Current Status of CT Imaging Before Common Transcatheter Interventions for Structural Heart Disease
Abstract
1. Introduction
2. Atrial Septal Defects
3. Transcatheter Aortic Valve Replacement
4. Mitral Valve Repair and Replacement
5. Transcatheter Tricuspid Valve Replacement
6. Transcatheter Left Atrial Appendage Occlusion
7. CT Before Pulmonary Vein Ablation
8. Conclusions
Funding
Conflicts of Interest
Acknowledgments
References
- Nicolay, S.; Salgado, R.A.; Shivalkar, B.; Van Herck, P.L.; Vrints, C.; Parizel, P.M. CT imaging features of atrioventricular shunts: What the radiologist must know. Insights Into Imaging 2016, 7, 119–129. [Google Scholar] [CrossRef][Green Version]
- Geva, T.; Martins, J.D.; Wald, R.M. Atrial septal defects. Lancet 2014, 383, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.G.; Gersh, B.J.; McGoon, M.D.; Mair, D.D.; Porter, C.-B.J.; Ilstrup, D.M.; McGoon, D.C.; Puga, F.J.; Kirklin, J.W.; Danielson, G.K. Long-Term Outcome after Surgical Repair of Isolated Atrial Septal Defect. N. Engl. J. Med. 1990, 323, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.P.; Fleming, G.A.; Chamberlain, R.C. Update on Transcatheter Device Closure of Congenital Septal Defects. Curr. Cardiol. Rep. 2023, 25, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Panjwani, B.; Singh, A.; Shah, A. CT and MR Imaging for Atrial Septal Defect Repair. Semin. Roentgenol. 2024, 59, 103–111. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Halpern, E.J.; Savage, M.P. Imaging of Adult Atrial Septal Defects with CT Angiography. JACC Cardiovasc. Imaging 2013, 6, 1342–1345. [Google Scholar] [CrossRef][Green Version]
- Yasunaga, D.; Hamon, M. MDCT of interatrial septum. Diagn. Interv. Imaging 2015, 96, 891–899. [Google Scholar] [CrossRef]
- Lindman, B.R.; Clavel, M.-A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, nrdp20166. [Google Scholar] [CrossRef] [PubMed]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic Stenosis in the Elderly Disease Prevalence and Number of Candidates for Transcatheter Aortic Valve Replacement: A Meta-Analysis and Modeling Study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Bleiziffer, S.; De Backer, O.; Delgado, V.; Arai, T.; Ziegelmueller, J.; Barbanti, M.; Sharma, R.; Perlman, G.Y.; Khalique, O.K.; et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. Circ. 2017, 69, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.S.; Siao, D.; Girard, S.E.; Duvernoy, C.; McCallister, B.D.; Gualano, S.K. Evaluation of Patients With Severe Symptomatic Aortic Stenosis Who Do Not Undergo Aortic Valve Replacement. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.A.; Budde, R.P.; Leiner, T.; Shivalkar, B.; Van Herck, P.L.; Op de Beeck, B.J.; Vrints, C.; Buijsrogge, M.P.; Stella, P.R.; Rodrigus, I.; et al. Transcatheter aortic valve replacement: Post-operative CT findings of Sapien and CoreValve transcatheter heart valves. Radiographics 2014, 34, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Francone, M.; Budde, R.P.; Bremerich, J.; Dacher, J.N.; Loewe, C.; Wolf, F.; Natale, L.; Pontone, G.; Redheuil, A.; Vliegenthart, R.; et al. CT and MR imaging prior to transcatheter aortic valve implantation: Standardisation of scanning protocols, measurements and reporting—A consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2019, 30, 2627–2650. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 13, 1–20. [Google Scholar] [CrossRef]
- Laakso, T.; Laine, M.; Moriyama, N.; Dahlbacka, S.; Airaksinen, J.; Virtanen, M.; Husso, A.; Tauriainen, T.; Niemelä, M.; Mäkikallio, T.; et al. Impact of paravalvular regurgitation on the mid-term outcome after transcatheter and surgical aortic valve replacement. Eur. J. Cardio-Thorac. Surg. 2020, 58, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Feuchtner, G.M.; Müller, S.; Bonatti, J.; Schachner, T.; Velik-Salchner, C.; Pachinger, O.; Dichtl, W. Sixty-Four Slice CT Evaluation of Aortic Stenosis Using Planimetry of the Aortic Valve Area. Am. J. Roentgenol. 2007, 189, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.G.; Novaro, G.M.; Blandon, R.J.; Whiteman, M.S.; Asher, C.R.; Kirsch, J. Aortic valve area: Meta-analysis of diagnostic performance of multi-detector computed tomography for aortic valve area measurements as compared to transthoracic echocardiography. Int. J. Cardiovasc. Imaging 2009, 25, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Burwash, I.G.; Pibarot, P. Cardiac Imaging for Assessing Low-Gradient Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 10, 185–202. [Google Scholar] [CrossRef]
- Pawade, T.; Sheth, T.; Guzzetti, E.; Dweck, M.R.; Clavel, M.-A. Why and How to Measure Aortic Valve Calcification in Patients With Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 1835–1848. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Flamm, S.D.; Schoenhagen, P.; Griffin, B.P.; Rodriguez, L.L.; Grimm, R.A.; Xu, B. Diagnostic and Prognostic Performance of Aortic Valve Calcium Score with Cardiac CT for Aortic Stenosis: A Meta-Analysis. Radiol. Cardiothorac. Imaging 2021, 3, e210075. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.R.; Clavel, M.A.; Messika-Zeitoun, D.; Cueff, C.; Malouf, J.; Araoz, P.A.; Mankad, R.; Michelena, H.; Vahanian, A.; Enriquez-Sarano, M. Sex Differences in Aortic Valve Calcification Measured by Multidetector Computed Tomography in Aortic Stenosis. Circ. Cardiovasc Imaging 2018, 6, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Simard, L.; Côté, N.; Dagenais, F.; Mathieu, P.; Couture, C.; Trahan, S.; Bossé, Y.; Mohammadi, S.; Pagé, S.; Joubert, P.; et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis. Circ. Res. 2017, 120, 681–691. [Google Scholar] [CrossRef]
- El Sabbagh, A.; Reddy, Y.N.; Nishimura, R.A. Mitral Valve Regurgitation in the Contemporary Era Insights Into Diagnosis, Management, and Future Directions. JACC Cardiovasc. Imaging 2018, 11, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Naoum, C.; Leipsic, J.; Cheung, A.; Ye, J.; Bilbey, N.; Mak, G.; Berger, A.; Dvir, D.; Arepalli, C.; Grewal, J.; et al. Mitral Annular Dimensions and Geometry in Patients With Functional Mitral Regurgitation and Mitral Valve Prolapse. JACC Cardiovasc. Imaging 2016, 9, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Dvir, D.; Cheung, A.; Levine, R.A.; Thompson, C.; Webb, J.G.; Leipsic, J. Mitral Annular Evaluation With CT in the Context of Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Imaging 2015, 8, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, P.N.; Leipsic, J.A.; Schoepf, U.J.; Dudek, D.; Schwarz, F.; Andreas, M.; Zlahoda-Huzior, A.; Thilo, C.; Renker, M.; Burt, J.R.; et al. CT in Transcatheter-delivered Treatment of Valvular Heart Disease. Radiology 2022, 304, 4–17. [Google Scholar] [CrossRef]
- Maggiore, P.; Anastasius, M.; Huang, A.L.; Blanke, P.; Leipsic, J. Transcatheter Mitral Valve Repair and Replacement: Current Evidence for Intervention and the Role of CT in Preprocedural Planning—A Review for Radiologists and Cardiologists Alike. Radiol. Cardiothorac. Imaging 2020, 2, e190106. [Google Scholar] [CrossRef]
- Blanke, P.; Naoum, C.; Dvir, D.; Bapat, V.; Ong, K.; Muller, D.; Cheung, A.; Ye, J.; Min, J.K.; Piazza, N.; et al. Predicting LVOT Obstruction in Transcatheter Mitral Valve Implantation. JACC Cardiovasc. Imaging 2016, 10, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, L.; Gabelloni, M.; Accogli, S.; Angelillis, M.; Costa, G.; Spontoni, P.; Petronio, A.S.; Caramella, D. Preprocedural planning of transcatheter mitral valve interventions by multidetector CT: What the radiologist needs to know. Eur. J. Radiol. Open 2018, 5, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kaewkes, D.; Patel, V.; Ochiai, T.; Flint, N.; Ahmad, Y.; Kim, I.; Koseki, K.; Sharma, R.; Joseph, J.; Yoon, S.-H.; et al. Usefulness of Computed Tomography to Predict Mitral Stenosis After Transcatheter Mitral Valve Edge-to-Edge Repair. Am. J. Cardiol. 2021, 153, 109–118. [Google Scholar] [CrossRef]
- Iliadis, C.; Baldus, S.; Kalbacher, D.; Boekstegers, P.; Schillinger, W.; Ouarrak, T.; Zahn, R.; Butter, C.; Zuern, C.S.; von Bardeleben, R.S.; et al. Impact of left atrial diameter on outcome in patients undergoing edge-to-edge mitral valve repair: Results from the German TRAnscatheter Mitral valve Interventions (TRAMI) registry. Eur. J. Hear. Fail. 2020, 22, 1202–1210. [Google Scholar] [CrossRef]
- Taramasso, M.; Pozzoli, A.; Guidotti, A.; Nietlispach, F.; Inderbitzin, D.T.; Benussi, S.; Alfieri, O.; Maisano, F. Percutaneous tricuspid valve therapies: The new frontier. Eur. Heart J. 2017, 38, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Taramasso, M.; O’Gara, P.T. Diagnosis and treatment of tricuspid valve disease: Current and future perspectives. Lancet 2016, 388, 2431–2442. [Google Scholar] [CrossRef]
- Fukuda, S.; Saracino, G.; Matsumura, Y.; Daimon, M.; Tran, H.; Greenberg, N.L.; Hozumi, T.; Yoshikawa, J.; Thomas, J.D.; Shiota, T. Three-Dimensional Geometry of the Tricuspid Annulus in Healthy Subjects and in Patients With Functional Tricuspid Regurgitation. Circulation 2006, 114, I492–I498. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.M.; Emrich, T.; Kreidel, F.; Kreitner, K.-F.; Schoepf, U.J.; Münzel, T.; von Bardeleben, R.S. Computed tomography imaging needs for novel transcatheter tricuspid valve repair and replacement therapies. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 601–610. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael, P.J.; Kamperidis, V.; Kong, W.K.; van Rosendael, A.R.; van der Kley, F.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Computed tomography for planning transcatheter tricuspid valve therapy. Eur. Heart J. 2017, 38, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; A Leo, L.; Paiocchi, V.L.; A Schlossbauer, S.; Borruso, M.G.; Pedrazzini, G.; Moccetti, T.; Ho, S.Y. Imaging-based tricuspid valve anatomy by computed tomography, magnetic resonance imaging, two and three-dimensional echocardiography: Correlation with anatomic specimen. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Maffei, E.; Messalli, G.; Martini, C.; Nieman, K.; Catalano, O.; Rossi, A.; Seitun, S.; I Guaricci, A.; Tedeschi, C.; Mollet, N.R.; et al. Left and right ventricle assessment with Cardiac CT: Validation study vs. Cardiac MR. Eur. Radiol. 2012, 22, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Naoum, C.; Blanke, P.; Cavalcante, J.L.; Leipsic, J. Cardiac Computed Tomography and Magnetic Resonance Imaging in the Evaluation of Mitral and Tricuspid Valve Disease. Circ. Cardiovasc. Imaging 2017, 10, e005331. [Google Scholar] [CrossRef]
- Zimetbaum, P. Atrial Fibrillation. Ann. Intern. Med. 2017, 166, ITC33. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Berti, S.; Iriart, X.; Saw, J.; Wang, D.D.; Cochet, H.; Chow, D.; Clemente, A.; De Backer, O.; Jensen, J.M.; et al. Expert Recommendations on Cardiac Computed Tomography for Planning Transcatheter Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2020, 13, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Rajwani, A.; Nelson, A.J.; Shirazi, M.G.; Disney, P.J.; Teo, K.S.; Wong, D.T.; Young, G.D.; Worthley, S.G. CT sizing for left atrial appendage closure is associated with favourable outcomes for procedural safety. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1361–1368. [Google Scholar] [CrossRef]
- Chun, S.H.; Suh, Y.J.; Han, K.; Park, S.J.; Shim, C.Y.; Hong, G.-R.; Lee, S.; Lee, S.-H.; Kim, Y.J.; Choi, B.W. Differentiation of left atrial appendage thrombus from circulatory stasis using cardiac CT radiomics in patients with valvular heart disease. Eur. Radiol. 2021, 31, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.; Habets, J.; Velthuis, B.K.; Cramer, M.J.; Loh, P. Double-contrast, single-phase computed tomography angiography for ruling out left atrial appendage thrombus prior to atrial fibrillation ablation. Int. J. Cardiovasc. Imaging 2017, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, R. Identifying and understanding the role of pulmonary vein activity in atrial fibrillation. Cardiovasc. Res. 2004, 64, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Cronin, P.; Sneider, M.B.; Kazerooni, E.A.; Kelly, A.M.; Scharf, C.; Oral, H.; Morady, F. MDCT of the Left Atrium and Pulmonary Veins in Planning Radiofrequency Ablation for Atrial Fibrillation: A How-To Guide. Am. J. Roentgenol. 2004, 183, 767–778. [Google Scholar] [CrossRef]
- Lacomis, J.M.; Wigginton, W.; Fuhrman, C.; Schwartzman, D.; Armfield, D.R.; Pealer, K.M. Multi–Detector Row CT of the Left Atrium and Pulmonary Veins before Radio-frequency Catheter Ablation for Atrial Fibrillation. RadioGraphics 2003, 23, S35–S48. [Google Scholar] [CrossRef] [PubMed]
- Eifer, D.A.; Nguyen, E.T.; Thavendiranathan, P.; Hanneman, K. Diagnostic Accuracy of Sex-Specific Chest CT Measurements Compared With Cardiac MRI Findings in the Assessment of Cardiac Chamber Enlargement. Am. J. Roentgenol. 2018, 211, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Zellerhoff, S.; Ullerich, H.; Lenze, F.; Meister, T.; Wasmer, K.; Mönnig, G.; Köbe, J.; Milberg, P.; Bittner, A.; Domschke, W.; et al. Damage to the Esophagus After Atrial Fibrillation Ablation. Circ. Arrhythmia Electrophysiol. 2010, 3, 155–159. [Google Scholar] [CrossRef]
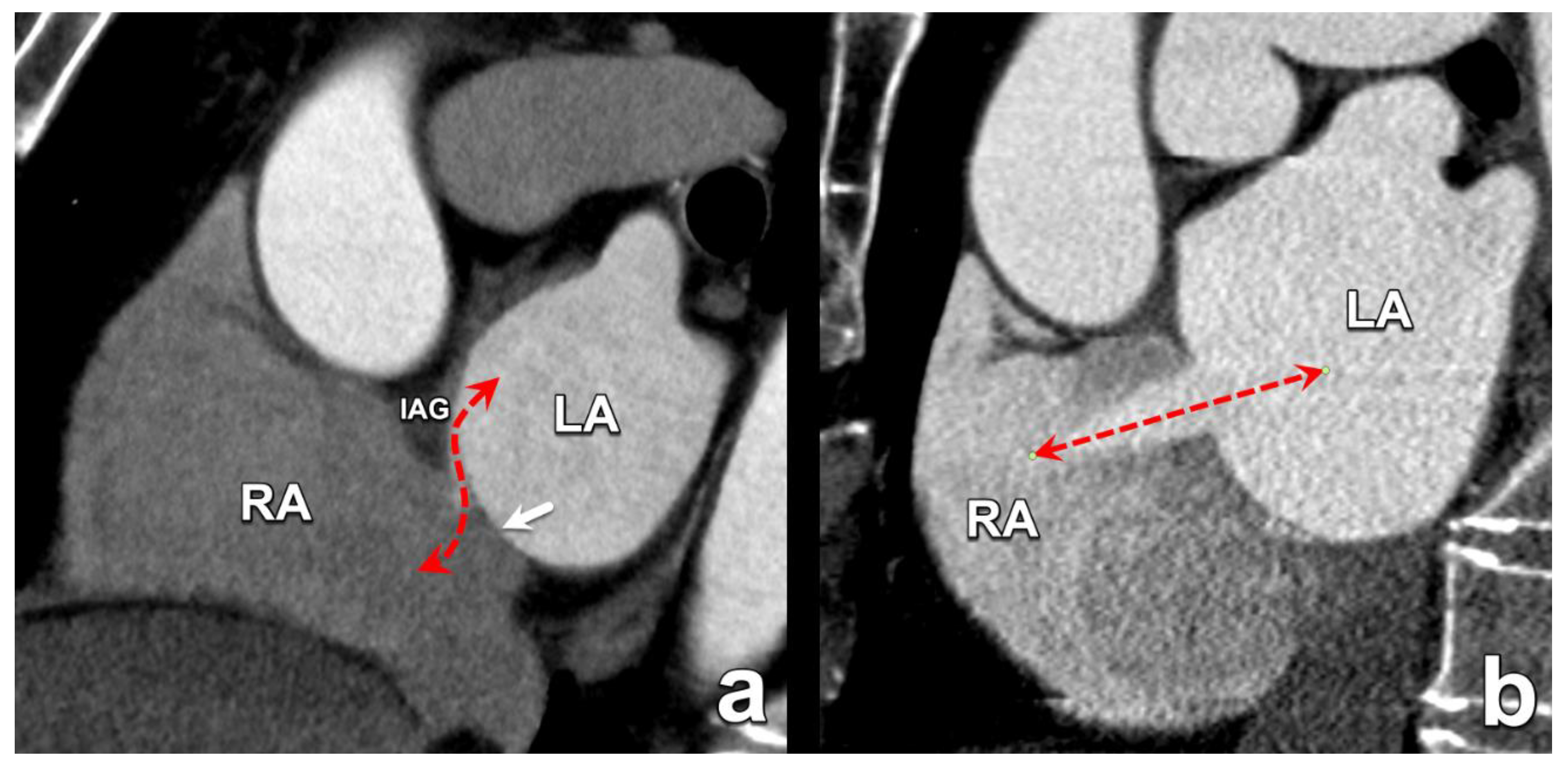

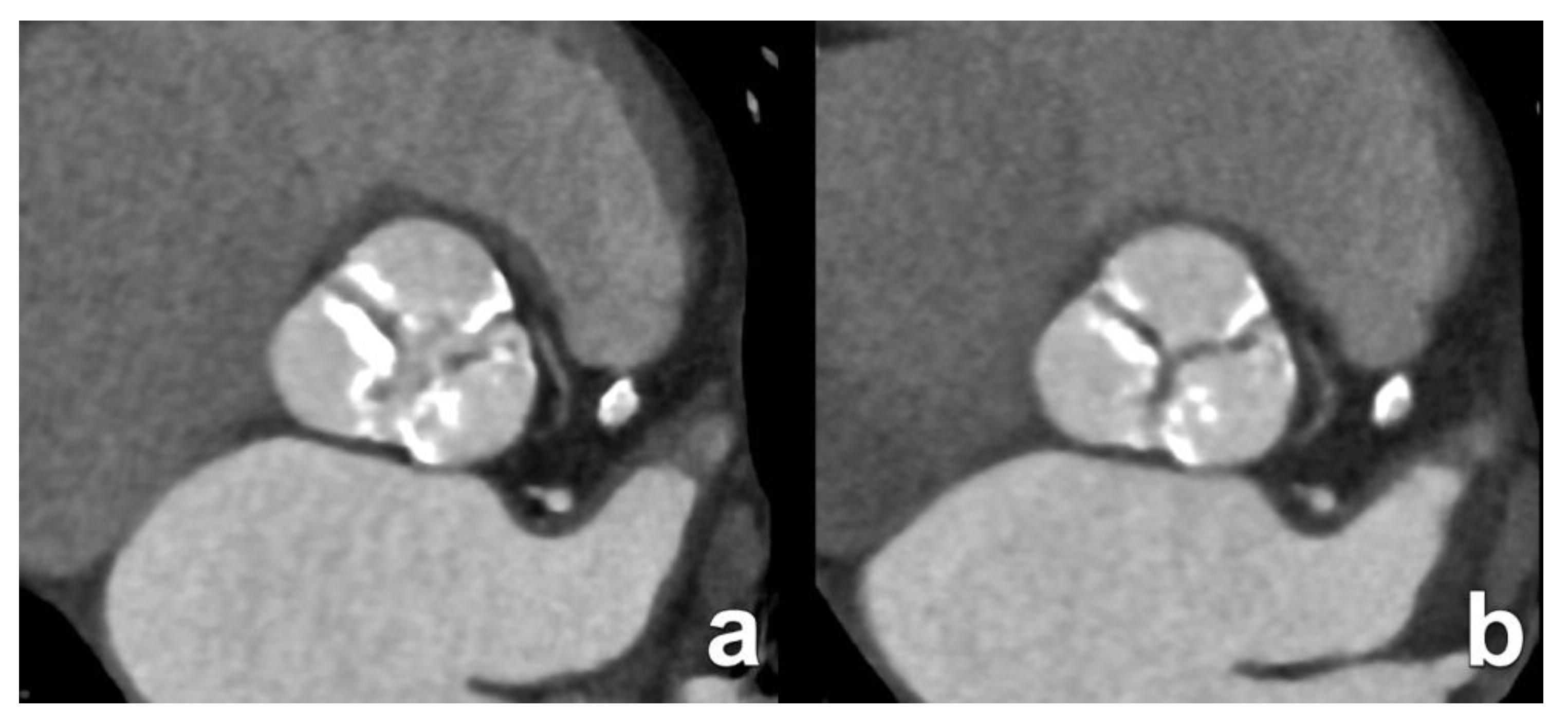

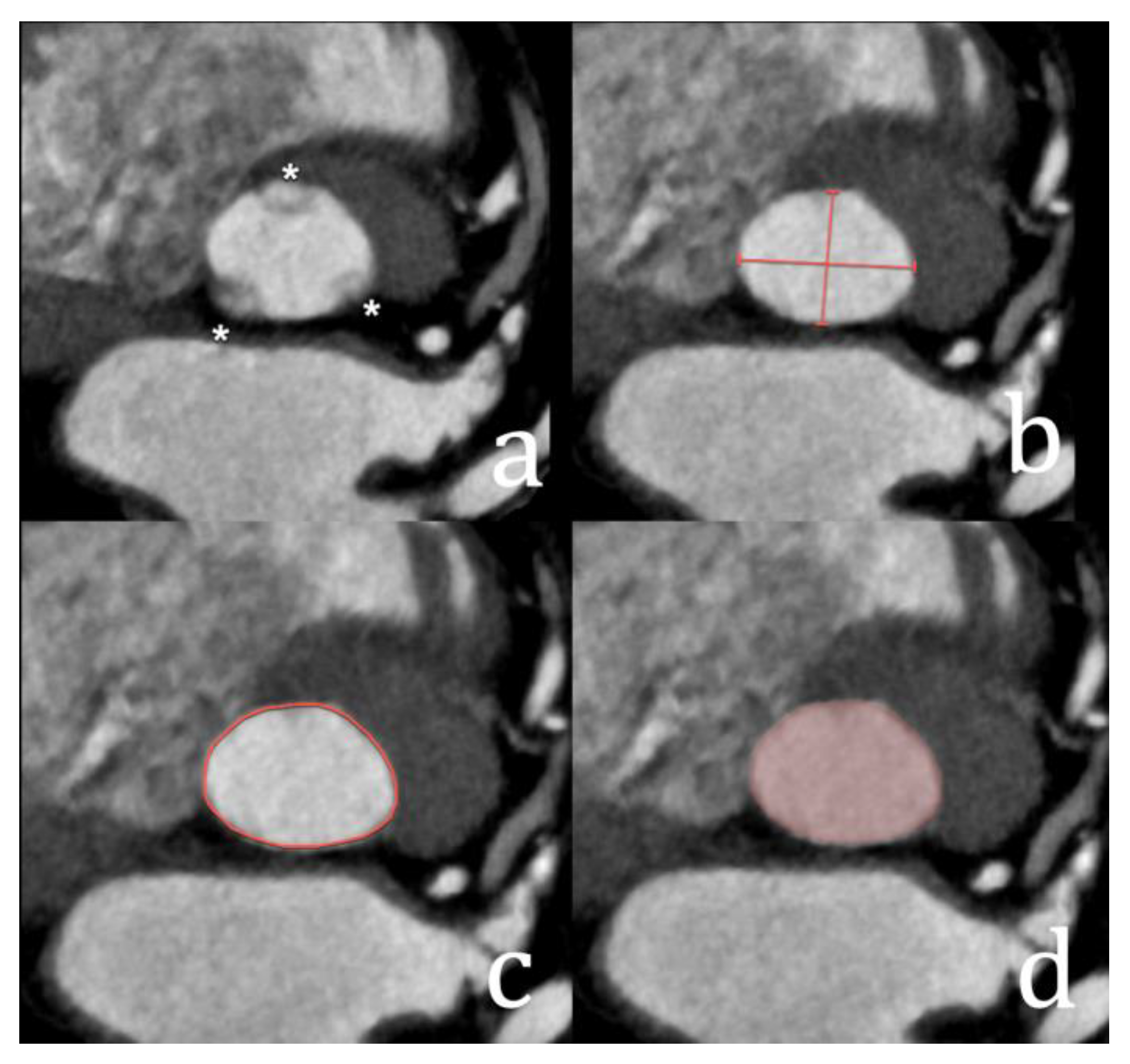

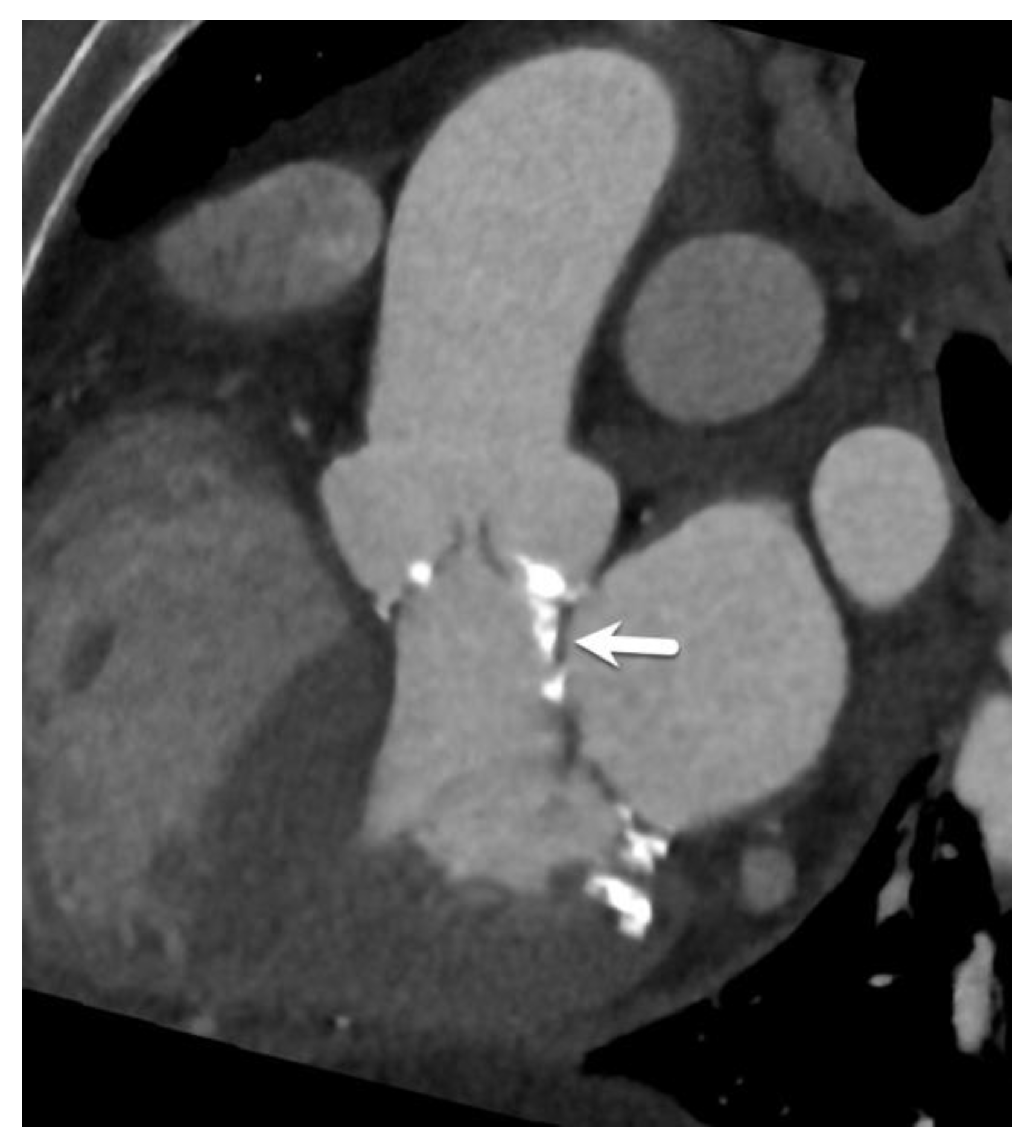





| Type of Interatrial Communication | Prevalence | Location | Associated Anomalies/Syndromes | Standard of Treatment |
|---|---|---|---|---|
| Ostium secundum ASD | 70–80% | Fossa ovalis of the atrial septum | More common in females (65%–70%) | Endovascular closure in suitable anatomy |
| Ostium primum ASD | 10–15% | Near the atrioventricular valves | Cleft mitral valve, partial atrioventricular septal defects, Down syndrome, DiGeorge syndrome, Ellis Van Creveld syndrome | Surgery |
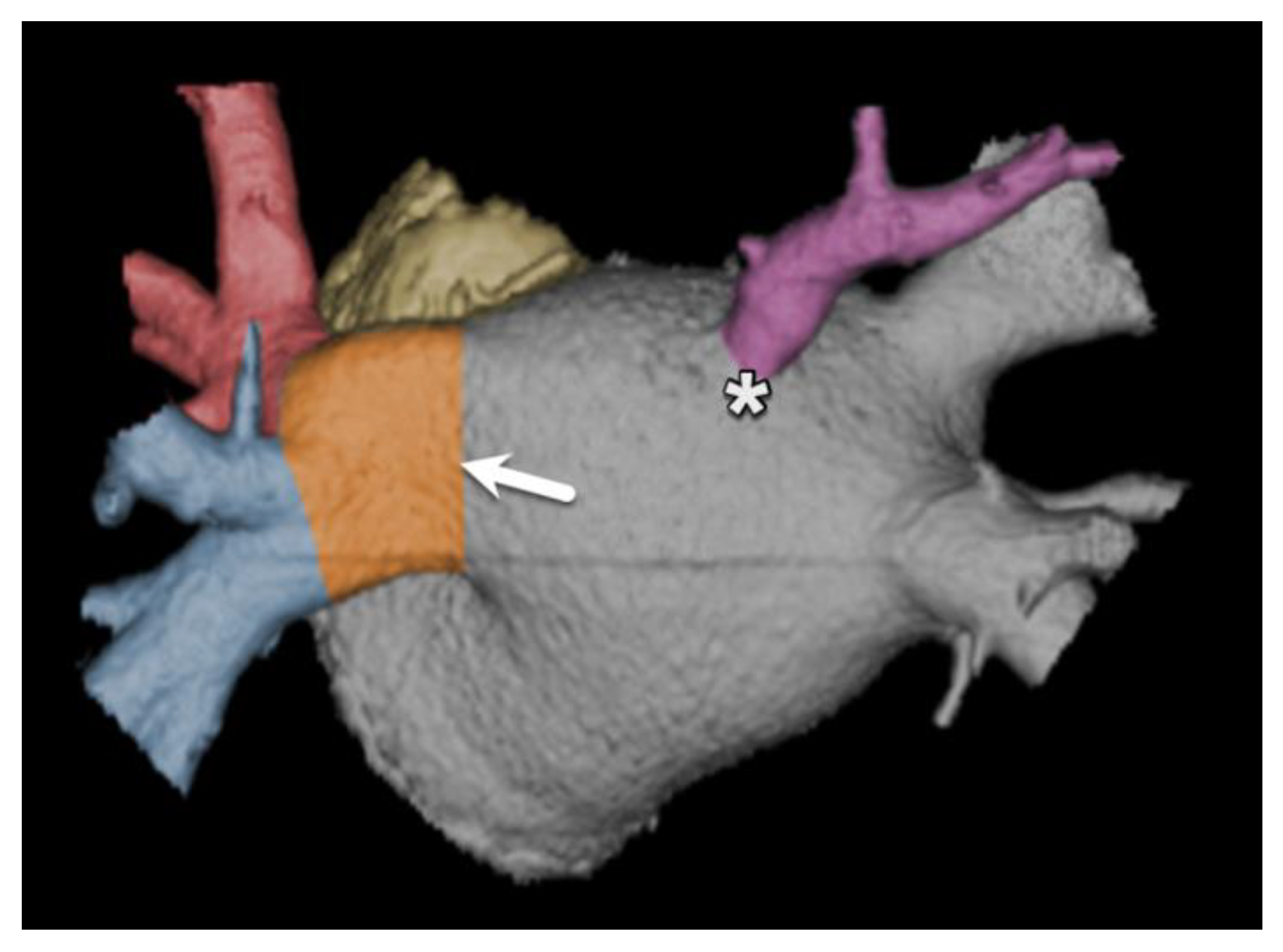
| Sinus venosus ASD | 5–10% | Deficiency of the common wall between the SVC and the right-sided pulmonary veins | Anomalous pulmonary venous connection | Surgery |
| Coronary sinus septal defect (unroofed coronary sinus) | 1% | Incomplete formation of the left arteriovenous fold | May be associated with a left-sided SVC | Surgery |
| Patent foramen ovale (PFO) | 25–30% of the general population | Fossa ovalis of the atrial septum | Cryptogenic stroke, migraine with aura, decompression sickness in divers | Usually no treatment required |
| Device | Maximum Defect Size | Minimum Rim Requirements | Other Considerations |
|---|---|---|---|
| Amplatzer Septal Occluder | Up to 38 mm | Generally 5 mm for all rims but can be used with deficient aortic rims | Avoid oversizing in deficient aortic rim cases |
| Occlutech Figulla Flex II | Up to 39 mm | Similar to the Amplatzer, generally 5 mm for all rims | May be more flexible in cases with deficient rims |
| Gore Cardioform Septal Occluder | Up to 18 mm | Requires adequate rims all around, especially in larger defects | Not suitable for large defects (>18 mm) evice size should be at least twice the defect size |
| CeraFlex ASD Occluder | Up to 40 mm | Similar to the Amplatzer, generally 5 mm for all rims | Flexible delivery system may help in difficult anatomies |
| Cocoon Septal Occluder | Up to 38 mm | Similar to the Amplatzer, generally 5 mm for all rims | Softer device may conform better to varying anatomies |
| Nit-Occlud ASD-R | Up to 28 mm | Requires adequate rims, especially for larger defects | “Reverse” configuration may help in some anatomies Not suitable for very large defects |
| Measurement | Description | Comments |
|---|---|---|
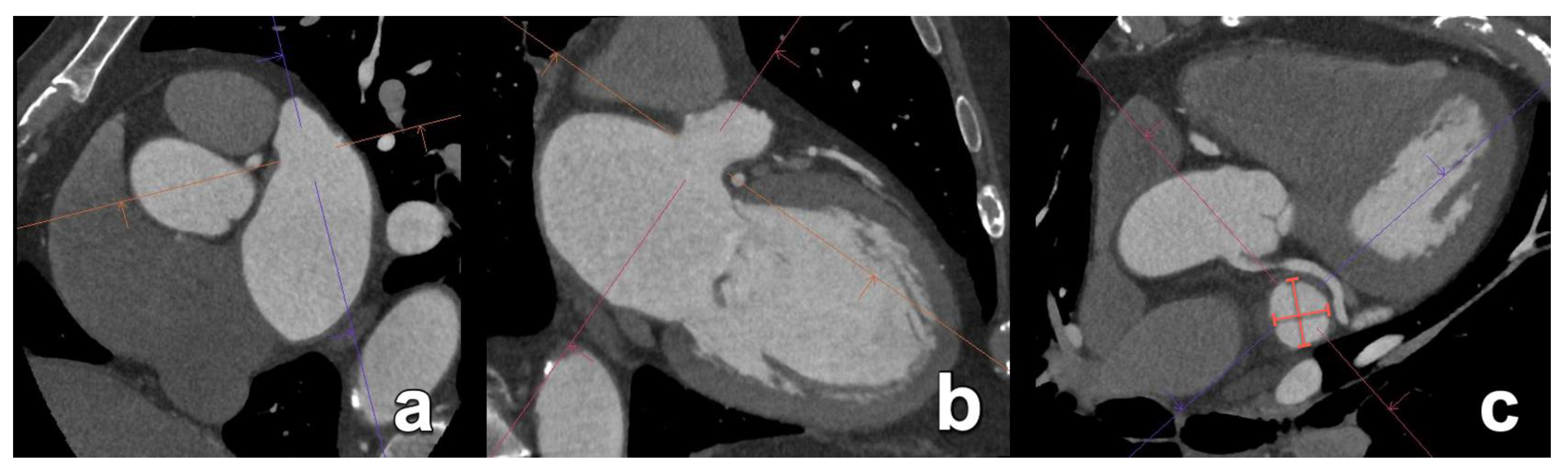
| Aortic annulus dimensions | Long- and short-axis diameters Area Perimeter | Sizing algorithms are device- and manufacturer-specific. |
| Aortic valve cuspidity | Comment on number of leaflets | The presence of a bicuspid valve may increase procedural complexity and increase the risk for permanent pacemaker implantation after the procedure. |
| Coronary ostia heights | Distance from annular plane to bottom of left and right coronary ostia | As a rule of thumb, a minimum diameter of 10 mm is considered the threshold to avid ostial coronary obstruction by migrated leaflet calcifications. |
| Sinus of Valsalva dimensions | Long- and short-axis cross-sectional diameters Largest cross-sectional diameter Commissure-to-cusp diameter Height of the coronary sinus | The aortic sinus needs to be wide enough to accommodate displaced native leaflet calcifications during the deployment of the THV. |
| Sinotubular junction diameter | Diameter at the sinotubular junction | |
| Tube angulation | Angles of the tube where all basal insertion points of the aortic leaflets are aligned in one plane | Knowledge of this tube angulation can reduce procedure time and the use of contrast material. |
| Ascending aorta diameter and wall calcifications | Diameter at 40 mm above annulus Presence and severity of aortic wall calcification | For transaortic access, the landing zone is about 60 mm above the annular plane. |
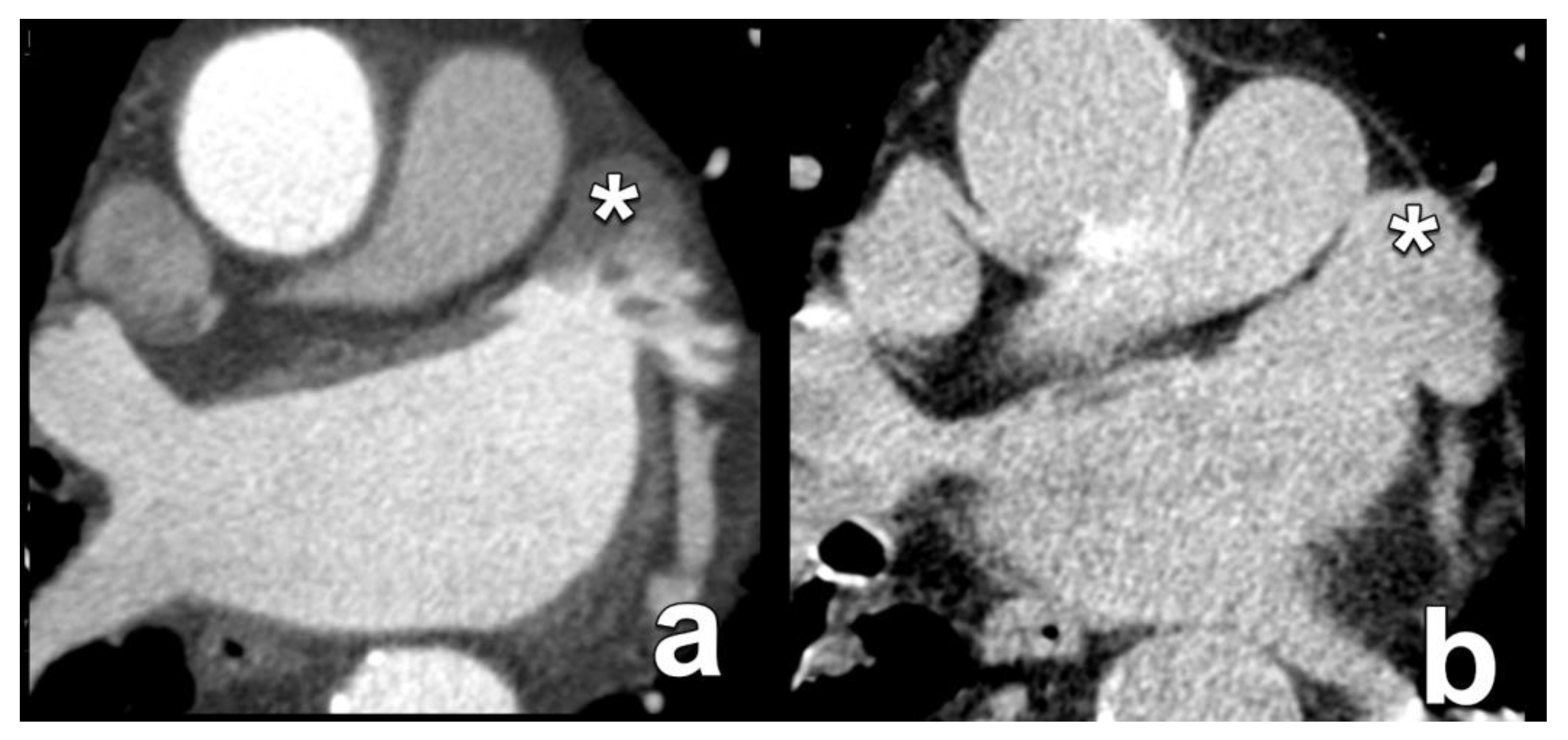
| Left ventricular outflow tract and subvalvular calcifications | Long- and short-axis diameters | The presence of prominent subvalvular calcification may interfere with THV deployment. |
| Aortic valve calcification | Quantification and distribution of leaflet calcification | The Agatston score is used to quantify leaflet calcifications. This is not routine and typically reserved for low-flow low-gradient cases with inconclusive echocardiography results. |
| Left ventricular basal septum thickness | Presence of basal left ventricular hypertrophy | A hypertrophic basal septum may contribute to device instability. |
| Peripheral access vessel diameters | Minimal luminal diameters of the iliofemoral arteries | A minimal luminal diameter of 5–6 mm is generally required, depending on the delivery system. |
| Aortic angulation | Angle between the LVOT and ascending aorta planes | A steep angulation increases procedural complexity. |
| Access Route | Description | Advantages | Disadvantages/Considerations |
|---|---|---|---|
| Transfemoral | Most common and preferred approach. Access is typically through the femoral artery. | Least invasive. Preferred when feasible. | Requires an adequate iliofemoral vessel size and a lack of severe tortuosity/calcification. |
| Transapical | Through the left ventricular apex via small chest incision. | Option when transfemoral route not possible. Direct access to the aortic valve. | Only applicable to balloon-expandable valves. More invasive. Requires a normal LV apex. A steeper angle may complicate procedure. |
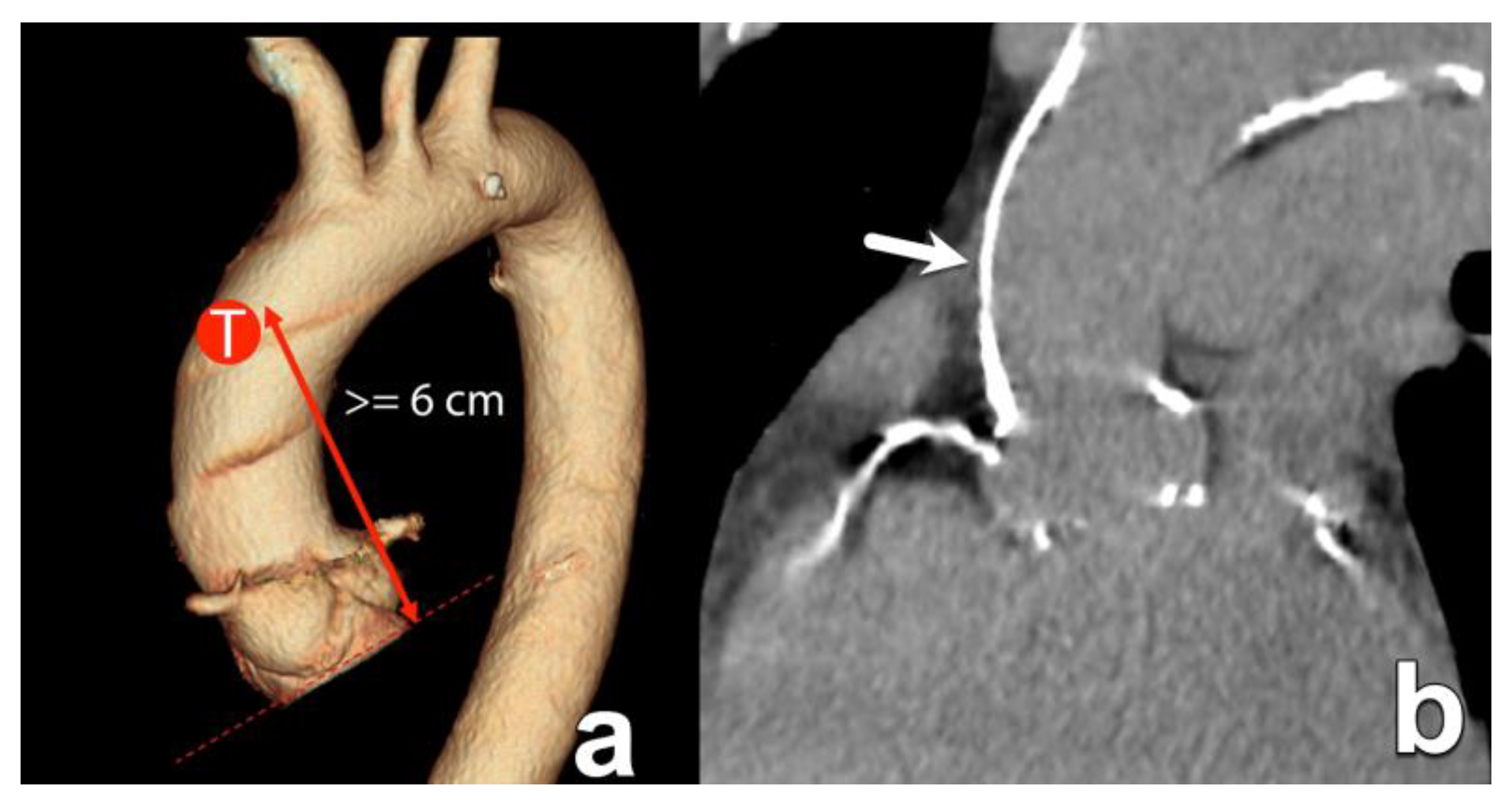
| Transaortic | Direct access to the ascending aorta via mini-sternotomy. | Option when transfemoral route not possible. | More invasive. The amount/location of ascending aorta wall calcification is important, typically at about 6 cm above the annular plane. |
| Subclavian/axillary | Through the subclavian or axillary artery. | Alternative when transfemoral route not possible. | Left side preferred due to angulation. |
| Transcarotid | Through the common carotid artery. | Alternative when other routes not possible. | Less commonly used. |
| Transcaval | Through the inferior vena cava and across the abdominal aorta. | Option in severe peripheral vascular disease. | Complex technique. Calcification-free window in aorta needed. Seldom used. |
| Likelihood of Severe Aortic Stenosis * | Men | Women |
|---|---|---|
| Highly likely | >3000 | >1600 |
| Likely | >2000 | >1200 |
| Unlikely | <1600 | <800 |
| Measurement | Description | Relevance |
|---|---|---|
| Mitral valve anatomy | Leaflet morphology, scallop identification (A1–A3, P1–P3) | Determines suitability for edge-to-edge repair |
| Coaptation depth | Distance from the annular plane to the point of leaflet coaptation | Should be ≤11 mm for optimal results |
| Coaptation length | Extent of the leaflet overlap | Ideally ≥2 mm for successful clipping |
| Flail gap | Distance between the flail segment and the opposing leaflet | Should be ≤10 mm |
| Flail width | Width of the flail segment | Should be ≤15 mm |
| Mitral valve area | Planimeter area of the mitral valve orifice | Should be ≥4 cm2 to avoid post-procedure mitral stenosis |
| Tenting height | Height of leaflet tenting in functional MR | Affects the likelihood of successful repair |
| Leaflet calcification | Extent and location of calcification | Heavy calcification may preclude successful clipping |
| Intercommissural distance | Distance between the anterior and posterior commissures | Aids in determining the number of clips needed |
| Left atrial size | Dimensions and volume of the left atrium | Affects the procedural approach |
| Interatrial septum | Thickness and location of the fossa ovalis | Guides the trans-septal puncture site |
| Neo-LVOT assessment | Simulated LVOT after clip placement | Assesses the risk of LVOT obstruction |
| Subvalvular apparatus | Papillary muscle and chorda anatomy | Ensures no interference with clip deployment |
| Device | Available Device Sizes (mm) | Applicable LAA Ostium Sizes (mm) | Landing Zone Diameter |
|---|---|---|---|
| Amulet | 16–34 | 11–31 | 12–15 mm distal to orifice |
| Watchman | 21–33 | 17–31 | 10–20 mm distal to ostium |
| Watchman FLX | 20–35 | 17–31.5 | 10–20 mm distal to ostium |
| Pulmonary veins |
|
| Left atrium |
|
| Interatrial septum |
|
| Other cardiac chambers |
|
| Oesophagus |
|
| Pericardium |
|
| Chest |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, R.; Cadour, F.; Cau, R.; Saba, L. Current Status of CT Imaging Before Common Transcatheter Interventions for Structural Heart Disease. Diagnostics 2025, 15, 97. https://doi.org/10.3390/diagnostics15010097
Salgado R, Cadour F, Cau R, Saba L. Current Status of CT Imaging Before Common Transcatheter Interventions for Structural Heart Disease. Diagnostics. 2025; 15(1):97. https://doi.org/10.3390/diagnostics15010097
Chicago/Turabian StyleSalgado, Rodrigo, Farah Cadour, Riccardo Cau, and Luca Saba. 2025. "Current Status of CT Imaging Before Common Transcatheter Interventions for Structural Heart Disease" Diagnostics 15, no. 1: 97. https://doi.org/10.3390/diagnostics15010097
APA StyleSalgado, R., Cadour, F., Cau, R., & Saba, L. (2025). Current Status of CT Imaging Before Common Transcatheter Interventions for Structural Heart Disease. Diagnostics, 15(1), 97. https://doi.org/10.3390/diagnostics15010097








