Usefulness of Diffusion-Weighted Imaging in Evaluating Acute Cellular Rejection and Monitoring Treatment Response in Liver Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Routine Biomarkers
2.3. Imaging Data Acquisition
2.4. Imaging Analysis
2.5. Histological Diagnosis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.L.; Kabiling, C.S.; Concejero, A.M. Why does living donor liver transplantation flourish in Asia? Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Lucey, M.R.; Furuya, K.N.; Foley, D.P. Liver Transplantation. N. Engl. J. Med. 2023, 389, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Tanaka, T.; Xiao, H.L.; Bittermann, T.; Dew, M.A.; Schnitzler, M.A.; Olthoff, K.M.; Locke, J.E.; Emre, S.; Hunt, H.F.; et al. Variation in adult living donor liver transplantation in the United States: Identifying opportunities for increased utilization. Clin. Transplant. 2023, 37, e14924. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Ganesh, S.; Jorgensen, D.; Tevar, A.; Ganoza, A.; Molinari, M.; Hughes, C. Adult Living Donor Versus Deceased Donor Liver Transplant (LDLT Versus DDLT) at a Single Center Time to Change Our Paradigm for Liver Transplant. Ann. Surg. 2019, 270, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.G.; Chen, C.L. Living donor liver transplantation in Taiwan-challenges beyond surgery. Hepatobiliary Surg. Nutr. 2016, 5, 145–150. [Google Scholar] [PubMed]
- Kwong, A.J.; Ebel, N.H.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Foutz, J.; Gauntt, K.; Cafarella, M.; et al. OPTN/SRTR 2020 Annual Data Report: Liver. Am. J. Transplant. 2022, 22, 204–309. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Yoo, H.Y. Graft and patient survival after adult live donor liver transplantation compared to a matched cohort who received a deceased donor transplantation. Liver Transplant. 2004, 10, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Francoz, C.; Berenguer, M.; Charlton, M.; Heimbach, J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin. Gastroenterol. Hepatol. 2023, 21, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, E.B. Liver transplantation: Current status and novel approaches to liver replacement. Gastroenterology 2001, 120, 749–762. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Rodríguez-Perálvarez, M.L.; Pageaux, G.P.; Sanchez-Fueyo, A.; Feng, S. Liver transplantation immunology: Immunosuppression, rejection, and immunomodulation. J. Hepatol. 2023, 78, 1199–1215. [Google Scholar] [CrossRef]
- Hubscher, S.G. Transplantation pathology. Semin. Diagn. Pathol. 2006, 23, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Neil, D.A.; Hubscher, S.G. Current views on rejection pathology in liver transplantation. Transpl. Int. 2010, 23, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Ormonde, D.G.; de Boer, W.B.; Kierath, A.; Bell, R.; Shilkin, K.B.; House, A.K.; Jeffrey, G.P.; Reed, W.D. Banff schema for grading liver allograft rejection: Utility in clinical practice. Liver Transpl. Surg. 1999, 5, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Demetris, A.J.; Batts, K.P.; Dhillon, A.P.; Ferrell, L.; Fung, J.; Geller, S.A.; Hart, J.; Hayry, P.; Hofmann, W.J.; Hubscher, S.; et al. Banff schema for grading liver allograft rejection: An international consensus document. Hepatology 1997, 25, 658–663. [Google Scholar]
- Rastogi, A.; Nigam, N.; Gayatri, R.; Bihari, C.; Pamecha, V. Biliary Epithelial Senescence in Cellular Rejection Following Live Donor Liver Transplantation. J. Clin. Exp. Hepatol. 2022, 12, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Saigal, S.; Bansal, R.K.; Saraf, N.; Gautam, D.; Soin, A.S. Acute and Chronic Rejection after Liver Transplantation: What A Clinician Needs to Know. J. Clin. Exp. Hepatol. 2017, 7, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.C.; Furth, E.E. Receiver Operating Characteristic Analysis of Serum Chemical-Parameters as Tests of Liver-Transplant Rejection and Correlation with Histology. Transplantation 1995, 59, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.N.; Nania, J.; Qiu, L.; Lewis, B.; Mais, D.D. Impact of Liver Biopsy Size on Histopathologic Evaluation of Liver Allograft Rejection. Arch. Pathol. Lab. Med. 2022, 146, 1530–1534. [Google Scholar] [CrossRef]
- Seiler, C.A.; Renner, E.L.; Czerniak, A.; Didonna, D.; Büchler, M.W.; Reichen, J. Early acute cellular rejection: No effect on late hepatic allograft function in man. Transpl. Int. 1999, 12, 195–201. [Google Scholar] [CrossRef]
- Perrakis, A.; Förtsch, T.; Niebling, N.; Croner, R.S.; Nissler, V.; Yedibela, S.; Lohmüller, C.; Zopf, S.; Kammerer, F.; Hohenberger, W.; et al. The Diagnostic Value of Systolic Acceleration Time and Resistive Index as Noninvasive Modality for Detection of Graft Rejection After Orthotopic Liver Transplantation. Clin. Gastroenterol. Hepatol. 2013, 45, 1961–1965. [Google Scholar] [CrossRef]
- Sugimoto, H.; Kato, K.; Hirota, M.; Takeda, S.; Kamei, H.; Nakamura, T.; Kiuchi, T.; Nakao, A. Serial Measurement of Doppler Hepatic Hemodynamic Parameters for the Diagnosis of Acute Rejection after Live Donor Liver Transplantation. Liver Transplant. 2009, 15, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Britton, P.D.; Lomas, D.J.; Coulden, R.A.; Farman, P.; Revell, S. The role of hepatic vein Doppler in diagnosing acute rejection following paediatric liver transplantation. Clin. Radiol. 1992, 45, 228–232. [Google Scholar] [CrossRef]
- Kok, T.; Slooff, M.J.; Peeters, P.M.; Zwaveling, J.H.; Bijleveld, C.M.; Gi-van Loon, C.E.; Klompmaker, I.J.; Haagsma, E.B. Changes in portal hemodynamics and acute rejection in the first 2 weeks after orthotopic liver transplantation. A prospective Doppler ultrasound study. Investig. Radiol. 1996, 31, 774–780. [Google Scholar] [CrossRef]
- Zalasin, S.; Shapiro, R.S.; Glajchen, N.; Stancato-Pasik, A. Liver transplant rejection: Value of hepatic vein Doppler waveform analysis. Abdom. Imaging 1998, 23, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mohapatra, N.; Borle, D.P.; Choudhury, A.; Sarin, S.; Gupta, E. Non invasive diagnosis of acute cellular rejection after liver transplantation—Current opinion. Transpl. Immunol. 2018, 47, 1–9. [Google Scholar] [CrossRef]
- Crespo, G.; Castro-Narro, G.; Garcia-Juarez, I.; Benitez, C.; Ruiz, P.; Sastre, L.; Colmenero, J.; Miquel, R.; Sanchez-Fueyo, A.; Forns, X.; et al. Usefulness of liver stiffness measurement during acute cellular rejection in liver transplantation. Liver Transpl. 2016, 22, 298–304. [Google Scholar] [CrossRef]
- Jang, J.K.; Kim, K.W.; Choi, S.H.; Jeong, S.Y.; Kim, J.H.; Yu, E.S.; Kwon, J.H.; Song, G.W.; Lee, S.G. CT of acute rejection after liver transplantation: A matched case-control study. Eur. Radiol. 2019, 29, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Miki, K.; Hirao, S.; Inoue, Y.; Ohtake, T.; Matsukura, A.; Aoyanagi, N.; Saiura, A.; Harihara, Y.; Takayama, T.; et al. Liver allograft functional reserve estimated by total asialoglycoprotein receptor amount using Tc-GSA liver scintigraphy. Transplant. Proc. 1998, 30, 3277–3278. [Google Scholar] [CrossRef]
- Brunot, B.; Petras, S.; Germain, P.; Vinee, P.; Constantinesco, A. Biopsy and Quantitative Hepatobiliary Scintigraphy in the Evaluation of Liver-Transplantation. J. Nucl. Med. 1994, 35, 1321–1327. [Google Scholar]
- Kim, J.S.; Moon, D.H.; Lee, S.G.; Lee, Y.J.; Park, K.M.; Hwang, S.; Lee, H.K. The usefulness of hepatobiliary scintigraphy in the diagnosis of complications after adult-to-adult living donor liver transplantation. Eur. J. Nucl. Med. 2002, 29, 473–479. [Google Scholar] [CrossRef]
- Engeler, C.M.; Kuni, C.C.; Nakhleh, R.; Engeler, C.E.; Ducret, R.P.; Boudreau, R.J. Liver-Transplant Rejection and Cholestasis—Comparison of Technetium 99m-Diisopropyl Iminodiacetic Acid Hepatobiliary Imaging with Liver-Biopsy. Eur. J. Nucl. Med. 1992, 19, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Taouli, B.; Ehman, R.L.; Reeder, S.B. Advanced MRI methods for assessment of chronic liver disease. AJR Am. J. Roentgenol. 2009, 193, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Curtis, W.A.; Fraum, T.J.; An, H.; Chen, Y.; Shetty, A.S.; Fowler, K.J. Quantitative MRI of Diffuse Liver Disease: Current Applications and Future Directions. Radiology 2019, 290, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, I.Y.; Catalano, O.A.; Caravan, P. Advances in functional and molecular MRI technologies in chronic liver diseases. J. Hepatol. 2020, 73, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Song, J.S. Non-Invasive Imaging Methods to Evaluate Non-Alcoholic Fatty Liver Disease with Fat Quantification: A Review. Diagnostics 2023, 13, 1852. [Google Scholar] [CrossRef] [PubMed]
- Pomohaci, M.D.; Grasu, M.C.; Dumitru, R.L.; Toma, M.; Lupescu, I.G. Liver Transplant in Patients with Hepatocarcinoma: Imaging Guidelines and Future Perspectives Using Artificial Intelligence. Diagnostics 2023, 13, 1663. [Google Scholar] [CrossRef]
- Lin, C.C.; Ou, H.Y.; Chuang, Y.H.; Chiang, H.J.; Yu, C.C.; Lazo, M.; Tsang, L.L.; Huang, T.L.; Lin, C.C.; Chen, C.L.; et al. Diffusion-Weighted Magnetic Resonance Imaging in Liver Graft Rejection. Transplant. Proc. 2018, 50, 2675–2678. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.J.; Chou, M.C.; Chuang, Y.H.; Li, C.W.; Lin, C.C.; Eng, H.L.; Chen, C.L.; Cheng, Y.F. Use of blood oxygen level-dependent magnetic resonance imaging to detect acute cellular rejection post-liver transplantation. Eur. Radiol. 2022, 32, 4547–4554. [Google Scholar] [CrossRef] [PubMed]
- Sandrasegaran, K.; Ramaswamy, R.; Ghosh, S.; Tahir, B.; Akisik, F.M.; Saxena, R.; Kwo, P. Diffusion-weighted MRI of the transplanted liver. Clin. Radiol. 2011, 66, 820–825. [Google Scholar] [CrossRef]
- Finotti, M.; Auricchio, P.; Vitale, A.; Gringeri, E.; Cillo, U. Liver transplantation for rare liver diseases and rare indications for liver transplant. Transl. Gastroenterol. Hepatol. 2021, 6, 27. [Google Scholar] [CrossRef]
- Ghelichi-Ghojogh, M.; Rajabi, A.; Mohammadizadeh, F.; Shojaie, L.; Vali, M.; Afrashteh, S.; Hassanipour, S.; Nikbakht, H.A.; Khezri, R.; Allah Kalteh, E.; et al. Survival Rate of Liver Transplantation in Asia: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2022, 51, 2207–2220. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.P.; Ferrarese, A.; Zanetto, A. Recent advances in understanding and managing liver transplantation. F1000Research 2016, 5, 2895. [Google Scholar] [CrossRef]
- Farges, O.; Saliba, F.; Farhamant, H.; Samuel, D.; Bismuth, A.; Reynes, M.; Bismuth, H. Incidence of rejection and infection after liver transplantation as a function of the primary disease: Possible influence of alcohol and polyclonal immunoglobulins. Hepatology 1996, 23, 240–248. [Google Scholar] [CrossRef]
- Dogan, N.; Hüsing-Kabar, A.; Schmidt, H.H.; Cicinnati, V.R.; Beckebaum, S.; Kabar, I. Acute allograft rejection in liver transplant recipients: Incidence, risk factors, treatment success, and impact on graft failure. J. Int. Med. Res. 2018, 46, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; Demetris, A.J.; Belle, S.H.; Seaberg, E.C.; Lake, J.R.; Zetterman, R.K.; Everhart, J.; Detre, K.M. Acute hepatic allograft rejection: Incidence, risk factors, and impact on outcome. Hepatology 1998, 28, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.U.; Bodian, C.A.; Gondolesi, G.E.; Schwartz, M.E.; Emre, S.; Roayaie, S.; Schiano, T.D. Marked differences in acute cellular rejection rates between living-donor and deceased-donor liver transplant recipients. Transplantation 2005, 80, 1072–1080. [Google Scholar] [CrossRef]
- Shaked, A.; Ghobrial, R.M.; Merion, R.M.; Shearon, T.H.; Emond, J.C.; Fair, J.H.; Fisher, R.A.; Kulik, L.M.; Pruett, T.L.; Terrault, N.A.; et al. Incidence and Severity of Acute Cellular Rejection in Recipients Undergoing Adult Living Donor or Deceased Donor Liver Transplantation. Am. J. Transplant. 2009, 9, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Eghtedari, M.; McKenzie, C.; Tang, L.C.Y.; Majumdar, A.; Kench, J.G. Banff 2016 Global Assessment and Quantitative Scoring for T Cell-Mediated Liver Transplant Rejection are Interchangeable. J. Transplant. 2023, 2023, 3103335. [Google Scholar] [CrossRef]
- Afzali, B.; Lechler, R.I.; Hernandez-Fuentes, M.P. Allorecognition and the alloresponse: Clinical implications. Tissue Antigens 2007, 69, 545–556. [Google Scholar] [CrossRef]
- Demetris, A.J.; Bellamy, C.; Hubscher, S.G.; O’Leary, J.; Randhawa, P.S.; Feng, S.; Neil, D.; Colvin, R.B.; McCaughan, G.; Fung, J.J.; et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am. J. Transplant. 2016, 16, 2816–2835. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ronca, V.; Wootton, G.; Milani, C.; Cain, O. The Immunological Basis of Liver Allograft Rejection. Front. Immunol. 2020, 11, 2155. [Google Scholar] [CrossRef]
- Badwei, N. Hepatic allograft rejection after liver transplantation: Clinicopathological debates! iLiver 2023, 2, 116–121. [Google Scholar] [CrossRef]
- Makhija, N.; Vikram, N.K.; Srivastava, D.N.; Madhusudhan, K.S. Role of Diffusion-Weighted Magnetic Resonance Imaging in the Diagnosis and Grading of Hepatic Steatosis in Patients with Non-alcoholic Fatty Liver Disease: Comparison with Ultrasonography and Magnetic Resonance Spectroscopy. J. Clin. Exp. Hepatol. 2021, 11, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Park, J.; Lee, J.M.; Grimm, R.; Kim, I.Y. Comparison of the Effects of Hepatic Steatosis on Monoexponential DWI, Intravoxel Incoherent Motion Diffusion-weighted Imaging and Diffusion Kurtosis Imaging. Acad. Radiol. 2021, 28 (Suppl. 1), S203–S209. [Google Scholar] [CrossRef]
- Sandrasegaran, K.; Akisik, F.M.; Lin, C.; Tahir, B.; Rajan, J.; Saxena, R.; Aisen, A.M. Value of diffusion-weighted MRI for assessing liver fibrosis and cirrhosis. AJR Am. J. Roentgenol. 2009, 193, 1556–1560. [Google Scholar] [CrossRef]
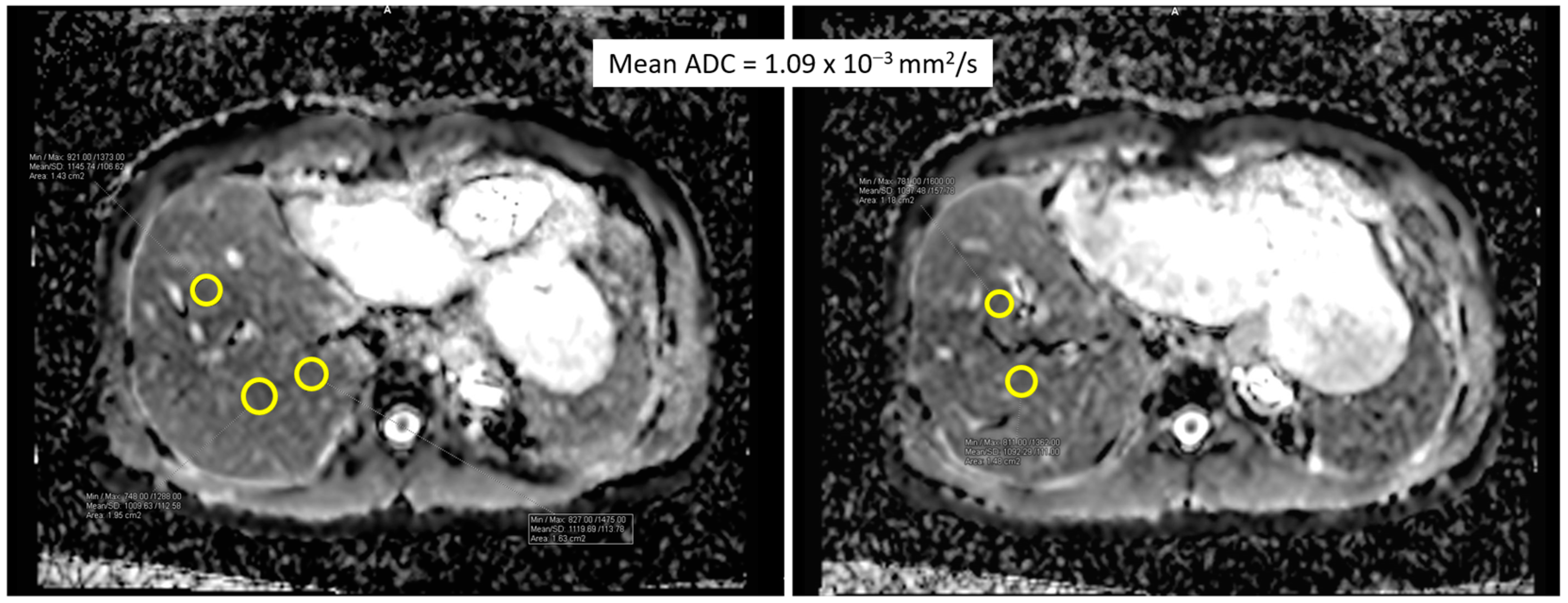

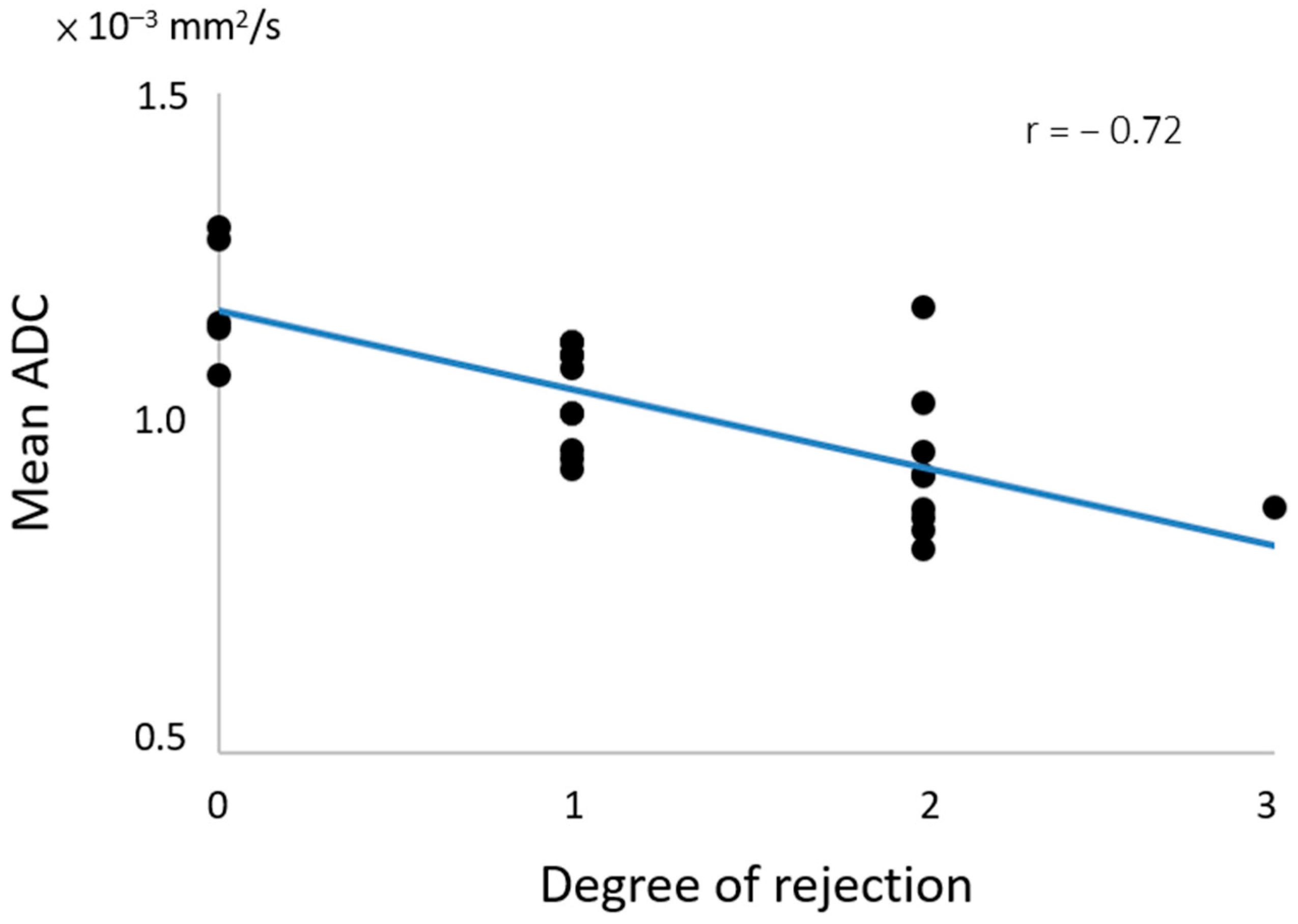
| Total n = 25 | |
|---|---|
| Male = 22 | |
| Female = 3 | |
| Mean age (range) = 53.56 (20–68 years) | |
| Graft type | |
| LDLT n = 18 | |
| Right n = 15 | |
| Left n = 3 | |
| DDLT n = 7 | |
| Whole liver n = 6 | |
| Right n = 1 | |
| Histopathology n = 25 | |
| Rejection n = 20 | Non-rejection n = 5 |
| Mild n = 10 | Cholangitis n = 3 |
| Moderate n = 9 | Cholangitis + mild (15%) fatty change n = 1 |
| Severe n = 1 | Minimal (<5%) fatty change n = 1 |
| Repeated MRI n = 25 Mean (range) interval between two MRI scans = 128 days (33–247 days) | |
| Rejection (n = 20) | Pre-Treatment | Post-Treatment | p Value |
|---|---|---|---|
| ADC (10−3 mm2/s) | 0.981 ± 0.109 * | 1.300 ± 0.199 | 0.001 |
| AST (U/L) | 544.8 ± 693.5 | 48.25 ± 38.9 | 0.000 |
| ALT (U/L) | 604.8 ± 393.8 | 52.6 ± 37.3 | 0.000 |
| T-bil (mg/dL) | 2.0 ± 2.0 | 1.2 ± 0.9 | 0.006 |
| PLT (1000/ μ L) | 149.8± 73.5 | 139.8 ± 46.5 | 0.267 |
| Non-rejection (n = 5) | |||
| ADC (10−3 mm2/s) | 1.182 ± 0.105 * | 1.346 ± 0.169 | 0.043 |
| AST (U/L) | 203.60 ± 121.80 | 42.2 ± 20.9 | 0.080 |
| ALT (U/L) | 416.20 ± 307.33 | 46.6 ± 24.7 | 0.043 |
| T-bil (mg/dL) | 3.50 ± 3.46 | 1.7 ± 1.6 | 0.006 |
| PLT (1000/ μ L) | 119.6 ± 50.6 | 142.4 ± 51.9 | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, H.-J.; Chuang, Y.-H.; Li, C.-W.; Lin, C.-C.; Eng, H.-L.; Chen, C.-L.; Cheng, Y.-F.; Chou, M.-C. Usefulness of Diffusion-Weighted Imaging in Evaluating Acute Cellular Rejection and Monitoring Treatment Response in Liver Transplant Recipients. Diagnostics 2024, 14, 807. https://doi.org/10.3390/diagnostics14080807
Chiang H-J, Chuang Y-H, Li C-W, Lin C-C, Eng H-L, Chen C-L, Cheng Y-F, Chou M-C. Usefulness of Diffusion-Weighted Imaging in Evaluating Acute Cellular Rejection and Monitoring Treatment Response in Liver Transplant Recipients. Diagnostics. 2024; 14(8):807. https://doi.org/10.3390/diagnostics14080807
Chicago/Turabian StyleChiang, Hsien-Jen, Yi-Hsuan Chuang, Chun-Wei Li, Chih-Che Lin, Hock-Liew Eng, Chao-Long Chen, Yu-Fan Cheng, and Ming-Chung Chou. 2024. "Usefulness of Diffusion-Weighted Imaging in Evaluating Acute Cellular Rejection and Monitoring Treatment Response in Liver Transplant Recipients" Diagnostics 14, no. 8: 807. https://doi.org/10.3390/diagnostics14080807
APA StyleChiang, H.-J., Chuang, Y.-H., Li, C.-W., Lin, C.-C., Eng, H.-L., Chen, C.-L., Cheng, Y.-F., & Chou, M.-C. (2024). Usefulness of Diffusion-Weighted Imaging in Evaluating Acute Cellular Rejection and Monitoring Treatment Response in Liver Transplant Recipients. Diagnostics, 14(8), 807. https://doi.org/10.3390/diagnostics14080807







