acid-binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 2007, 18, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.Y.H.; Seto, W.K.; Fung, J.; Chok, S.H.; Chan, S.C.; Chan, G.C.W.; Fung, Y.M.; Mao, C.T. Serum and urinary biomarkers that predict hepatorenal syndrome in patients with advanced cirrhosis. Dig. Liver Dis. 2017, 49, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Puthumana, J.; Ariza, X.; Belcher, J.M.; Graupera, I.; Ginès, P.; Parikh, C.R. Urine Interleukin 18 and Lipocalin 2 Are Biomarkers of Acute Tubular Necrosis in Patients with Cirrhosis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1003–1013.e3. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Kwon, J.H.; Kim, Y.S.; Nam, S.W.; Park, J.W.; Kim, H.Y.; Kim, C.W.; Shin, S.K.; Chon, Y.E.; Jang, E.S.; et al. The role of urinary N-acetyl-β-D-glucosaminidase in cirrhotic patients with acute kidney injury: Multicenter, prospective cohort study. J. Clin. Med. 2021, 10, 4328. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, H.A.; Seo, Y.S.; Lee, Y.R.; Yim, S.Y.; Lee, Y.S.; Suh, S.J.; Jung, Y.K.; Kim, J.H.; An, H.; et al. Assessment and prediction of acute kidney injury in patients with decompensated cirrhosis with serum cystatin C and urine N-acetyl-β-D-glucosaminidase. J. Gastroenterol. Hepatol. 2019, 34, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.M.; Sanyal, A.J.; Peixoto, A.J.; Perazella, M.A.; Lim, J.; Thiessen-Philbrook, H.; Ansari, N.; Coca, S.G.; Garcia-Tsao, G.; Parikh, C.R. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 2014, 60, 622–632. [Google Scholar] [CrossRef]
- Francoz, C.; Durand, F.; Kahn, J.A.; Genyk, Y.S.; Nadim, M.K. Hepatorenal syndrome. Clin. J. Am. Soc. Nephrol. 2019, 14, 774–781. [Google Scholar] [CrossRef]
- Platt, J.F.; Ellis, J.H.; Rubin, J.M.; Merion, R.M.; Lucey, M.R. Renal Duplex Doppler Ultrasonography: A Noninvasive Predictor of Kidney Dysfunction and Hepatorenal Failure in Liver Disease Kidney dysfunction commonly develops in patients. Hepatology 1994, 20, 362–369. [Google Scholar] [CrossRef]
- Platt, J.F. Duplex Doppler Evaluation of Native Kidney Dysfunction: Obstructive and Nonobstructive Disease. AJR Am. J. Roentgenol. 1992, 158, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Platt, J.F.; Rubin, J.M.; Ellis, J.H. Acute renal failure: Possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology 1991, 179, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Platt, J.F.; Rubin, J.M.; Ellis, J.H. Distinction between obstructive and nonobstructive pyelocaliectasis with duplex Doppler sonography. AJR Am. J. Roentgenol. 1989, 153, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Parvey, H.R. Case Report Image-Directed Doppler Sonography of the Intrarenal Arteries in Acute Renal Vein Thrombosis. J. Clin. Ultrasound 1990, 18, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Velez, J.C.Q.; Petkovich, B.; Karakala, N.; Huggins, J.T. Point-of-Care Echocardiography Unveils Misclassification of Acute Kidney Injury as Hepatorenal Syndrome. Am. J. Nephrol. 2019, 50, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, E.M.; Oo, Z.; Kaptein, M.J. Hepatorenal syndrome misdiagnosis may be reduced using inferior vena cava ultrasound to assess intravascular volume and guide management. Ren. Fail. 2023, 45, 2185468. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.G.; Schelleman, A.; Goodwin, M.D.; Bailey, M.; Eastwood, G.M.; Bellomo, R. Contrast-enhanced ultrasound evaluation of the renal microcirculation response to terlipressin in hepato-renal syndrome: A preliminary report. Ren. Fail. 2015, 37, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Paratore, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Gasbarrini, A.; Zocco, M.A. Dynamic contrast enhanced ultrasound in gastrointestinal diseases: A current trend or an indispensable tool? World J. Gastroenterol. 2023, 29, 4021–4035. [Google Scholar] [CrossRef]
- Lim, A.K.P.; Patel, N.; Eckersley, R.J.; Goldin, R.D.; Thomas, H.C.; Cosgrove, D.O.; Taylor-Robinson, S.D.; Blomley, M.J.K. Hepatic vein transit time of SonoVue: A comparative study with levovist. Radiology 2006, 240, 130–135. [Google Scholar] [CrossRef]
- Staub, F.; Tournoux-Facon, C.; Roumy, J.; Chaigneau, C.; Morichaut-Beauchant, M.; Levillain, P.; Prevost, C.; Aubé, C.; Lebigot, J.; Oberti, F.; et al. Liver fibrosis staging with contrast-enhanced ultrasonography: Prospective multicenter study compared with METAVIR scoring. Eur. Radiol. 2009, 19, 1991–1997. [Google Scholar] [CrossRef]
- Kim, M.Y.; Suk, K.T.; Baik, S.K.; Kim, H.A.; Kim, Y.J.; Cha, S.H.; Kwak, H.R.; Cho, M.Y.; Park, H.J.; Jeon, H.K.; et al. Hepatic vein arrival time as assessed by contrast-enhanced ultrasonography is useful for the assessment of portal hypertension in compensated cirrhosis. Hepatology 2012, 56, 1053–1062. [Google Scholar] [CrossRef]
- Leong, S.S.; Wong, J.H.; Md Shah, M.N.; Vijayananthan, A.; Jalalonmuhali, M.; Ng, K.H. Shear wave elastography in the evaluation of renal parenchymal stiffness in patients with chronic kidney disease. Br. J. Radiol. 2018, 91, 20180235. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.; Loberant, N.; Abbas, N.; Fadi, H.; Shadia, H.; Khazim, K. Shear wave elastography imaging for assessing the chronic pathologic changes in advanced diabetic kidney disease. Ther. Clin. Risk Manag. 2016, 12, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Bob, F.; Bota, S.; Sporea, I.; Sirli, R.; Popescu, A.; Schiller, A. Relationship between the estimated glomerular filtration rate and kidney shear wave speed values assessed by acoustic radiation force impulse elastography: A pilot study. J. Ultrasound Med. 2015, 34, 649–654. [Google Scholar] [CrossRef]
- Cui, G.; Yang, Z.; Zhang, W.; Li, B.; Sun, F.; Xu, C.; Wang, K. Evaluation of acoustic radiation force impulse imaging for the clinicopathological typing of renal fibrosis. Exp. Ther. Med. 2013, 7, 233–235. [Google Scholar] [CrossRef] [PubMed]
- He, W.Y.; Jin, Y.J.; Wang, W.P.; Li, C.L.; Ji, Z.B.; Yang, C. Tissue elasticity quantification by acoustic radiation force impulse for the assessment of renal allograft function. Ultrasound Med. Biol. 2014, 40, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Xu, H.X.; Fu, H.J.; Peng, A.; Zhang, Y.F.; Liu, L.N. Acoustic Radiation Force Impulse Imaging for Noninvasive Evaluation of Renal Parenchyma Elasticity: Preliminary Findings. PLoS ONE 2013, 8, e68925. [Google Scholar] [CrossRef] [PubMed]
- Grenier, N.; Poulain, S.; Lepreux, S.; Gennisson, J.L.; Dallaudière, B.; Lebras, Y.; Bavu, E.; Servais, A.; Meas-Yedid, V.; Piccoli, M.; et al. Quantitative elastography of renal transplants using supersonic shear imaging: A pilot study. Eur. Radiol. 2012, 22, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Ogata, A.; Tanaka, K.; Ide, Y.; Sankoda, A.; Kawakita, C.; Nishikawa, M.; Ohmori, K.; Kinomura, M.; Shimada, N.; et al. Acoustic radiation force impulse elastography of the kidneys: Is shear wave velocity affected by tissue fibrosis or renal blood flow? J. Ultrasound Med. 2014, 33, 793–801. [Google Scholar] [CrossRef]
- Yoo, M.G.; Jung, D.C.; Oh, Y.T.; Park, S.Y.; Han, K. Usefulness of multiparametric ultrasound for evaluating structural abnormality of transplanted kidney: Can we predict histologic abnormality on renal biopsy in advance? Am. J. Roentgenol. 2017, 209, W139–W144. [Google Scholar] [CrossRef]
- Marticorena Garcia, S.R.; Guo, J.; Dürr, M.; Denecke, T.; Hamm, B.; Sack, I.; Fischer, T. Comparison of ultrasound shear wave elastography with magnetic resonance elastography and renal microvascular flow in the assessment of chronic renal allograft dysfunction. Acta Radiol. 2018, 59, 1139–1145. [Google Scholar] [CrossRef]
- Warner, L.; Yin, M.; Glaser, K.J.; Woollard, J.A.; Carrascal, C.A.; Korsmo, M.J.; Crane, J.A.; Ehman, R.L.; Lerman, L.O. Noninvasive In Vivo Assessment of Renal Tissue Elasticity during Graded Renal Ischemia Using MR Elastography. 2011. Available online: www.investigativeradiology.com (accessed on 1 August 2011).
- Gennisson, J.L.; Grenier, N.; Combe, C.; Tanter, M. Supersonic Shear Wave Elastography of In Vivo Pig Kidney: Influence of Blood Pressure, Urinary Pressure and Tissue Anisotropy. Ultrasound Med. Biol. 2012, 38, 1559–1567. [Google Scholar] [CrossRef]
- Castelein, J.; Pamplona, C.; Armstrong Junior, R.; Vidal dos Santos, M.; Sack, I.; Dierckx, R.; Moers, C.; Borra, R. Effects of kidney perfusion on renal stiffness and tissue fluidity measured with tomoelastography in an MRI-compatible ex vivo model. Front. Bioeng. Biotechnol. 2023, 11, 1236949. [Google Scholar] [CrossRef]
- Lang, S.T.; Guo, J.; Bruns, A.; Dürr, M.; Braun, J.; Hamm, B.; Sack, I.; Garcia, S.R.M. Multiparametric Quantitative MRI for the Detection of IgA Nephropathy Using Tomoelastography, DWI, and BOLD Imaging. Investig. Radiol. 2019, 54, 669–674. [Google Scholar] [CrossRef]
- Low, G.; Owen, N.E.; Joubert, I.; Patterson, A.J.; Graves, M.J.; Alexander, G.J.M.; Lomas, D.J. Magnetic resonance elastography in the detection of hepatorenal syndrome in patients with cirrhosis and ascites. Eur. Radiol. 2015, 25, 2851–2858. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, H.; Gao, C.; Gu, Y.; Liu, Y.; Yuan, Y.; Wu, X. Value of shear wave elastography in predicting hepatorenal syndrome in patients with liver cirrhosis and ascites. Int. J. Clin. Pract. 2021, 75, e14811. [Google Scholar] [CrossRef]
| Classification of Kidney Injury in Patients with Cirrhosis | |
|---|---|
| Acute kidney injury (AKI) Increase in sCr of ≥50% from baseline or an increase in sCr of ≥0.3 mg/dL in <48 h. |
|
| Chronic kidney disease (CKD) | Glomerular filtration rate (GFR) of <60 mL/min for >3 months (MDRD6 formula) HRS type 2 is a specific form of CKD |
| Acute-on-chronic kidney disease | Increase in sCr of ≥50% from baseline or an increase in sCr of ≥0.3 mg/dL in <48 h in a patient with cirrhosis whose GFR is <60 mL/min for >3 months (MDRD6 formula) |
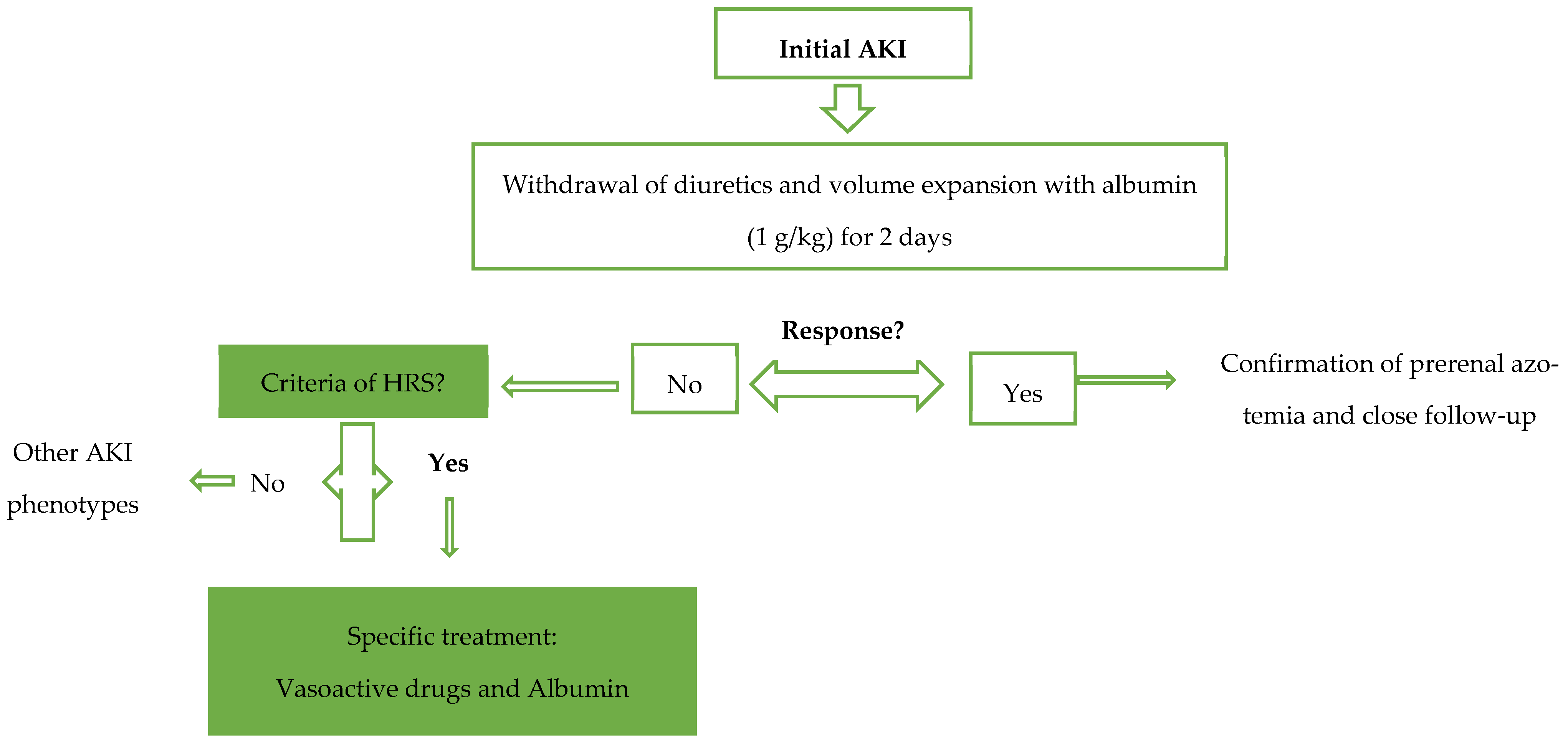
| International Club of Ascites Diagnostic Criteria for Hepatorenal Syndrome |
|---|
| Diagnosis of cirrhosis and ascites Diagnosis of acute kidney injury (AKI) according to ICA-AKI criteria No response after 48 h of diuretic withdrawal and plasma volume expansion (not necessarily with albumin at 1 g per kg of body weight) Absence of shock No current or recent use of nephrotoxic agents No signs of structural kidney injuries, defined as the following:
|
| Biomarker | Place of Origin | Fluid Tested | Time of Expression | Clinical Relevance |
|---|---|---|---|---|
| CysC [30,34,35] | Nucleated cells | Serum | 12–24 h | Correlation with renal function, 5-year survival, 1-year AKI development, 3-month mortality, HRS development |
| NGAL [39,40] | Loop of Henle and collecting ducts | Urine/serum | <12 h | AKI phenotype (differentiation of ATN), HRS development, 3-month mortality, AKI progression |
| KIM-1 [41,43] | Proximal tubular cells | Urine | <12 h | Discriminating ATN |
| FABPs [44,45] | Proximal tubular cells, hepatocytes | Urine | <12 h | Discriminating ATN |
| IL-18 [23,46,47] | Monocytes, macrophages, dendritic cells | Urine | <12 h | Discriminating ATN, 3-month mortality |
| NAG [48,49] | Proximal tubular cells | Urine | 12 h | Discriminating HRS-AKI, predicting the 3-month transplant-free survival |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tăluță, C.; Ștefănescu, H.; Crișan, D. Seeing and Sensing the Hepatorenal Syndrome (HRS): The Growing Role of Ultrasound-Based Techniques as Non-Invasive Tools for the Diagnosis of HRS. Diagnostics 2024, 14, 938. https://doi.org/10.3390/diagnostics14090938
Tăluță C, Ștefănescu H, Crișan D. Seeing and Sensing the Hepatorenal Syndrome (HRS): The Growing Role of Ultrasound-Based Techniques as Non-Invasive Tools for the Diagnosis of HRS. Diagnostics. 2024; 14(9):938. https://doi.org/10.3390/diagnostics14090938
Chicago/Turabian StyleTăluță, Cornelia, Horia Ștefănescu, and Dana Crișan. 2024. "Seeing and Sensing the Hepatorenal Syndrome (HRS): The Growing Role of Ultrasound-Based Techniques as Non-Invasive Tools for the Diagnosis of HRS" Diagnostics 14, no. 9: 938. https://doi.org/10.3390/diagnostics14090938
APA StyleTăluță, C., Ștefănescu, H., & Crișan, D. (2024). Seeing and Sensing the Hepatorenal Syndrome (HRS): The Growing Role of Ultrasound-Based Techniques as Non-Invasive Tools for the Diagnosis of HRS. Diagnostics, 14(9), 938. https://doi.org/10.3390/diagnostics14090938





