Abstract
Fertility potential ever more diminishes due to the complex, multifactorial, and still not entirely clarified process of reproductive aging in women and men. Gamete quality and reproductive lifespan are compromised by biologic factors like mitochondrial dysfunction, increased oxidative stress (OS), and incremental telomere shortening. Clinically confirmed biomarkers, including follicle-stimulating hormone (FSH) and anti-Müllerian hormone (AMH), are used to estimate ovarian reserve and reproductive status, but these markers have limited predictive validity and an incomplete representation of the complexity of reproductive age. Recent advances in artificial intelligence (AI) have the capacity to address the integration and interpretation of disparate and complex sets of data, like imaging, molecular, and clinical, for consideration. AI methodologies that improve the accuracy of reproductive outcome predictions and permit the construction of personalized treatment programs are machine learning (ML) and deep learning. To promote fertility evaluations, here, as part of its critical discussion, the roles of mitochondria, OS, and telomere biology as latter-day biomarkers of reproductive aging are presented. We also address the current status of AI applications in reproductive medicine, promises for the future, and applications involving embryo selection, multi-omics set integration, and estimation of reproductive age. Finally, to ensure that AI technology is used ethically and responsibly for reproductive care, model explainability, heterogeneity of data, and other ethical issues remain as residual concerns.
1. Introduction
Reproductive aging of females and males is an intricate, multifactorial process that has significant effects on fertility, outcomes of pregnancy, and health of the offspring [1,2]. It is described in females as decreased quantity and quality of oocytes resulting in diminished ovarian reserve (DOR), high aneuploidy rates, and decreased reproductive capability. It is described in males as alterations in the quality of the sperm, including oxidative injury, DNA fragmentation, and alterations of telomere length [3]. The epidemiological tendency of postponed birth has widened the medical relevance of reproductive aging, placing great priority on precise diagnosis techniques for the estimation of reproductive lifespan as well as tailoring treatment regimens.
Conventional markers, including antral follicle count (AFC), FSH, and AMH exhibit limited predictive accuracy for reproductive outcomes, although they provide valuable insights into ovarian reserve [4,5]. Conversely, conventional semen analysis in males primarily assesses morphology and motility, often overlooking the underlying cellular and molecular alterations associated with reproductive aging. The quality of gametes and embryos is largely determined by three interrelated biological processes, mitochondrial dysfunction, oxidative stress (OS), and telomere biology. Recent research in reproductive biology has identified these processes as key contributors to reproductive decline and poorer reproductive outcomes. Telomere shortening functions as a cellular aging clock that restricts reproductive longevity, OS degrades DNA, proteins, and lipids necessary for gamete function, and mitochondrial impairment lowers ATP synthesis and increases reactive oxygen species (ROS) generation. Compared to traditional clinical indicators, these events collectively offer a more thorough biological basis for reproductive aging [6]. Despite the promise for these indicators to improve the assessment of reproductive aging, their integration into clinical practice is limited by the complexity and diversity of the information they provide [7,8].
AI, particularly ML and deep learning techniques, offers effective and efficient remedies to these challenges. Such technologies can process and integrate molecular profiles, clinical measures, imaging variables, and other varied information to develop forecasting systems that are more precise and robust than conventional statistical approaches [9]. Applications of AI in reproductive medicine are expanding quickly, ranging from sperm quality analysis to ovarian reserve prediction and IVF embryo selection. However, there is still little research on how artificial intelligence might be used to analyze and correlate biomarkers of reproductive aging, such as mitochondrial competence, OS measurements, and telomere dynamics [10].
We discuss the current knowledge of telomere biology, mitochondria, and OS as important indicators of reproductive aging. Subsequently, the application of AI in reproductive medicine is explored, with an emphasis on how these technologies may revolutionize the diagnosis and management of reproductive aging. The challenges of algorithm interpretability, data heterogeneity, and ethical issues in the use of AI-driven solutions are finally covered.
2. Mitochondrial Dysfunction in Reproductive Aging
2.1. The Central Role of Mitochondria in Reproductive Aging
Cells generate energy through oxidative phosphorylation (OXPHOS), which is controlled by vital organelles called mitochondria. Gamete mitochondrial activity is particularly crucial given the high energy demands of oocyte maturation, fertilization, and early embryonic development [11,12]. Oocyte mitochondria provide adenosine triphosphate (ATP) and regulate calcium homeostasis, apoptosis, and redox balance, which are critical for maintaining developmental competence and genetic stability. Sperm mitochondrial activity maintains motility and the integrity of the paternal DNA [13]. Even within the same individual throughout time, it is crucial to understand that every gamete cohort—whether from males or females—represents a distinct metabolic and functional profile. Due to this variability, a significant therapeutic window exists during which couples still producing gametes can benefit from treatments that enhance OXPHOS, improve mitochondrial function, and reduce OS. The function, quality, and viability of gametes in the context of reproductive age may be enhanced by focusing on these pathways, which could increase fertility potential and enhance the results of ART.
Reduced fertility is a result of reproductive aging-related decreases in mitochondrial quantity and quality. The accumulation of mitochondrial DNA (mtDNA) mutations, a decrease in mitochondrial membrane potential, and reduced ATP production are hallmarks of aging oocytes. These changes impair meiotic spindle formation, increasing aneuploidy rates and decreasing embryo quality. In a similar vein, elderly sperm with mitochondrial malfunction exhibit decreased motility and greater DNA breakage [14,15].
There are several methods for directly measuring mitochondrial activity in gametes. Transmission electron microscopy (TEM) to examine morphology, ATP assays for energy production, and JC-1 or TMRE labeling to determine mitochondrial membrane potential are frequently used to analyze mitochondrial activity in sperm. ATP quantification, ROS imaging to identify OS, and TEM or confocal imaging to analyze structure and distribution are used to evaluate oocyte mitochondrial function [16]. ATP measurements and oxygen consumption rate (respirometry) are utilized to assess mitochondrial function in granulosa and cumulus cells. These assessments provide essential insights into gamete quality, fertilization potential, and early embryo developmental competence [17].
Gamete-Specific Impacts of Mitochondrial Dysfunction
The mitochondria in the midpiece are crucial for spermatozoa to produce the ATP required for motility and capacitation. The mitochondrial membrane potential (MMP) is decreased by aging-related mitochondrial dysfunction, though, and this results in impaired motility, a reduced acrosome reaction, and increased DNA fragmentation because of the overproduction of ROS. In sperm, abnormal mitochondrial morphology and compromised mtDNA integrity have been linked to poor embryo development and decreased fertilization ability.
Granulosa and cumulus cells’ mitochondria are essential for oocyte maturation, antioxidant defense, and steroidogenesis in ovarian follicles. Functional follicular mitochondria reduce the oocyte’s developmental capacity by affecting its metabolic and redox environment. Mitochondrial DNA alterations in follicular cells have been linked to decreased ovarian reserve, whereas decreased ATP generation in cumulus cells can disrupt nutrition and signal exchange with the contained oocyte.
The energy requirements of oocytes, which have the most mitochondria in the body, peak during meiotic spindle construction and fertilization. A decrease in oocyte mitochondrial quality with age raises the probability of aneuploidy, impairs spindle formation, and lowers ATP availability. Furthermore, the development of the embryo is further jeopardized by the accumulation of mtDNA mutations and structural damage.
2.2. Mitochondrial Biomarkers of Reproductive Aging
An assortment of mitochondrial features has been determined to include potential indicators for assessing reproductive age. Among these, the mitochondrial DNA copy number (mtDNA-CN) has garnered significant attention [18]. Studies have indicated a relationship between mtDNA-CN in oocytes and embryos and both developmental competence and implantation capability. Reports of alterations in mtDNA-CN levels in the granulosa cells and follicular fluid of older women have raised the possibility that it could serve as a surrogate indication of oocyte quality [19].
Another possible biomarker for mitochondrial function is the mitochondrial membrane potential, or MMP. Higher apoptotic susceptibility and decreased energy production are indicated by reduced MMP in aged gametes [20]. Although JC-1 staining and other fluorometric assays have been used to measure MMP, its clinical applicability is currently primarily in the experimental stage [21].
The production of ROS is a third crucial mitochondrial characteristic. An increased ROS is mostly caused by dysfunctional mitochondria, which exacerbate OS and cause DNA damage, lipid peroxidation, and protein oxidation in gametes [22]. ROS levels in seminal plasma and follicular fluid have been frequently associated with poor reproductive outcomes, including decreased fertilization rates and embryo quality [23].
Finally, a direct measure of mitochondrial energy generation is the concentration of ATP. Aging oocytes with lower ATP levels experience challenges with cytoskeletal dynamics and meiotic spindle assembly, leading to chromosomal defects and unsuccessful fertilization. Measuring the ATP content of oocytes and embryos can provide insights into their developmental potential, even though it is technically challenging [24,25]. Table 1 summarizes the key mitochondrial biomarkers implicated in reproductive aging, including their biological roles, assessment methods, clinical relevance, and current limitations. However, these conventional methods are inherently invasive and destructive, rendering the assessed gametes unusable for ART. Recent developments in AI-driven image processing, spectroscopy, and metabolic profiling present the potential for non-invasive, point-of-care, and real-time gamete assessment. For instance, deep learning algorithms and high-resolution, label-free imaging modalities can evaluate subcellular characteristics without endangering gamete viability, potentially allowing integration into ART workflows for prompt clinical decision-making.

Table 1.
Key mitochondrial biomarkers implicated in reproductive aging, including their biological roles, assessment methods, clinical relevance, and current limitations.
2.3. Current Challenges in Clinical Translation
Several limitations prevent mitochondrial biomarkers from becoming commonplace in the clinic, although they have good potential. One of the most significant restrictions is that there is no standard assay for the quantitative measurement of aspects like MMP and mtDNA-CN. Variation between the sensitivity of tests, treatment of samples, as well as the interpretation of results, can all cause inconsistent results between studies [30].
Another obstacle is obtaining the invasive embryo or oocyte samples required for direct analysis of the mitochondria. While less invasive, the validity of surrogate samples such as cumulus cells and follicular fluid as predictors of oocyte mitochondrial health remains under study [31]. Age, obesity, smoking, environmental contaminants, metabolic illnesses, and other confounding factors that may independently impact mitochondrial function make it more difficult to interpret biomarker data. These elements emphasize the need for advanced analytical tools capable of integrating several data dimensions while accounting for these confounders [32].
2.4. Potential of AI in Mitochondrial Biomarker Analysis
Given its capacity to integrate and interpret complex mitochondrial datasets, AI holds significant potential for addressing many of these challenges [33]. Through the identification of complex patterns and interactions among variables such as mtDNA-CN, ROS levels, and patient demographics, machine learning algorithms can predict oocyte quality and fertility results more precisely than traditional statistical methods [34].
Analysis of data from high-resolution mitochondrial imaging has demonstrated the effectiveness of deep learning methods, particularly convolutional neural networks (CNNs). These methods could be used to detect subtle structural or functional abnormalities in mitochondria that are not obvious to human observers [35].
Furthermore, a promising approach to thorough modeling of reproductive aging is AI-driven multi-omics integration [36]. Through the integration of proteomics, metabolomics, and mitochondrial genomes data, artificial intelligence frameworks can offer a comprehensive perspective on mitochondrial health and its correlation with reproductive capacity [37]. Personalized fertility treatment plans and non-invasive, precise prediction tools may be made possible by such methods [38,39].
3. OS as a Biomarker
3.1. Role of OS in Reproductive Aging
An imbalance between the generation of ROS and the antioxidant defense systems that counteract them results in OS [40]. Normal reproductive activities, including folliculogenesis, oocyte maturation, sperm capacitation, and embryo development, depend on physiological levels of ROS, whereas excessive ROS creation damages cells. This damage includes DNA fragmentation, protein oxidation, and lipid peroxidation, all of which are especially harmful to the reproductive system’s highly specialized cells [41,42].
OS has been linked to age-related declines in ovarian reserve and oocyte quality in women [43]. Increased ROS buildup and decreased antioxidant capability in aging ovaries are linked to meiotic errors, aneuploidy, and granulosa cell death. OS is a major factor in men’s sperm quality declining with age, leading to DNA breakage, decreased motility, and compromised fertilization ability [44].
OS in gametes can be directly assessed using a variety of techniques. While DNA fragmentation can be evaluated using TUNEL or Comet assays, ROS levels in sperm are often quantified using DCFDA labeling or chemiluminescence tests. Oxidative balance in oocytes is assessed by ROS imaging and antioxidant enzyme activity assays (e.g., SOD, catalase), and oxidative damage can also be deduced from DNA oxidation indicators or lipid peroxidation products. Similar biochemical tests are used to evaluate ROS levels and antioxidant capacity in granulosa and cumulus cells, which shed light on the follicular microenvironment and its capacity to promote oocyte development [45].
Gamete-Specific Impacts of OS
For functions including capacitation and hyperactivation, sperm require physiological amounts of ROS; nevertheless, too much ROS can harm sperm proteins, lipids, and DNA, decreasing motility and increasing morphological abnormalities. Male infertility, especially in older men, has been closely linked to elevated OS in seminal plasma.
Granulosa and cumulus cells’ ability to function is hampered in ovarian follicles by OS in the follicular fluid, which changes the microenvironment surrounding the oocyte. In ART cycles, lower fertilization rates and lower-quality embryos are associated with elevated lipid peroxidation products in the follicle, such as malondialdehyde (MDA).
Oocytes are particularly vulnerable to oxidative injury due to their large cyto-plasmic volume and prolonged meiotic arrest. The consequences of aging on reproductive capacity can be exacerbated by excessive ROS, which can cause spindle integrity disruption, mitochondrial malfunction, and telomere shortening.
3.2. OS Biomarkers in Reproductive Aging
Several biomarkers have been proposed for the evaluation of OS within the reproductive system. ROS levels in follicular fluid, seminal plasma, or cumulus cells can be directly measured to ascertain the oxidative environment surrounding gametes [46,47]. Reduced implantation success, lower fertilization rates, and poorer embryo quality have been linked to increased ROS concentrations in ART [48,49].
Indirect indicators of oxidative damage are the markers of lipid peroxidation MDA and 4-hydroxynonenal (4-HNE). These have been reported to be present in increased quantities within infertile men’s seminal plasma and women suffering from DOR’s follicular fluid [50]. Oxidation of reproductive proteins required for gametic functionality is also suggested by markers of protein oxidation, such as advanced oxidation protein products (AOPPs) [51].
Measuring antioxidant capacity with specific enzymes such as catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD) or total antioxidant capacity (TAC) can yield more details on the oxidative equilibrium. These antioxidant defenses have been shown to decline with age and infertility in both sexes [52]. Table 2 summarizes the major biomarkers of OS associated with reproductive aging. Gametes meant for ART cannot be immediately subjected to OS evaluations since the majority of them still require destructive testing or invasive sampling. By combining label-free imaging, fast spectroscopic readouts, and non-invasive metabolic profiling, advances in AI-assisted analysis provide a mechanism to evaluate oxidative status in real time at the point of treatment while maintaining gamete integrity.

Table 2.
Major biomarkers of OS relevant to reproductive aging, categorized by type (direct oxidative markers, lipid peroxidation, protein oxidation, and antioxidant status). Assessment methods, associations with gamete and embryo quality, and current challenges in clinical application are included.
3.3. Current Challenges in Biomarker Utilization
Despite growing evidence that OS contributes to reproductive aging, the therapeutic use of OS biomarkers remains limited [57]. The variation in assay techniques used to assess ROS and antioxidant levels is a major issue that contributes to contradictory research outcomes. The assessment of OS is complicated by its dynamic character, which varies in response to clinical states, environmental exposures, and lifestyle factors [58].
Additionally, the intrusive collection of follicular fluid or cumulus cells for OS testing limits its usual use in reproductive clinics [59]. Men’s semen collection is less intrusive, but interpretation is made more difficult by the variety of OS indicators in seminal plasma. A crucial first step in integrating biomarker assays into reproductive medicine is standardizing them and creating clinically meaningful reference ranges [60,61,62].
3.4. Potential of AI in OS Biomarker Analysis
By combining intricate datasets and identifying patterns that are difficult to identify using traditional techniques, artificial intelligence holds the potential to revolutionize the research of OS biomarkers [63]. To create prediction models of reproductive outcomes, ML algorithms can integrate clinical indicators, OS markers, antioxidant levels, and patient lifestyle factors [64].
Fluorescent ROS probes in gametes or embryos are examples of high-content OS imaging that deep learning techniques could examine to find minute oxidative signatures linked to aging [65]. AI frameworks can facilitate the integration of mitochondrial and telomere biomarkers with OS data, enabling a more comprehensive assessment of gamete quality and reproductive potential. Non-invasive, customized methods to track OS and improve the results of reproductive treatments may be made possible by these advancements [10].
4. Telomere Biology in Fertility
4.1. Telomere Shortening and Reproductive Lifespan
Telomeres, repetitive nucleotide sequences that cap the ends of chromosomes, function as a biological clock for cellular aging by progressively shortening with each cell division. The reduction in reproductive lifespan is closely related to this shortening [66]. Oocyte telomere attrition in females is linked to greater rates of infertility and miscarriage, as well as decreased ovarian reserve and oocyte quality. Male is adversely affected by spermatozoa with shortened telomeres, which have been linked to reduced sperm motility, increased DNA fragmentation, and lower fertilization rates [67].
Several well-established methods can be used to evaluate the integrity and length of the telomeres in gametes. While fluorescence in situ hybridization (FISH) can show telomere distribution, qPCR or terminal restriction fragment (TRF) analysis are typically used to quantify telomere length in sperm. Q-FISH is frequently used to assess oocyte telomeres because it enables the direct assessment of telomere length and the identification of dangerously short telomeres. The ability of granulosa and cumulus cells to support oocyte development and overall reproductive potential can be determined by measuring their telomere length using qPCR or FISH [68]. Current methods for measuring telomeres in gametes usually involve destructive sampling, which precludes the evaluated cells from being used in other clinical settings. Without sacrificing the viability of gametes for ART, quick, real-time telomere evaluation at the point of treatment may be possible by combining non-invasive optical or molecular profiling with AI-driven picture and data processing.
Gamete-Specific Telomere Dynamics
As paternal age increases, telomerase activity in spermatogonial stem cells tends to maintain or even increase telomere length in sperm. Nonetheless, telomeric DNA can still be harmed by severe OS, which can lead to fragmentation of sperm DNA and decreased embryo viability.
Follicle somatic cells in ovarian follicles, such as cumulus and granulosa cells, gradually shorten their telomeres as they age. Oocyte quality may be compromised if these supporting cells’ telomeres are shortened, which could affect their capacity to protect and nourish the oocyte.
Due to the low telomerase activity after birth, oocytes show gradual telomere attrition throughout a woman’s reproductive lifespan. Chromosome mis-segregation, meiotic spindle instability, and an elevated risk of miscarriage are all associated with oocytes with short telomeres. This attrition is further exacerbated by mitochondrial dysfunction and OS, creating a synergistic pathway that contributes to reproductive decline.
4.2. Male vs. Female Gametes: Telomere Dynamics
Male and female gametes have distinct telomere length dynamics as a result of differences in gametogenesis and telomerase activity. Since spermatogonial stem cells contain active telomerase, sperm telomeres often lengthen as paternal age increases [69]. However, oocytes lack considerable telomerase activity after birth, resulting in increasing telomere shortening with maternal age. This sexual dimorphism identifies distinct molecular mechanisms in gamete aging and emphasizes the significance of considering sex-specific telomere biology in reproductive research [70].
4.3. AI Approaches to Telomere Length Prediction
AI and ML techniques have emerged as very efficient methods for the analysis of telomere length from complex genomic and clinical data [71]. By integrating clinical traits and multi-omics data, AI models can precisely predict telomere length, enabling early detection of reproductive aging and risk stratification for infertility. These techniques may improve reproductive outcomes and allow for tailored fertility treatments by directing decisions regarding the timing and strategies of interventions [72].
5. AI in Reproductive Medicine
5.1. Current Applications of AI
AI has accelerated the transformation of reproductive medicine by enhancing clinical decision-making and diagnostic precision [73]. IVF selection is streamlined by embryo grading systems that assess embryo morphology and developmental potential using deep learning algorithms and sophisticated image analysis [74]. Furthermore, by examining patient clinical data and treatment parameters, prediction models have been created to forecast IVF success rates. AI-driven sperm quality evaluations in male fertility use pattern recognition and computer vision to detect sperm motility, morphology, and concentration more reliably and accurately than conventional techniques [75].
5.2. AI for Multi-Omics Integration
Reproductive biology is composed of complex interactions of the genome, proteasome, and metabolome. By integrating multi-omics datasets, AI techniques reveal new biomarkers and pathways involved in infertility and reproductive aging [76]. Extracting patterns and relationships between disparate sets of data that are routinely ignored by conventional analytical techniques, machine learning programs provide detailed insights into the health of gametes, embryo development, and processes of aging. Personalized reproductive health might be advanced by the integrated AI framework by customizing drugs based upon complex biological realities [77].
5.3. Case Studies and Existing AI Models in Reproductive Aging
Despite being a relatively recent focus, reproductive aging has prompted the development of several AI models. For example, by analyzing hormone patterns in combination with genetic markers, several studies have used machine learning to predict the decline of ovarian reserve [78]. Other models use imaging data to assess ovarian follicle quality and forecast when menopause will begin. These case studies, while still in their infancy, show how AI may advance our knowledge of and strategy for dealing with reproductive aging, opening the door for therapeutic applications that might prolong windows of fertility or maximize timing for fertility preservation [78]. Table 3 summarizes the key applications of AI in reproductive medicine.

Table 3.
Key applications of AI in reproductive medicine.
5.4. AI for Integrating Complex Datasets in Reproductive Aging
The biological processes involved in reproductive aging are intricately linked and include OS, telomere attrition, and mitochondrial dysfunction. These processes are measured in a variety of gametes and supporting cells. Conventional studies cannot fully represent the biological complexity of the generated datasets since they are massive, multidimensional, and frequently non-linear [82]. AI provides the computing power to merge these diverse datasets—clinical, molecular, imaging, and functional—into logical models that can identify trends linked to less successful reproductive outcomes. To predict oocyte or sperm quality, aneuploidy risk, and embryo developmental potential, for example, machine learning can combine patient-specific reproductive features with data on mitochondrial activity, ROS levels, and telomere length. AI promotes tailored approaches to slow fertility decline and allows for a more comprehensive understanding of reproductive aging by combining intricate measures into useful insights (Figure 1) [72,83].
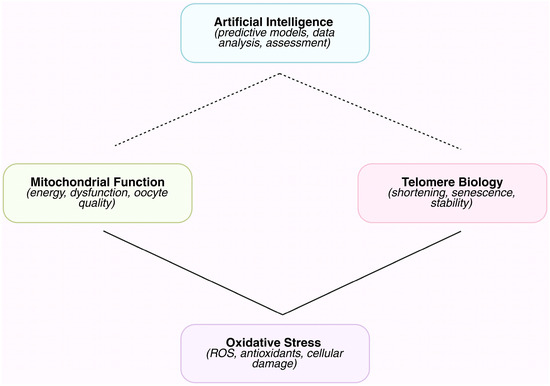
Figure 1.
Schematic overview of the interplay between AI approaches and key mechanisms involved in reproductive aging. AI tools are applied to assess, predict, and monitor mitochondrial function, OS, and telomere biology. Solid arrows represent well-established mechanistic links, while dashed arrows indicate emerging or hypothesized connections. This schematic highlights how AI can integrate multifactorial biological data to provide insights into reproductive aging.
6. Challenges and Future Directions
6.1. Data Heterogeneity and Standardization
AI applications in reproductive aging are emerging, with existing models representing only the initial stages of development. ML has been used in several studies to predict DOR by examining hormone patterns in conjunction with genetic markers. Other models utilize imaging data to predict the onset of menopause and assess the health of ovarian follicles [77]. These early-stage case studies demonstrate how AI can improve our understanding and treatment of reproductive aging. This may lead to therapeutic applications that extend windows of viability or enhance timing for fertility maintenance [38].
6.2. Utilization of AI Models
Even though AI models, particularly deep learning networks, have a high predictive accuracy, their “black-box” nature makes clinical implementation difficult. Understanding how models make decisions, or explainability, is necessary to win over patients’ and physicians’ trust. Incorporating transparent reasoning techniques and developing interpretable AI frameworks will enable validation, regulatory approval, and ethical application in fertility care [84].
6.3. Explainability of AI Models
Reproductive medicine has used a number of different machine learning techniques, each with special benefits based on the kind and complexity of the data. CNNs are especially good at evaluating imaging data, including sperm morphology evaluations and time-lapse embryo movies. Their layered architecture makes it possible to identify tiny morphological variations that could be hard for human observers to notice by automatically extracting hierarchical image attributes. A CNN trained on more than 12,000 annotated embryo photos outperformed traditional morphological grading in one case study in terms of implantation prediction accuracy.
For tabular clinical data, such as patient information, hormone levels, and genetic markers, tree-based ensemble models like Random Forest are frequently utilized. Random Forest can handle diverse datasets with both continuous and categorical variables and minimize overfitting by merging several decision trees. For instance, a Random Forest model with significant cross-clinical generalizability was trained on a multi-center dataset of 2000 IVF cycles and was able to predict clinical pregnancy rates.
This strategy is expanded by gradient boosting algorithms like XGBoost, which construct trees one after the other with an emphasis on fixing the mistakes of the earlier ones. High prediction performance, effective management of missing data, and the capacity to identify intricate non-linear relationships are all hallmarks of XGBoost. With an area under the ROC curve (AUC) above 0.85 in validation cohorts, XGBoost models have been utilized in reproductive aging research to combine genetic, hormonal, and lifestyle data to estimate the likelihood of decreased ovarian reserve.
For these models to function at their best, access to well-annotated datasets is still essential. Training material can be obtained from publicly accessible sources, such as the Human Fertilization and Embryology Authority (HFEA) database for IVF results and curated imaging libraries for embryo development. To enable solid, repeatable AI research in reproductive medicine, collaborative data-sharing activities are necessary, as many high-quality datasets are still proprietary or limited.
6.4. Ethical and Legal Considerations
The application of AI in reproductive medicine raises important ethical and legal concerns [85]. Ensuring fair and responsible AI use requires addressing patient privacy, data security, informed consent, and biases in training data. Regulations specific to reproductive health applications are required to protect patient rights and advance equitable access to cutting-edge AI technologies [86].
6.5. Personalized Fertility Medicine Powered by AI
AI might transform future fertility treatment by supplying extremely personalized drugs [38]. Multi-dimensional information, like genetic and behavioral character traits, might be combined by AI to maximize therapy timing, personalize therapies based on each unique patient profile, and enhance results. Effective validation, ethical oversight, and continued innovation can unlock AI-led personalized fertility care [87].
6.6. Telomere Biology in Reproductive Aging: Measurement, Mechanisms, and Therapeutic Potential
Telomere length is increasingly recognized as an important biomarker of reproductive aging. It can be measured through different methods, each with their own advantages and disadvantages. Rapid, high-throughput analysis is possible using qPCR, although it only provides estimates of relative length. Although it allows for absolute length values, TRF analysis is less appropriate for small reproductive samples and necessitates substantial amounts of DNA. Individual gametes or embryos can be observed using Q-FISH, but more recent methods like telomere shortest length assay (TeSLA) and single telomere length analysis (STELA) improve the detection of critically short telomeres, which may be the most clinically relevant [88].
There is growing recognition that telomere dynamics differ by sex. Male sperm telomeres can be maintained or even extended over time by telomerase activity during spermatogenesis, while female oocytes incur progressive telomere attrition, which is made worse by mitochondrial malfunction and OS. With androgens affecting telomere stability through oxidative pathways and estrogen having been demonstrated to increase telomerase activity, hormonal regulation may also play a role [69].
Pharmacologic telomerase activators, antioxidant supplements, and lifestyle modifications to reduce OS are examples of potential telomere-targeted therapies that have shown some initial promise in preclinical animals. Though they are being investigated, more sophisticated strategies—like gene therapy to increase telomerase expression—need to be carefully evaluated for safety because of possible carcinogenic concerns. Using non-invasive biomarker screening to choose gametes or embryos with longer telomeres may improve the results of ART. Long-term safety and ethical issues must be weighed against these promising treatments, particularly when it comes to younger patient populations [89,90].
6.7. Limitations and Ethical Challenges of AI in Reproductive Medicine
Despite the promising advances in AI applications for reproductive medicine, there are important limitations that must be addressed before these technologies can be widely adopted in clinical practice. Given that AI models significantly depend on the caliber and representativeness of their training data, dataset bias is one of the main issues. Due to a lack of diversity in variables including age, ethnicity, and clinical conditions, many of the datasets currently in use may produce skewed forecasts that exacerbate healthcare inequities. For instance, when applied to underrepresented groups, models that were primarily trained on data from particular populations may perform poorly, which could lead to unequal outcomes for fertility care [91].
Model validation is another important concern. Thorough testing across several independent cohorts is necessary to determine the generalizability and dependability of AI models. However, single-center data and small sample sizes are common in reproductive medicine research, which restricts external validity. Comparing and evaluating various models is made more difficult by the lack of defined procedures for data gathering and result measurement [92].
Practical difficulties can arise when integrating AI models into clinical settings. Early-stage research occasionally ignores important steps like integration with current electronic health record systems, guaranteeing compatibility with clinical workflows, and offering sufficient user training. Furthermore, many AI algorithms are “black-box” in nature, which limits physicians’ capacity to fully understand the predictions and confidently apply them to clinical decision-making [91].
Informed consent, algorithm openness, and patient privacy are ethical issues. The utilization of their data and the creation of AI-generated suggestions may not always be clear to patients. Additionally, AI may be used in ways that compromise patient autonomy or inadvertently reinforce preexisting biases [93].
Finally, securing governmental approval, protecting data, and defining who is responsible for AI blunders are some of the obstacles to real-world application. For example, it remains unclear who would be held accountable—the healthcare facility, the AI developer, or the clinician—if an AI model forecasts embryo viability inaccurately, resulting in a failed IVF cycle. Clinical professionals, data scientists, ethicists, and regulators must work together to address these intricate issues and guarantee that AI tools be used in reproductive care in a way that is safe, efficient, and morally sound [94].
7. Conclusions
This review underscores the essential link between mitochondrial dysfunction, OS, telomere biology, and fertility by illustrating the ways in which these interconnected processes determine gamete quality and reproductive duration for females and males. Key mechanisms of reproductive aging and poorer reproductive outcomes can be explained by telomere shortening acting as a cellular aging clock, OS causing molecular damage that compromises fertilization and embryo development, and mitochondrial impairment decreasing energy production and increasing ROS generation. By facilitating advanced analysis of sperm, precise IVF chance prognosis, better embryo grading, and incorporation of sophisticated multi-omics data, artificial intelligence has transformed reproductive medicine. Aging can now be better understood and managed due to these advances.
Data heterogeneity, model explainability requirements, and substantial ethical and regulatory challenges are just some of the challenges for the implementation of AI technology in this industry. AI technology solutions have to overcome these challenges for them to remain trustworthy, fair, and therapeutically valuable.
AI-centered methods of the future have unprecedented potential to provide customized diagnosis, customized fertility care, and precise reproductive age determination. It would take an interdisciplinary approach and innovation in technology to realize the full potential of AI and ultimately improve fertility and reproductive health outcomes across varied groups.
Author Contributions
Conceptualization, E.M. and E.D.; methodology, T.G. and S.S.; validation, I.T., C.P. and K.L.; investigation, A.P., A.Z. and A.G.; data curation, E.M.; writing—original draft preparation, E.M., T.G. and S.S.; writing—review and editing, A.P., A.Z., A.G., I.T., C.P., K.L. and E.D.; visualization, A.P.; supervision, A.P. and A.G.; project administration, E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moghadam, A.R.E.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 2022, 26, 105–122. [Google Scholar] [CrossRef]
- Jimbo, M.; Kunisaki, J.; Ghaed, M.; Yu, V.; Flores, H.A.; Hotaling, J.M. Fertility in the aging male: A systematic review. Fertil. Steril. 2022, 118, 1022–1034. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, N.D.; Dang, H.N.T.; Nguyen, Q.H.V. Does diminished ovarian reserve impact oxidation-reduction potential in follicular fluid? J. Int. Med. Res. 2025, 53, 3000605251349926. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, A.; Esteves, S.C.; Yarali, H.; Vuong, L.N.; Dahan, M.H. Antimullerian hormone and antral follicle count thresholds for hyperresponse risk assessment in in vitro fertilization: A Hyperresponse Risk Assessment consensus study. Fertil. Steril. 2025, 123, 827–837. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Mullerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Wang, C.; Swerdloff, R.S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 2014, 102, 1502–1507. [Google Scholar] [CrossRef]
- Cohen, J.; Silvestri, G.; Paredes, O.; Martin-Alcala, H.E.; Chavez-Badiola, A.; Alikani, M.; Palmer, G.A. Artificial intelligence in assisted reproductive technology: Separating the dream from reality. Reprod. Biomed. Online 2025, 50, 104855. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhu, Z.; Wang, L.; Li, C.; Sun, L.; Wang, W.; Gong, W. Biomarkers of Aging and Relevant Evaluation Techniques: A Comprehensive Review. Aging Dis. 2024, 15, 977–1005. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Hanassab, S.; Abbara, A.; Yeung, A.C.; Voliotis, M.; Tsaneva-Atanasova, K.; Kelsey, T.W.; Trew, G.H.; Nelson, S.M.; Heinis, T.; Dhillo, W.S. The prospect of artificial intelligence to personalize assisted reproductive technology. NPJ Digit. Med. 2024, 7, 55. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Seli, E. The role of mitochondrial dynamics in oocyte and early embryo development. Semin. Cell Dev. Biol. 2024, 159–160, 52–61. [Google Scholar] [CrossRef]
- Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology 2020, 9, 269. [Google Scholar] [CrossRef]
- Phan, O.T.H.; Trinh, T.T.C. Mitochondria in the reproduction system and mitotherapy in assisted reproductive technology: The importance of mitochondria selection. Biomed. Res. Ther. 2025, 12, 7054–7081. [Google Scholar] [CrossRef]
- Ju, W.; Zhao, Y.; Yu, Y.; Zhao, S.; Xiang, S.; Lian, F. Mechanisms of mitochondrial dysfunction in ovarian aging and potential interventions. Front. Endocrinol. 2024, 15, 1361289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, F. Effects of adverse fertility-related factors on mitochondrial DNA in the oocyte: A comprehensive review. Reprod. Biol. Endocrinol. 2023, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Overchuk, M.; Chappell, V.A.; Kemal Ruhi, M.; Sinawang, P.D.; Nguyen Hoang, T.T.; Akin, D.; Demirci, U.; Franco, W.; Fenton, S.E.; et al. Methods to Evaluate Changes in Mitochondrial Structure and Function in Cancer. Cancers 2023, 15, 2564. [Google Scholar] [CrossRef]
- Venturas, M.; Yang, X.; Sakkas, D.; Needleman, D. Noninvasive metabolic profiling of cumulus cells, oocytes, and embryos via fluorescence lifetime imaging microscopy: A mini-review. Hum. Reprod. 2023, 38, 799–810. [Google Scholar] [CrossRef]
- Kupsco, A.; Bloomquist, T.R.; Hu, H.; Reddam, A.; Tang, D.; Goldsmith, J.; Rundle, A.G.; Baccarelli, A.A.; Herbstman, J.B. Mitochondrial DNA copy number dynamics and associations with the prenatal environment from birth through adolescence in a population of Dominican and African American children. Mitochondrion 2023, 69, 140–146. [Google Scholar] [CrossRef]
- Zhang, D.; Keilty, D.; Zhang, Z.F.; Chian, R.C. Mitochondria in oocyte aging: Current understanding. Facts Views Vis. Obgyn 2017, 9, 29–38. [Google Scholar]
- Lu, J.; Wu, L.; Wang, X.; Zhu, J.; Du, J.; Shen, B. Detection of Mitochondria Membrane Potential to Study CLIC4 Knockdown-induced HN4 Cell Apoptosis In Vitro. J. Vis. Exp. 2018, 137, e56317. [Google Scholar] [CrossRef]
- Baig, J.; Pradeepkiran, J.A.; Reddy, P.H. Methods to Study Mitochondria: Techniques Used to Study the Effects of Age-Related Diseases Including Alzheimer’s. Curr. Protoc. 2023, 3, e631. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Coskun, S.; Al-Rouqi, R.; Al-Rajudi, T.; Eltabache, C.; Abduljabbar, M.; Al-Hassan, S. Oxidative stress and DNA damage status in couples undergoing in vitro fertilization treatment. Reprod. Fertil. 2021, 2, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Galeska, E.; Kowalczyk, A.; Wrzecinska, M.; Garcia, M.C.; Czerniawska-Piatkowska, E.; Gwozdziewicz, S.; Witkiewicz, W.; Dobrzanski, Z. The Importance of Mitochondrial Processes in the Maturation and Acquisition of Competences of Oocytes and Embryo Culture. Int. J. Mol. Sci. 2025, 26, 4098. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Yang, S.C.; Yu, E.J.; Park, J.K.; Kim, T.H.; Eum, J.H.; Paek, S.K.; Hwang, J.Y.; Lyu, S.W.; Kim, J.Y.; Lee, W.S.; et al. The Ratio of Mitochondrial DNA to Genomic DNA Copy Number in Cumulus Cell May Serve as a Biomarker of Embryo Quality in IVF Cycles. Reprod. Sci. 2021, 28, 2495–2502. [Google Scholar] [CrossRef]
- Uribe, P.; Villegas, J.V.; Boguen, R.; Treulen, F.; Sanchez, R.; Mallmann, P.; Isachenko, V.; Rahimi, G.; Isachenko, E. Use of the fluorescent dye tetramethylrhodamine methyl ester perchlorate for mitochondrial membrane potential assessment in human spermatozoa. Andrologia 2017, 49, e12753. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.T.; Rhodes, C.J. Reactive Oxygen Species (ROS) in Metabolic Disease-Don’t Shoot the Metabolic Messenger. Int. J. Mol. Sci. 2025, 26, 2622. [Google Scholar] [CrossRef]
- Hu, L.L.; Liao, M.H.; Liu, Y.X.; Xing, C.H.; Nong, L.L.; Yang, F.L.; Sun, S.C. Loss of AMPK activity induces organelle dysfunction and oxidative stress during oocyte aging. Biol. Direct 2024, 19, 29. [Google Scholar] [CrossRef]
- Koller, A.; Fazzini, F.; Lamina, C.; Rantner, B.; Kollerits, B.; Stadler, M.; Klein-Weigel, P.; Fraedrich, G.; Kronenberg, F. Mitochondrial DNA copy number is associated with all-cause mortality and cardiovascular events in patients with peripheral arterial disease. J. Intern. Med. 2020, 287, 569–579. [Google Scholar] [CrossRef]
- Lemseffer, Y.; Terret, M.E.; Campillo, C.; Labrune, E. Methods for Assessing Oocyte Quality: A Review of Literature. Biomedicines 2022, 10, 2184. [Google Scholar] [CrossRef]
- Singh, S.; Kriti, M.; Anamika, K.S.; Sarma, D.K.; Verma, V.; Nagpal, R.; Mohania, D.; Tiwari, R.; Kumar, M. Deciphering the complex interplay of risk factors in type 2 diabetes mellitus: A comprehensive review. Metabol. Open 2024, 22, 100287. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Uchihashi, T.; Kawata, S.; Nishiura, A.; Yamamoto, T.; Hiraoka, S.I.; Yokota, Y.; Isomura, E.T.; Kogo, M.; Tanaka, S.; et al. Role and Potential of Artificial Intelligence in Biomarker Discovery and Development of Treatment Strategies for Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2025, 26, 4346. [Google Scholar] [CrossRef]
- Ferreira, T.; Rodriguez, S. Mitochondrial DNA: Inherent Complexities Relevant to Genetic Analyses. Genes 2024, 15, 617. [Google Scholar] [CrossRef]
- Iqbal, M.S.; El-Ashram, S.; Hussain, S.; Khan, T.; Huang, S.; Mehmood, R.; Luo, B. Efficient cell classification of mitochondrial images by using deep learning. J. Opt. 2019, 48, 113–122. [Google Scholar] [CrossRef]
- Theodorakis, N.; Feretzakis, G.; Tzelves, L.; Paxinou, E.; Hitas, C.; Vamvakou, G.; Verykios, V.S.; Nikolaou, M. Integrating Machine Learning with Multi-Omics Technologies in Geroscience: Towards Personalized Medicine. J. Pers. Med. 2024, 14, 931. [Google Scholar] [CrossRef]
- Morabito, A.; De Simone, G.; Pastorelli, R.; Brunelli, L.; Ferrario, M. Algorithms and tools for data-driven omics integration to achieve multilayer biological insights: A narrative review. J. Transl. Med. 2025, 23, 425. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Teke, J.; Adeleye, K.K.; Weerasinghe, K.; Maidoki, M.; Clement David-Olawade, A. Artificial intelligence in in-vitro fertilization (IVF): A new era of precision and personalization in fertility treatments. J. Gynecol. Obstet. Hum. Reprod. 2025, 54, 102903. [Google Scholar] [CrossRef] [PubMed]
- Potiris, A.; Alyfanti, E.; Drakaki, E.; Mavrogianni, D.; Karampitsakos, T.; Machairoudias, P.; Topis, S.; Zikopoulos, A.; Skentou, C.; Panagopoulos, P.; et al. The Contribution of Proteomics in Understanding Endometrial Protein Expression in Women with Recurrent Implantation Failure. J. Clin. Med. 2024, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef]
- Potiris, A.; Moustakli, E.; Trismpioti, E.; Drakaki, E.; Mavrogianni, D.; Matsas, A.; Zikopoulos, A.; Sfakianakis, A.; Tsakiridis, I.; Dagklis, T.; et al. From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health. Metabolites 2025, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory assessment of oxidative stress in semen. Arab. J. Urol. 2018, 16, 77–86. [Google Scholar] [CrossRef]
- Castleton, P.E.; Deluao, J.C.; Sharkey, D.J.; McPherson, N.O. Measuring Reactive Oxygen Species in Semen for Male Preconception Care: A Scientist Perspective. Antioxidants 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zhang, L. The Impact of Follicular Fluid Oxidative Stress Levels on the Outcomes of Assisted Reproductive Therapy. Antioxidants 2023, 12, 2117. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Katopodis, P.; Domali, E.; Potiris, A.; Stavros, S.; Zachariou, A. Impact of Reductive Stress on Human Infertility: Underlying Mechanisms and Perspectives. Int. J. Mol. Sci. 2024, 25, 11802. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wen, S.; Lin, Y.; Yang, X.; Liu, Z.; Quan, S.; Song, Y. Advanced oxidation protein products change biological behaviors of rat endometrial epithelial cells by activating ERK/P38 signaling pathways. Biol. Open 2020, 9, bio048876. [Google Scholar] [CrossRef]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity-Relevance, methods and clinical implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef] [PubMed]
- Babić, N.; Peyrot, F. Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations. Magnetochemistry 2019, 5, 13. [Google Scholar] [CrossRef]
- Mihalas, B.P.; De Iuliis, G.N.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci. Rep. 2017, 7, 6247. [Google Scholar] [CrossRef] [PubMed]
- Campara, K.; Rodrigues, P.; Viero, F.T.; da Silva, B.; Trevisan, G. A systematic review and meta-analysis of advanced oxidative protein products levels (AOPP) levels in endometriosis: Association with disease stage and clinical implications. Eur. J. Pharmacol. 2025, 996, 177434. [Google Scholar] [CrossRef]
- Silvestrini, A.; Mancini, A. The Double-Edged Sword of Total Antioxidant Capacity: Clinical Significance and Personal Experience. Antioxidants 2024, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Khoo, M.I.; Ismail, E.H.E.; Hussain, N.H.N.; Zin, A.A.M.; Noordin, L.; Abdullah, S.; Mahdy, Z.A.; Lah, N. Oxidative stress biomarkers in pregnancy: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 93. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jedrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Stavros, S.; Sofikitis, N.; Georgiou, I.; Zachariou, A. Integrative Assessment of Seminal Plasma Biomarkers: A Narrative Review Bridging the Gap between Infertility Research and Clinical Practice. J. Clin. Med. 2024, 13, 3147. [Google Scholar] [CrossRef]
- Vashisht, A.; Gahlay, G.K. Understanding seminal plasma in male infertility: Emerging markers and their implications. Andrology 2024, 12, 1058–1077. [Google Scholar] [CrossRef]
- Stavros, S.; Potiris, A.; Molopodi, E.; Mavrogianni, D.; Zikopoulos, A.; Louis, K.; Karampitsakos, T.; Nazou, E.; Sioutis, D.; Christodoulaki, C.; et al. Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. Int. J. Mol. Sci. 2024, 25, 10167. [Google Scholar] [CrossRef]
- Arya, S.S.; Dias, S.B.; Jelinek, H.F.; Hadjileontiadis, L.J.; Pappa, A.M. The convergence of traditional and digital biomarkers through AI-assisted biosensing: A new era in translational diagnostics? Biosens. Bioelectron. 2023, 235, 115387. [Google Scholar] [CrossRef]
- Peng, J.; Geng, X.; Zhao, Y.; Hou, Z.; Tian, X.; Liu, X.; Xiao, Y.; Liu, Y. Machine learning algorithms in constructing prediction models for assisted reproductive technology (ART) related live birth outcomes. Sci. Rep. 2024, 14, 32083. [Google Scholar] [CrossRef] [PubMed]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Lu, J.; Cheng, L.; Wu, D.; Li, L.; Zhang, S.; Lai, X.; Xu, L. The relationship between telomere length and aging-related diseases. Clin. Exp. Med. 2025, 25, 72. [Google Scholar] [CrossRef] [PubMed]
- Fragkiadaki, P.; Kouvidi, E.; Angelaki, A.; Nikolopoulou, D.; Vakonaki, E.; Tsatsakis, A. Evaluation of telomere length and telomerase activity on predicting in vitro fertilization treatment outcomes. J. Assist. Reprod. Genet. 2024, 41, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere length: A review of methods for measurement. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef]
- Fice, H.E.; Robaire, B. Telomere Dynamics Throughout Spermatogenesis. Genes 2019, 10, 525. [Google Scholar] [CrossRef]
- Chico-Sordo, L.; Cordova-Oriz, I.; Polonio, A.M.; LS, S.M.; Medrano, M.; Garcia-Velasco, J.A.; Varela, E. Reproductive aging and telomeres: Are women and men equally affected? Mech. Ageing Dev. 2021, 198, 111541. [Google Scholar] [CrossRef]
- Bakare, O.S. AI-Driven Multi-Omics Integration for Precision Medicine in Complex Disease Diagnosis and Treatment. Int. J. Res. Publ. Rev. 2025, 6, 5070–5084. [Google Scholar] [CrossRef]
- Findikli, N.; Houba, C.; Pening, D.; Delbaere, A. The Role of Artificial Intelligence in Female Infertility Diagnosis: An Update. J. Clin. Med. 2025, 14, 3127. [Google Scholar] [CrossRef]
- Wu, Y.C.; Chia-Yu Su, E.; Hou, J.H.; Lin, C.J.; Lin, K.B.; Chen, C.H. Artificial intelligence and assisted reproductive technology: A comprehensive systematic review. Taiwan J. Obstet. Gynecol. 2025, 64, 11–26. [Google Scholar] [CrossRef]
- AlSaad, R.; Abusarhan, L.; Odeh, N.; Abd-Alrazaq, A.; Choucair, F.; Zegour, R.; Ahmed, A.; Aziz, S.; Sheikh, J. Deep learning applications for human embryo assessment using time-lapse imaging: Scoping review. Front. Reprod. Health 2025, 7, 1549642. [Google Scholar] [CrossRef] [PubMed]
- Baldán, F.J.; García-Gil, D.; Fernandez-Basso, C. Revolutionizing Sperm Analysis with AI: A Review of Computer-Aided Sperm Analysis Systems. Computation 2025, 13, 132. [Google Scholar] [CrossRef]
- Yetgin, A. Revolutionizing multi-omics analysis with artificial intelligence and data processing. Quant. Biol. 2025, 13, e70002. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial intelligence in reproductive medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef]
- Miao, H.; Liu, S.; Wang, Z.; Ke, Y.; Cheng, L.; Yu, W.; Yu, D.; Zhang, K.; Gao, Y.; Sun, Z. Artificial intelligence-derived retinal age gap as a marker for reproductive aging in women. NPJ Digit. Med. 2025, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.Y.; Liu, C.F.; Chung, M.T.; Tsai, Y.C. Artificial intelligence model to predict pregnancy and multiple pregnancy risk following in vitro fertilization-embryo transfer (IVF-ET). Taiwan J. Obstet. Gynecol. 2022, 61, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Ghayda, R.A.; Cannarella, R.; Calogero, A.E.; Shah, R.; Rambhatla, A.; Zohdy, W.; Kavoussi, P.; Avidor-Reiss, T.; Boitrelle, F.; Mostafa, T.; et al. Artificial Intelligence in Andrology: From Semen Analysis to Image Diagnostics. World J. Mens. Health 2024, 42, 39–61. [Google Scholar] [CrossRef]
- Worheide, M.A.; Krumsiek, J.; Kastenmuller, G.; Arnold, M. Multi-omics integration in biomedical research—A metabolomics-centric review. Anal. Chim. Acta 2021, 1141, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Derevyanko, A.; Skowronska, A.; Skowronski, M.T.; Kordowitzki, P. The Interplay between Telomeres, Mitochondria, and Chronic Stress Exposure in the Aging Egg. Cells 2022, 11, 2612. [Google Scholar] [CrossRef]
- Hew, Y.; Kutuk, D.; Duzcu, T.; Ergun, Y.; Basar, M. Artificial Intelligence in IVF Laboratories: Elevating Outcomes Through Precision and Efficiency. Biology 2024, 13, 988. [Google Scholar] [CrossRef]
- Wadden, J.J. Defining the undefinable: The black box problem in healthcare artificial intelligence. J. Med. Ethics 2022, 48, 764–768. [Google Scholar] [CrossRef]
- Rolfes, V.; Bittner, U.; Gerhards, H.; Krussel, J.S.; Fehm, T.; Ranisch, R.; Fangerau, H. Artificial Intelligence in Reproductive Medicine—An Ethical Perspective. Geburtshilfe Frauenheilkd 2023, 83, 106–115. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Jingwa, K.A.; Okoye, O.K.; John Okah, M.; Ladele, J.A.; Farah, A.H.; Alimi, H.A. Ethical implications of AI and robotics in healthcare: A review. Medicine 2023, 102, e36671. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.K.; Elshaikh, R.H.; Shalabi, M.G.; Abbas, A.M.; Prabhakar, P.K.; Babker, A.M.A.; Choudhary, R.K.; Gaur, V.; Choudhary, A.S.; Agarwal, S. Role of Artificial Intelligence and Personalized Medicine in Enhancing HIV Management and Treatment Outcomes. Life 2025, 15, 745. [Google Scholar] [CrossRef] [PubMed]
- Fasching, C.L. Telomere length measurement as a clinical biomarker of aging and disease. Crit. Rev. Clin. Lab. Sci. 2018, 55, 443–465. [Google Scholar] [CrossRef]
- Dragoumani, K.; Kletsas, D.; Chrousos, G.P.; Vlachakis, D.; Balatsos, N.A.A. Molecular and Environmental Modulators of Aging: Interplay Between Inflammation, Epigenetics, and RNA Stability. Genes 2025, 16, 796. [Google Scholar] [CrossRef]
- Boccardi, V.; Marano, L. Aging, Cancer, and Inflammation: The Telomerase Connection. Int. J. Mol. Sci. 2024, 25, 8542. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Spooner, B.; Isherwood, J.; Lane, M.; Orrock, E.; Dennison, A. A Systematic Review of the Barriers to the Implementation of Artificial Intelligence in Healthcare. Cureus 2023, 15, e46454. [Google Scholar] [CrossRef] [PubMed]
- Cabitza, F.; Campagner, A.; Soares, F.; Garcia de Guadiana-Romualdo, L.; Challa, F.; Sulejmani, A.; Seghezzi, M.; Carobene, A. The importance of being external. methodological insights for the external validation of machine learning models in medicine. Comput. Methods Programs Biomed. 2021, 208, 106288. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, K.; Akhlaghpour, S.; Fatehi, F. Patient consent for the secondary use of health data in artificial intelligence (AI) models: A scoping review. Int. J. Med. Inform. 2025, 198, 105872. [Google Scholar] [CrossRef] [PubMed]
- Cestonaro, C.; Delicati, A.; Marcante, B.; Caenazzo, L.; Tozzo, P. Defining medical liability when artificial intelligence is applied on diagnostic algorithms: A systematic review. Front. Med. 2023, 10, 1305756. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).