A Narrative Review of Biomarkers and Imaging in the Diagnosis of Acute Aortic Syndrome
Abstract
1. Introduction
2. Methodology
3. Epidemiology and Risk Factors
4. Pathologic Definitions
4.1. Dissection, Intramural Hematoma, and Penetrating Aortic Ulcer
4.2. Genetic-Associated Aortic Syndromes
4.3. Autoimmune Aortitis
5. Diagnosis
5.1. Laboratory Testing
5.1.1. D-Dimer
5.1.2. Homocysteine
5.1.3. Brain Natriuretic Peptide
5.1.4. Other Novel Potential Biomarkers
5.2. Imaging
5.2.1. X-Ray and Electrocardiography
5.2.2. Echocardiography
5.2.3. Computed Tomography
5.2.4. Magnetic Resonance Imaging (MRI)
6. Immuno-Metabolic Workup
7. Discussion
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, Y.; DeAnda, A., Jr. Revisiting the Death and Autopsy of King George II. Aorta 2021, 9, 196–198. [Google Scholar] [CrossRef]
- Vilacosta, I.; Aragoncillo, P.; Cañadas Román, S.; Ferreirós, J.A.; Rodríguez, J. Acute aortic syndrome: A new look at an old conundrum. Postgrad. Med. J. 2010, 86, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, V.; Parakh, A.; Prabhakar, A.M.; Hedgire, S. Acute aortic syndromes and aortic emergencies. Cardiovasc. Diagn. Ther. 2018, 8, S82–S96. [Google Scholar] [CrossRef] [PubMed]
- Vilacosta, I.; San Román, J.A.; Di Bartolomeo, R.; Eagle, K.; Estrera, A.L.; Ferrera, C.; Kaji, S.; Nienaber, C.A.; Riambau, V.; Schäfers, H.J.; et al. Acute aortic syndrome revisited: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 78, 2106–2125. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Dentamaro, I.; Masi, F.; Carbonara, S.; Ricci, G. Advances in the diagnosis of acute aortic syndromes: Role of imaging techniques. Vasc. Med. 2016, 21, 239–250. [Google Scholar] [CrossRef]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. ESC Scientific Document Group. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef]
- DeMartino, R.R.; Sen, I.; Huang, Y.; Bower, T.C.; Oderich, G.S.; Pochettino, A.; Greason, K.; Kalra, M.; Johnstone, J.; Shuja, F.; et al. Population-based assessment of the incidence of aortic dissection, intramural hematoma, and penetrating ulcer, and its associated mortality from 1995 to 2015. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004689. [Google Scholar] [CrossRef]
- Elbadawi, A.; Elgendy, I.Y.; Jimenez, E.; Omer, M.A.; Shahin, H.I.; Ogunbayo, G.O.; Paniagua, D.; Jneid, H. Trends and outcomes of elective thoracic aortic repair and acute thoracic aortic syndromes in the United States. Am. J. Med. 2021, 134, 902–909.e5. [Google Scholar] [CrossRef]
- Xu, W.; Haran, C.; Dean, A.; Lim, E.; Bernau, O.; Mani, K.; Khanafer, A.; Pitama, S.; Khashram, M. Acute aortic syndrome: Nationwide study of epidemiology, management, and outcomes. Br. J. Surg. 2023, 110, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Coutinho, T.; Chu, M.W.A.; Ouzounian, M. Sex differences in thoracic aortic disease: A review of the literature and a call to action. J. Thorac. Cardiovasc. Surg. 2020, 160, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Sokolis, D.P.; Iliopoulos, D.C. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J. Mech. Behav. Biomed. Mater. 2014, 34, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Stevens, L.M.; Ouzounian, M.; El-Hamamsy, I.; Bouhout, I.; Dagenais, F.; Cartier, A.; Peterson, M.D.; Boodhwani, M.; Guo, M.; et al. Sex-related differences in patients undergoing thoracic aortic surgery. Evidence from the Canadian thoracic aortic collaborative. Circulation 2019, 139, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Braatz, E.; Olsson, C.; Dalén, M.; Nielsen, S.J.; Jeppsson, A.; Stenman, M. Early clinical outcomes in men and women undergoing proximal thoracic aortic surgery: A Swedish population-based cohort study. J. Thorac. Cardiovasc. Surg. 2024, 168, 39396613. [Google Scholar] [CrossRef] [PubMed]
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K. The International registry of acute aortic dissection (IRAD): New insights into an old disease. JAMA 2000, 283, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute aortic syndromes: Diagnosis and management, an update. Eur. Heart J. 2018, 39, 739–749d. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: https://www.who.int/news/item/27-06-2023-landmark-road-safety-leader-s-summit-to-combat-road-crash-deaths-worldwide (accessed on 15 November 2024).
- Naidoo, S.; Hardcastle, T.C. Traumatic injury to the great vessels of the chest. Mediastinum 2021, 5, 26. [Google Scholar] [CrossRef]
- Vilacosta, I.; Ferrera, C.; San Román, A. Acute aortic syndrome. Med. Clin. 2024, 162, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Macura, K.J.; Corl, F.M.; Fishman, E.K.; Bluemke, D.A. Pathogenesis in acute aortic syndromes: Aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am. J. Roentgenol. 2003, 181, 309–316. [Google Scholar] [CrossRef]
- Chin, A.S.; Willemink, M.J.; Kino, A.; Hinostroza, V.; Sailer, A.M.; Fischbein, M.P.; Mitchell, R.S.; Berry, G.J.; Miller, D.C.; Fleischmann, D. Acute limited intimal tears of the thoracic aorta. J. Am. Coll. Cardiol. 2018, 71, 2773–2785. [Google Scholar] [CrossRef]
- Sundt, T.M. Intramural Hematoma and Penetrating Ulcer. In Advances in Understanding Aortic Diseases; Kazui, T., Takamoto, S., Eds.; Springer: Tokyo, Japan, 2009; p. 25. [Google Scholar] [CrossRef]
- Uchida, K.; Imoto, K.; Karube, N.; Minami, T.; Cho, T.; Goda, M.; Suzuki, S.; Masuda, M. Intramural haematoma should be referred to as thrombosed-type aortic dissection. Eur. J. Cardiothorac. Surg. 2013, 44, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pacini, D.; Foà, A.; Corsini, A.; Agostini, V.; Corti, B.; Di Marco, L.; Leone, A.; Lorenzini, M.; Reggiani, L.B.; et al. Redefining the histopathologic profile of acute aortic syndromes: Clinical and prognostic implications. J. Thorac. Cardiovasc. Surg. 2018, 156, 1776–1785.e6. [Google Scholar] [CrossRef] [PubMed]
- Vilacosta, I.; San Román, J.A.; Aragoncillo, P.; Ferreirós, J.; Mendez, R.; Graupner, C.; Batlle, E.; Serrano, J.; Pinto, A.; Oyonarte, J.M. Penetrating atherosclerotic aortic ulcer: Documentation by transesophageal echocardiography. J. Am. Coll. Cardiol. 1998, 32, 83–89. [Google Scholar] [CrossRef]
- Albornoz, G.; Coady, M.A.; Roberts, M.; Davies, R.R.; Tranquilli, M.; Rizzo, J.A.; Elefteriades, J.A. Familial thoracic aortic aneurysms and dissections—Incidence, modes of inheritance, and phenotypic patterns. Ann. Thorac. Surg. 2006, 82, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, C.G.E.; Dekker, S.; Bons, L.R.; Geenen, L.W.; Gökalp, A.L.; Takkenberg, J.J.M.; Mokhles, M.M.; Bekkers, J.A.; Boersma, E.; Bouwens, E.; et al. Novel biomarkers associated with thoracic aortic disease. Int. J. Cardiol. 2023, 378, 115–122. [Google Scholar] [CrossRef]
- Vyas, R.; Iris Ravindran, J.; Victoria Schmitt, L.; Osei-Bonsu, J.; Jaarah, N.; Munir, W.; Bashir, M.; Idhrees, M. Predictors and correlators of acute aortic syndrome: A critical review of current evidence. J. Heart Valve Dis. 2024, 29, 16–29. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the diagnosis and management of aortic disease: A Report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Pluchinotta, F.R.; Andreas, M.; Blanc, P.; Citro, R.; Limongelli, G.; Della Corte, A.; Parikh, A.; Frigiola, A.; Lerakis, S.; et al. Aortitis. Vascul. Pharmacol. 2016, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Fulbright, J.W.; Hunder, G.G.; Evans, J.M.; Goronzy, J.J. Treatment of giant cell arteritis: Interleukin-6 as a biologic marker of disease activity. Arthritis Rheum. 2000, 43, 1041–1048. [Google Scholar] [CrossRef]
- Mwipatayi, B.P.; Jeffery, P.C.; Beningfield, S.J.; Matley, P.J.; Naidoo, N.G.; Kalla, A.A.; Kahn, D. Takayasu arteritis: Clinical features and management: Report of 272 cases. ANZ J. Surg. 2005, 75, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Shchetynska-Marinova, T.; Amendt, K.; Sadick, M.; Keese, M.; Sigl, M. Aortitis—An interdisciplinary challenge. Vivo 2021, 35, 41–52. [Google Scholar] [CrossRef]
- Schmidt, J.; Sunesen, K.; Kornum, J.B.; Duhaut, P.; Thomsen, R.W. Predictors for pathologically confirmed aortitis after resection of the ascending aorta: A 12-year Danish nationwide population-based cross-sectional study. Arthritis Res. Ther. 2011, 13, R87. [Google Scholar] [CrossRef] [PubMed]
- Benhuri, B.; ELJack, A.; Kahaleh, B.; Chakravarti, R. Mechanism and biomarkers in aortitis—A review. J. Mol. Med. 2020, 98, 11–23. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Banceu, C.M.; Banceu, D.M.; Kauvar, D.S.; Popentiu, A.; Voth, V.; Liebrich, M.; Halic Neamtu, M.; Oprean, M.; Cristutiu, D.; Harpa, M.; et al. Acute Aortic Syndromes from Diagnosis to Treatment—A Comprehensive Review. J. Clin. Med. 2024, 13, 1231. [Google Scholar] [CrossRef]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Ann. Thorac. Surg. 2024, 118, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.M.; Hermann, L.K.; Booher, A.M.; Nienaber, C.A.; Williams, D.M.; Kazerooni, E.A.; Froehlich, J.B.; O’Gara, P.T.; Montgomery, D.G.; Cooper, J.V.; et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: Results from the international registry of acute aortic dissection. Circulation 2011, 123, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Eagle, K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Vagnarelli, F.; Corsini, A.; Bugani, G.; Lorenzini, M.; Longhi, S.; Bacchi Reggiani, M.L.; Biagini, E.; Graziosi, M.; Cinti, L.; Norscini, G.; et al. Troponin T elevation in acute aortic syndromes: Frequency and impact on diagnostic delay and misdiagnosis. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, H.; Qiu, Z.; Cheng, X. Prognostic role of neutrophil-to-lymphocyte ratio in aortic disease: A meta-analysis of observational studies. J. Cardiothorac. Surg. 2020, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Bedel, C.; Selvi, F. Association of platelet to lymphocyte and neutrophil to lymphocyte ratios with in-hospital mortality in patients with type A acute aortic dissection. Braz. J. Cardiovasc. Surg. 2019, 34, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Henry, B.M.; Hsieh, C.C.; Maruna, P.; Omara, M.; Lindner, J. Prognostic Role of admission c-reactive protein level as a predictor of in-hospital mortality in type-A acute aortic dissection: A meta-analysis. Vasc. Endovasc. Surg. 2019, 53, 547–557. [Google Scholar] [CrossRef]
- Wren, J.; Goodacre, S.; Pandor, A.; Essat, M.; Clowes, M.; Cooper, G.; Hinchliffe, R.; Reed, M.J.; Thomas, S.; Wilson, S. Diagnostic Accuracy of alternative biomarkers for acute aortic syndrome: A systematic review. Emerg. Med. J. 2024, 41, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Giusti, B.; Porciani, M.C.; Brunelli, T.; Evangelisti, L.; Fedi, S.; Gensini, G.F.; Abbate, R.; Sani, G.; Yacoub, M.; Pepe, G. Phenotypic variability of cardiovascular manifestations in Marfan Syndrome. Possible role of hyperhomocysteinemia and C677T MTHFR gene polymorphism. Eur. Heart J. 2003, 24, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Sbarouni, E.; Georgiadou, P.; Analitis, A.; Chaidaroglou, A.; Marathias, A.; Degiannis, D.; Voudris, V. High homocysteine and low folate concentrations in acute aortic dissection. Int. J. Cardiol. 2013, 168, 463–466. [Google Scholar] [CrossRef]
- Golledge, J.; Velu, R.; Quigley, F.; Jenkins, J.; Singh, T.P. The predictive value of four serum biomarkers for major adverse events in patients with small abdominal aortic aneurysm. J. Vasc. Surg. 2023, 77, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Zhou, X.L.; Li, J.J.; Luo, F.; Zhang, L.; Gao, L.G.; Wang, L.P.; Song, L.; Sun, K.; Zou, Y.B.; et al. Plasma concentrations of interleukin-6, C-reactive protein, tumor necrosis factor-α and matrix metalloproteinase-9 in aortic dissection. Clin. Chim. Acta 2012, 413, 198–202. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Chen, L.; Yan, L.L.; Qiu, Z.; Shen, Y.; Xie, X.; Xie, L. Efficacy of interleukin-6 in combination with D-dimer in predicting early poor postoperative prognosis after acute Stanford type A aortic dissection. J. Cardiothorac. Surg. 2020, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, J.; Fang, M.; Yang, J.; Wang, X.; Wang, S.; Yang, L. Procalcitonin, interleukin-6 and C-reactive protein levels predict renal adverse outcomes and mortality in patients with acute type A aortic dissection. Front. Surg. 2022, 9, 902108. [Google Scholar] [CrossRef]
- Suzuki, T.; Katoh, H.; Kurabayashi, M.; Yazaki, Y.; Nagai, R. Biochemical diagnosis of aortic dissection by raised concentrations of creatine kinase BB-isozyme. Lancet 1997, 350, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Katoh, H.; Tsuchio, Y.; Hasegawa, A.; Kurabayashi, M.; Ohira, A.; Hiramori, K.; Sakomura, Y.; Kasanuki, H.; Hori, S.; et al. Diagnostic implications of elevated levels of smooth-muscle myosin heavy-chain protein in acute aortic dissection. The smooth muscle myosin heavy chain study. Ann. Intern. Med. 2000, 133, 537–541. [Google Scholar] [CrossRef]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Salerno Uriarte, J.A.; de Luca Tupputi Schinosa, L.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) Investigators. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur. Heart J. 2008, 29, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Lian, R.; Zhang, T.; Liu, J.; Zhang, G.; Hu, T.; Li, G.; Zhang, S.; Zhang, G. Routine use of a pocket-sized handheld echoscopic device plus a biomarker by emergency medicine residents with an early screening algorithm for suspected Type A acute aortic syndrome. J. Clin. Med. 2023, 12, 1346. [Google Scholar] [CrossRef] [PubMed]
- Koullias, G.J.; Ravichandran, P.; Korkolis, D.P.; Rimm, D.L.; Elefteriades, J.A. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2004, 78, 2106–2110; discussion 2110–2111. [Google Scholar] [CrossRef]
- Giachino, F.; Loiacono, M.; Lucchiari, M.; Manzo, M.; Battista, S.; Saglio, E.; Lupia, E.; Moiraghi, C.; Hirsch, E.; Mengozzi, G.; et al. Rule out of acute aortic dissection with plasma matrix metalloproteinase 8 in the emergency department. Crit. Care 2013, 17, R33. [Google Scholar] [CrossRef] [PubMed]
- Proietta, M.; Tritapepe, L.; Cifani, N.; Ferri, L.; Taurino, M.; Del Porto, F. MMP-12 as a new marker of Stanford-A acute aortic dissection. Ann. Med. 2014, 46, 44–48. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Choi, J.C.; Minard, C.G.; Hou, X.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. Matrix metalloproteinase levels in chronic thoracic aortic dissection. J. Surg. Res. 2014, 189, 348–358. [Google Scholar] [CrossRef]
- Vianello, E.; Dozio, E.; Rigolini, R.; Marrocco-Trischitta, M.M.; Tacchini, L.; Trimarchi, S.; Corsi Romanelli, M.M. Acute phase of aortic dissection: A pilot study on CD40L, MPO, and MMP-1, -2, 9, and TIMP-1 circulating levels in elderly patients. Immun. Ageing 2016, 13, 9. [Google Scholar] [CrossRef]
- Zhang, W.H.; Qiao, C.H.; Zhang, X.; Luo, H.; Sun, X.K. The expression of MMP-7 in serum and aneurysm tissues of patients with abdominal aortic aneurysm associated with hypertension and the clinical efficacy of endovascular exclusion. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4623–4631. [Google Scholar] [PubMed]
- Li, T.; Jing, J.J.; Yang, J.; Sun, L.P.; Gong, Y.H.; Xin, S.J.; Yuan, Y. Serum levels of matrix metalloproteinase 9 and toll-like receptor 4 in acute aortic dissection: A case-control study. BMC Cardiovasc. Disord. 2018, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, D.; Yu, J.; Jiang, W.; Liu, Y.; Li, F.; Li, W.; Zeng, R.; Liao, X.; Wan, Z. Prognostic value of interleukin-33, sST2, myeloperoxidase, and matrix metalloproteinase-9 in acute aortic dissection. Front. Cardiovasc. Med. 2023, 9, 1084321. [Google Scholar] [CrossRef]
- Irqsusi, M.; Dong, L.A.; Rodepeter, F.R.; Ramzan, R.; Talipov, I.; Ghazy, T.; Günther, M.; Vogt, S.; Rastan, A.J. The Role of Matrix Metalloproteinases in Thoracic Aortic Disease: Are They Indicators for the Pathogenesis of Dissections? Biomedicines 2024, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, X.; Gao, H.; Yuan, H.; Hu, R.; Jia, L.; Zhu, J.; Sun, L.; Zhang, H.; Huang, L.; et al. Magnitude of Soluble ST2 as a Novel Biomarker for Acute Aortic Dissection. Circulation 2018, 137, 259–269. [Google Scholar] [CrossRef]
- Morello, F.; Bartalucci, A.; Bironzo, M.; Santoro, M.; Pivetta, E.; Ianniello, A.; Rumbolo, F.; Mengozzi, G.; Lupia, E. Prospective diagnostic accuracy study of plasma soluble ST2 for diagnosis of acute aortic syndromes. Sci. Rep. 2020, 10, 3103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, L.; Dai, C.; Zhang, Y.; Han, P.; Huang, Y.; Liu, H.; Wang, L. Diagnostic potential of soluble ST2 and D-dimer for Stanford Type B aortic dissection and intramural aortic hematoma. Microvasc. Res. 2024, 151, 104623. [Google Scholar] [CrossRef]
- Sodeck, G.; Laggner, H.; Schillinger, M.; Ehrlich, M.P.; Endler, G.; Herkner, H.; Laggner, A. D-dimer in ruling out acute aortic dissection: A systematic review and prospective cohort study. Eur. Heart J. 2007, 28, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, W.; Wang, L. Biomarkers in aortic dissection: Diagnostic and prognostic value from clinical research. Chin. Med. J. 2024, 137, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Alfonso, F.; Boileau, C.; Dirsch, O.; Eber, B.; Haverich, A.; Rakowski, H.; Struyven, J.; Radegran, K.; Sechtem, U.; et al. Diagnosis and management of aortic dissection: Task Force on Aortic Dissection, European Society of Cardiology. Eur. Heart J. 2001, 22, 1642–1681. [Google Scholar] [CrossRef]
- Weber, T.; Högler, S.; Auer, J.; Berent, R.; Lassnig, E.; Kvas, E.; Eber, B. D-dimer in acute aortic dissection. Chest 2003, 123, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Naber, C.K.; Bruch, C.; Kröger, K.; von Birgelen, C.; Schmermund, A.; Wichert, M.; Bartel, T.; Mann, K.; Erbel, R. Value of plasma fibrin D-dimers for detection of acute aortic dissection. J. Am. Coll. Cardiol. 2004, 44, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Salerno Uriarte, J.A.; De Luca Tupputi Schinosa, L.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. IRAD-Bio Investigators. Diagnosis of acute aortic dissection by D-dimer: The International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009, 119, 2702–2707. [Google Scholar] [CrossRef]
- Yao, J.; Bai, T.; Yang, B.; Sun, L. The diagnostic value of D-dimer in acute aortic dissection: A meta-analysis. J. Cardiothorac. Surg. 2021, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A. D-dimer testing in laboratory practice. Clin. Chem. 2011, 57, 1256–1262. [Google Scholar] [CrossRef]
- Nazerian, P.; Mueller, C.; Soeiro, A.M.; Leidel, B.A.; Salvadeo, S.A.T.; Giachino, F.; Vanni, S.; Grimm, K.; Oliveira, M.T., Jr.; Pivetta, E.; et al. ADvISED Investigators. Diagnostic Accuracy of the Aortic Dissection Detection Risk Score Plus D-Dimer for Acute Aortic Syndromes: The ADvISED Prospective Multicenter Study. Circulation 2018, 137, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Essat, M.; Pandor, A.; Goodacre, S.; Ren, S.; Clowes, M.; Bima, P.; Toyofuku, M.; McLatchie, R.; Bossone, E. Diagnostic accuracy of the aortic dissection detection risk score alone or with D-dimer for acute aortic syndromes: Systematic review and meta-analysis. PLoS ONE 2024, 19, e0304401. [Google Scholar] [CrossRef] [PubMed]
- Essat, M.; Goodacre, S.; Pandor, A.; Ren, S.; Ren, S.; Clowes, M. Diagnostic Accuracy of D-Dimer for Acute Aortic Syndromes: Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2024, 84, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, R.; Kimura, N.; Mieno, M.; Hori, D.; Itoh, S.; Akiyoshi, K.; Yuri, K.; Tanno, K.; Kawahito, K.; Yamaguchi, A. Characteristics and treatment outcomes of acute Type A aortic dissection with elevated D-dimer concentration. J. Am. Heart Assoc. 2018, 7, e009144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, Z.; Wen, M.; Zhang, H.; Wang, L.; Zhang, N.; Li, L.; Luo, W.; Jiang, W.; Zhang, H.; et al. Association between preoperative D-dimer with morphologic features and surgical outcomes of acute type A aortic dissection. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae193. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Q.; Li, C.; Wu, J.; Kuang, J.; Yang, J.; Fan, R. Significant prediction of in-hospital major adverse events by D-Dimer level in patients with acute Type A aortic dissection. Front. Cardiovasc. Med. 2022, 9, 821928. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Abrishami, S.; Subramanian, L.; Mahadevaiah, A.; Vyas, A.; Jain, A.; Nathaniel, S.; Gnanaguruparan, S.; Desai, R. Impact of D-dimer on in-hospital mortality following aortic dissection: A systematic review and meta-analysis. World J. Cardiol. 2024, 16, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.D.; Foucault-Bertaud, A.; Genovesio, C.; Dignat-George, F.; Lamy, E.; Charpiot, P. Homocysteine modulates the proteolytic potential of human arterial smooth muscle cells through a reactive oxygen species dependent mechanism. Mol. Cell. Biochem. 2010, 335, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Chae, I.H.; Kim, H.S.; Park, Y.B.; Choi, Y.S.; Lee, Y.W.; Park, S.J.; Cha, Y.J. Differential effects of homocysteine on porcine endothelial and vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2002, 39, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H. Homocysteine—From disease biomarker to disease prevention. J. Intern. Med. 2021, 290, 826–854. [Google Scholar] [CrossRef]
- Takagi, H.; Umemoto, T. Homocysteinemia is a risk factor for aortic dissection. Med. Hypotheses 2005, 64, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Leip, E.P.; Benjamin, E.J.; Wilson, P.W.; Sutherland, P.; Omland, T.; Vasan, R.S. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am. J. Cardiol. 2002, 90, 254–258. [Google Scholar] [CrossRef]
- Sbarouni, E.; Georgiadou, P.; Marathias, A.; Geroulanos, S.; Kremastinos, D.T. D-dimer and BNP levels in acute aortic dissection. Int. J. Cardiol. 2007, 122, 170–172. [Google Scholar] [CrossRef]
- Sodeck, G.; Domanovits, H.; Schillinger, M.; Janata, K.; Thalmann, M.; Ehrlich, M.P.; Endler, G.; Laggner, A. Pre-operative N-terminal pro-brain natriuretic peptide predicts outcome in type A aortic dissection. J. Am. Coll. Cardiol. 2008, 51, 1092–1097. [Google Scholar] [CrossRef]
- Goyal, A.; Jain, H.; Usman, M.; Zuhair, V.; Sulaiman, S.A.; Javed, B.; Mubbashir, A.; Abozaid, A.M.; Passey, S.; Yakkali, S.; et al. A comprehensive exploration of novel biomarkers for the early diagnosis of aortic dissection. Hell. J. Cardiol. 2024, in press. [CrossRef] [PubMed]
- Forrer, A.; Schoenrath, F.; Torzewski, M.; Schmid, J.; Franke, U.F.W.; Göbel, N.; Aujesky, D.; Matter, C.M.; Lüscher, T.F.; Mach, F.; et al. Novel blood biomarkers for a diagnostic workup of acute aortic dissection. Diagnostics 2021, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Peng, Z.; Chai, X.; Zhu, Q.; Yang, G.; Zhao, Q.; Zhou, S. Potential biomarkers for early diagnosis of acute aortic dissection. Heart Lung 2015, 44, 205–208. [Google Scholar] [CrossRef]
- Yuan, S.M. Profiles and predictive values of interleukin-6 in aortic dissection: A review. Braz. J. Cardiovasc. Surg. 2019, 34, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.J.; Zhu, J.J.; Li, X.M.; Yang, Y.N. Predictive value of interleukin-6 for mortality in patients with acute aortic dissection. Chin. J. Clin. Electron. Ed. 2015, 9, 3184–3187. [Google Scholar] [CrossRef]
- Song, D.H.; Choi, J.H.; Lee, J.Y. Predicting acute aortic syndrome using aortic dissection detection risk score, D-dimer, and X-ray. Heliyon 2023, 9, e20578. [Google Scholar] [CrossRef]
- Morello, F.; Santoro, M.; Fargion, A.T.; Grifoni, S.; Nazerian, P. Diagnosis and management of acute aortic syndromes in the emergency department. Intern. Emerg. Med. 2021, 16, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, P.; Pivetta, E.; Veglia, S.; Cavigli, E.; Mueller, C.; de Matos Soeiro, A.; Leidel, B.A.; Lupia, E.; Rutigliano, C.; Wussler, D.; et al. ADvISED Investigators. Integrated Use of Conventional Chest Radiography Cannot Rule Out Acute Aortic Syndromes in Emergency Department Patients at Low Clinical Probability. Acad. Emerg. Med. 2019, 26, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.A.; Albaroudi, B.; Shaban, E.E.; Alkahlout, B.H.; Yigit, Y.; Elnabawy, W.; Basharat, K.; Almarri, N.D.; Azad, A.M. Comparison between transthoracic echocardiography and transoesophageal echocardiography in the diagnosis of acute aortic dissection from an emergency perspective: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 10, 1283703. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Yang, F.; Fujikawa, T.; Wong, M.C.; Yu, S.C.; Underwood, M.J.; Lee, A.P. Pocket-Size Mobile Echocardiographic Screening of Thoracic Aortic Aneurysms in Hypertensive Patients. Ann. Thorac. Surg. 2021, 111, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Sitges, M.; Jondeau, G.; Nijveldt, R.; Pepi, M.; Cuellar, H.; Pontone, G.; Bossone, E.; Groenink, M.; Dweck, M.R.; et al. Multimodality imaging in thoracic aortic diseases: A clinical consensus statement from the European Association of Cardiovascular Imaging and the European Society of Cardiology working group on aorta and peripheral vascular diseases. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e65–e85. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Czerny, M.; Lerakis, S.; Rodríguez-Palomares, J.; Kukar, N.; Ranieri, B.; Russo, V.; Punzo, B.; Cocchia, R.; Cademartiri, F.; et al. Imaging and Biomarkers in Acute Aortic Syndromes: Diagnostic and Prognostic Implications. Curr. Probl. Cardiol. 2021, 46, 100654. [Google Scholar] [CrossRef]
- Sathiadoss, P.; Haroon, M.; Wongwaisayawan, S.; Krishna, S.; Sheikh, A.M. Multidetector Computed Tomography in Traumatic and Nontraumatic Aortic Emergencies: Emphasis on Acute Aortic Syndromes. Can. Assoc. Radiol. J. 2020, 71, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Pourafkari, L.; Tajlil, A.; Ghaffari, S.; Parvizi, R.; Chavoshi, M.; Kolahdouzan, K.; Khaki, N.; Parizad, R.; Hobika, G.G.; Nader, N.D. The frequency of initial misdiagnosis of acute aortic dissection in the emergency department and its impact on outcome. Intern. Emerg. Med. 2017, 12, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Mastrodicasa, D.; Codari, M.; Bäumler, K.; Sandfort, V.; Shen, J.; Mistelbauer, G.; Hahn, L.D.; Turner, V.L.; Desjardins, B.; Willemink, M.J.; et al. Artificial Intelligence Applications in Aortic Dissection Imaging. Semin. Roentgenol. 2022, 57, 357–363. [Google Scholar] [CrossRef]
- Hu, Y.; Xiang, Y.; Zhou, Y.J.; He, Y.; Yang, S.; Du, X.; Den, C.; Xu, Y.; Wang, G.; Ding, Z.; et al. Rapid and Accurate Diagnosis of Acute Aortic Syndrome Using Non-contrast CT: A Large-scale, Retrospective, Multi-center and AI-based Study. arXiv 2024, arXiv:2406.15222. [Google Scholar] [CrossRef]
- Quintana, R.A.; Taylor, W.R. Cellular mechanisms of aortic aneurysm formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Li, Y.; Zhang, C.; Li, Y.; LeMaire, S.A.; Shen, Y.H. Programmed cell death in aortic aneurysm and dissection: A potential therapeutic target. J. Mol. Cell. Cardiol. 2022, 163, 67–80. [Google Scholar] [CrossRef]
- Wang, Y.X.; Martin-McNulty, B.; da Cunha, V.; Vincelette, J.; Lu, X.; Feng, Q.; Halks-Miller, M.; Mahmoudi, M.; Schroeder, M.; Subramanyam, B.; et al. Fasudil, a Rho-kinase inhibitor, attenuates angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice by inhibiting apoptosis and proteolysis. Circulation 2005, 111, 2219–2226. [Google Scholar] [CrossRef]
- Xiong, W.; Mactaggart, J.; Knispel, R.; Worth, J.; Zhu, Z.; Li, Y.; Sun, Y.; Baxter, B.T.; Johanning, J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009, 202, 128–134. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Zhao, L.; Wang, L.; Li, Y.; Cho, K.; Tao, C.; Jiang, B. Targeted depletion of monocyte/macrophage suppresses aortic dissection with the spatial regulation of MMP-9 in the aorta. Life Sci. 2020, 254, 116927. [Google Scholar] [CrossRef] [PubMed]
- Moltzer, E.; Essers, J.; van Esch, J.H.; Roos-Hesselink, J.W.; Danser, A.H. The role of the renin-angiotensin system in thoracic aortic aneurysms: Clinical implications. Pharmacol. Ther. 2011, 131, 50–60. [Google Scholar] [CrossRef]
- Zeng, Q.; Rong, Y.; Li, D.; Wu, Z.; He, Y.; Zhang, H.; Huang, L. Identification of serum biomarker in acute aortic dissection by global and targeted metabolomics. Ann. Vasc. Surg. 2020, 68, 497–504. [Google Scholar] [CrossRef]
- Wang, Q.; Yesitayi, G.; Liu, B.; Siti, D.; Ainiwan, M.; Aizitiaili, A.; Ma, X. Targeting metabolism in aortic aneurysm and dissection: From basic research to clinical applications. Int. J. Biol. Sci. 2023, 19, 3869–3891. [Google Scholar] [CrossRef]
- Balint, B.; Jepchumba, V.K.; Guéant, J.L.; Guéant-Rodriguez, R.M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wu, Y.; Zhang, Y.; Youn, J.Y.; Cai, H. Combination of folic acid with nifedipine is completely effective in attenuating aortic aneurysm formation as a novel oral medication. Redox Biol. 2022, 58, 102521. [Google Scholar] [CrossRef]
- Van Hove, J.L.K.; Freehauf, C.L.; Ficicioglu, C.; Pena, L.D.M.; Moreau, K.L.; Henthorn, T.K.; Christians, U.; Jiang, H.; Cowan, T.M.; Young, S.P.; et al. Biomarkers of oxidative stress, inflammation, and vascular dysfunction in inherited cystathionine β-synthase deficient homocystinuria and the impact of taurine treatment in a phase 1/2 human clinical trial. J. Inherit. Metab. Dis. 2019, 42, 424–437. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Wang, X.; Zhang, H.; Liu, E.; Su, X. Homocysteine up-regulates endothelin type A receptor in vascular smooth muscle cells through Sirt1/ERK1/2 signaling pathway. Microvasc. Res. 2017, 114, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Q.; Cao, Y.; Si, J.; Li, J.; Cao, K.; Pang, X. Probucol decreases homocysteine-stimulated CRP production in rat aortic smooth muscle cells via regulating HO-1/NADPH oxidase/ROS/p38 pathway. Acta Biochim. Biophys. Sin. 2021, 53, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.; Ding, Y.; Wang, Q.; Zhang, W.; Song, P.; Zou, M.H. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ. Res. 2014, 114, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Sun, C.; Zhu, H.; Zhai, M.; Zhang, L.; Jiang, L.; Hou, P.; Li, J.; Li, K.; Liu, Z.; et al. Melatonin protects against thoracic aortic aneurysm and dissection through SIRT1-dependent regulation of oxidative stress and vascular smooth muscle cell loss. J. Pineal Res. 2020, 69, e12661. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Kanuri, S.H.; Mehta, J.L. Role of Ox-LDL and LOX-1 in Atherogenesis. Curr. Med. Chem. 2019, 26, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Jovin, I.S.; Duggal, M.; Ebisu, K.; Paek, H.; Oprea, A.D.; Tranquilli, M.; Rizzo, J.; Memet, R.; Feldman, M.; Dziura, J.; et al. Comparison of the effect on long-term outcomes in patients with thoracic aortic aneurysms of taking versus not taking a statin drug. Am. J. Cardiol. 2012, 109, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Meital, L.T.; Schulze, K.; Magee, R.; O’Donnell, J.; Jha, P.; Meital, C.Y.; Donkin, R.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Long chain omega-3 polyunsaturated fatty acids improve vascular stiffness in abdominal aortic aneurysm: A randomized controlled trial. Nutrients 2020, 13, 138. [Google Scholar] [CrossRef]
- Nettersheim, F.S.; Lemties, J.; Braumann, S.; Geißen, S.; Bokredenghel, S.; Nies, R.; Hof, A.; Winkels, H.; Freeman, B.A.; Klinke, A.; et al. Nitro-oleic acid reduces thoracic aortic aneurysm progression in a mouse model of Marfan syndrome. Cardiovasc. Res. 2022, 118, 2211–2225. [Google Scholar] [CrossRef]
- Tsuruda, T.; Hatakeyama, K.; Nagamachi, S.; Sekita, Y.; Sakamoto, S.; Endo, G.J.; Nishimura, M.; Matsuyama, M.; Yoshimura, K.; Sato, Y.; et al. Inhibition of development of abdominal aortic aneurysm by glycolysis restriction. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bae, K.H.; Byun, J.K.; Lee, S.; Kim, J.G.; Lee, I.K.; Jung, G.S.; Lee, Y.M.; Park, K.G. Lactate dehydrogenase-A is indispensable for vascular smooth muscle cell proliferation and migration. Biochem. Biophys. Res. Commun. 2017, 492, 41–47. [Google Scholar] [CrossRef]
- He, Y.; Ma, C.; Xing, J.; Wang, S.; Ji, C.; Han, Y.; Zhang, J. Serum amyloid A protein as a potential biomarker in predicting acute onset and association with in-hospital death in acute aortic dissection. BMC Cardiovasc. Disord. 2019, 19, 282. [Google Scholar] [CrossRef]
- Peng, S.; Larsson, A.; Wassberg, E.; Gerwins, P.; Thelin, S.; Fu, X.; Westermark, P. Role of aggregated medin in the pathogenesis of thoracic aortic aneurysm and dissection. Lab. Investig. 2007, 87, 1195–1205. [Google Scholar] [CrossRef]
- Merlini, G.; Westermark, P. The systemic amyloidoses: Clearer understanding of the molecular mechanisms offers hope for more effective therapies. J. Intern. Med. 2004, 255, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Chen, Y.; Li, K.; Zhan, R.; Zhao, M.; Xu, Y.; Lin, Z.; Fu, Y.; He, Q.; Tang, P.C.; et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur. Heart J. 2021, 42, 4373–4385. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Atkins, A.D.; Reardon, M.J.; Atkins, M.D. Endovascular Management of the Ascending Aorta: State of the Art. Methodist DeBakey Cardiovasc. J. 2023, 19, 29–37. [Google Scholar] [CrossRef]
- Giudici, A.; Spronck, B.; Wilkinson, I.B.; Khir, A.W. Tri-layered constitutive modelling unveils functional differences between the pig ascending and lower thoracic aorta. J. Mech. Behav. Biomed. Mater. 2023, 141, 105752. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef]
- Sterpetti, A.V.; Gabriele, R.; Sapienza, P.; Marzo, L.D.; Borrelli, V. Mortality and burden related with aortic aneurysms and dissections: The importance of information and education. Curr. Probl. Cardiol. 2024, 49, 102384. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Half of patients with acute aortic dissection in England die before reaching a specialist centre. BMJ 2020, 368, m304. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; Thordsen, S.; Thomas, T.; Mackey-Bojack, S.M.; Duncanson, E.R.; Nwuado, D.; Garberich, R.F.; Harris, K.M. Clinical and pathologic findings of aortic dissection at autopsy: Review of 336 cases over nearly 6 decades. Am. Heart J. 2019, 209, 108–115. [Google Scholar] [CrossRef]
- Ramos Gonzalez-Cristobal, I.; Ferrera, C.; Carrero, A.; Gutierrez, F.; Gonzalez Del Castillo, J.; Dominguez, M.J.; Martinez, I.; Cobiella, J.; Noriega, F.J.; Viana-Tejedor, A.; et al. Impact of misdiagnosis on the treatment and prognosis of acute aortic syndrome. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, zuae036.118. [Google Scholar] [CrossRef]
- Carrel, T.; Sundt, T.M., 3rd; von Kodolitsch, Y.; Czerny, M. Acute aortic dissection. Lancet 2023, 401, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, S.; Wong, C.W.; Schwarz, K.; Borovac, J.A.; Lo, T.; Gunning, M.; Phan, T.; Patwala, A.; Barker, D.; Mallen, C.D.; et al. Misdiagnosis of aortic dissection: A systematic review of the literature. Am. J. Emerg. Med. 2022, 53, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Yamamoto, Y.; Okamatsu, H.; Okabe, M. D-dimer is helpful for differentiating acute aortic dissection and acute pulmonary embolism from acute myocardial infarction. Hell. J. Cardiol. 2011, 52, 123–127. [Google Scholar] [PubMed]
- Kaito, D.; Yamamoto, R.; Nakama, R.; Hashizume, K.; Ueno, K.; Sasaki, J. D-dimer for screening of aortic dissection in patients with ST-elevation myocardial infarction. Am. J. Emerg. Med. 2022, 59, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Kirchberger, I.; Warm, T.D.; Hyhlik-Dürr, A.; Goßlau, Y.; Linseisen, J. Elevated plasma D-dimer concentrations in adults after an outpatient-treated COVID-19 infection. Viruses 2022, 14, 2441. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Bima, P.; Castelli, M.; Nazerian, P. Acute aortic syndromes: An internist’s guide to the galaxy. Eur. J. Intern. Med. 2022, 106, 45–53. [Google Scholar] [CrossRef]
- Morello, F.; Bima, P.; Castelli, M.; Capretti, E.; de Matos Soeiro, A.; Cipriano, A.; Costantino, G.; Vanni, S.; Leidel, B.A.; Kaufmann, B.A.; et al. Diagnosis of acute aortic syndromes with ultrasound and D-dimer: The PROFUNDUS study. Eur. J. Intern. Med. 2024, 128, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Nazerian, P.; Lupia, E.; Castelli, M.; Mills, N.L.; Mueller, C. Study group on biomarkers of the ESC association for acute cardiovascular care. Biomarkers for diagnosis and prognostication of acute aortic syndromes. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.P.; Goldstein, J.M.; Latson, L.A., Jr.; Azour, L.; Gozansky, E.K.; Moore, W.; Patel, S.; Hutchinson, B. Chest CT angiography for acute aortic pathologic conditions: Pearls and pitfalls. Radiographics 2021, 41, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, J.G.; Rodrigues, J.C.L.; Roditi, G. Emergency CT misdiagnosis in acute aortic syndrome. Br. J. Radiol. 2021, 94, 20201294. [Google Scholar] [CrossRef]
- Lanzafame, L.R.M.; Bucolo, G.M.; Muscogiuri, G.; Sironi, S.; Gaeta, M.; Ascenti, G.; Booz, C.; Vogl, T.J.; Blandino, A.; Mazziotti, S.; et al. Artificial Intelligence in Cardiovascular CT and MR Imaging. Life 2023, 13, 507. [Google Scholar] [CrossRef]
- Marume, K.; Noguchi, T.; Kaichi, R.; Yano, T.; Matsuyama, M.; Nagamine, Y.; Mori, T.; Mikami, T.; Ikebe, S.; Takae, M.; et al. Women with acute aortic dissection have higher prehospital mortality than men. JACC Adv. 2023, 2, 100623. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A.; Barrett, P.W.; Kopf, G.S. Litigation in nontraumatic aortic diseases—A tempest in the malpractice maelstrom. Cardiology 2008, 109, 263–272. [Google Scholar] [CrossRef]
- Proietti, R.; Field, M.; McKay, V.; Lip, G.Y.H.; Kuduvalli, M. Screening for the vulnerable aorta: Targeting high-risk groups in the population. Br. J. Cardiol. 2023, 30, 25. [Google Scholar] [CrossRef] [PubMed]
- Almasi, M.Y.; Pirola, S.; Sasidharan, S.; Fisichella, S.M.; Redaelli, A.; Jarral, O.A.; O’Regan, D.P.; Oo, A.Y.; Moore, J.E., Jr.; Xu, X.Y.; et al. High wall shear stress can predict wall degradation in ascending aortic aneurysms: An integrated biomechanics study. Front. Bioeng. Biotechnol. 2021, 9, 750656. [Google Scholar] [CrossRef]
- Neiroukh, D.; Hajdarpasic, A.; Ayhan, C.; Sultan, S.; Soliman, O. Gut microbial taxonomy and its role as a biomarker in aortic diseases: A systematic review and future perspectives. J. Clin. Med. 2024, 13, 6938. [Google Scholar] [CrossRef] [PubMed]
- Pukaluk, A.; Sommer, G.; Holzapfel, G.A. Multimodal experimental studies of the passive mechanical behavior of human aortas: Current approaches and future directions. Acta Biomater. 2024, 178, 1–12. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, R.; Zhuang, D.; Zhu, J.; Zheng, H.; Chen, J. The recent 10-year landscape of aortic dissection research: A bibliometric analysis. J. Thorac. Dis. 2021, 13, 1592–1602. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, Z.; Shi, Y.; Zhu, H. Diagnostic biomarkers and aortic dissection: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 497. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, A.; Koenen, R.R. Extracellular vesicles as biomarkers in cardiovascular disease; Chances and risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Stendahl, J.C.; Sinusas, A.J. Nanoparticles for cardiovascular imaging and therapeutic delivery, Part 1: Compositions and features. J. Nucl. Med. 2015, 56, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
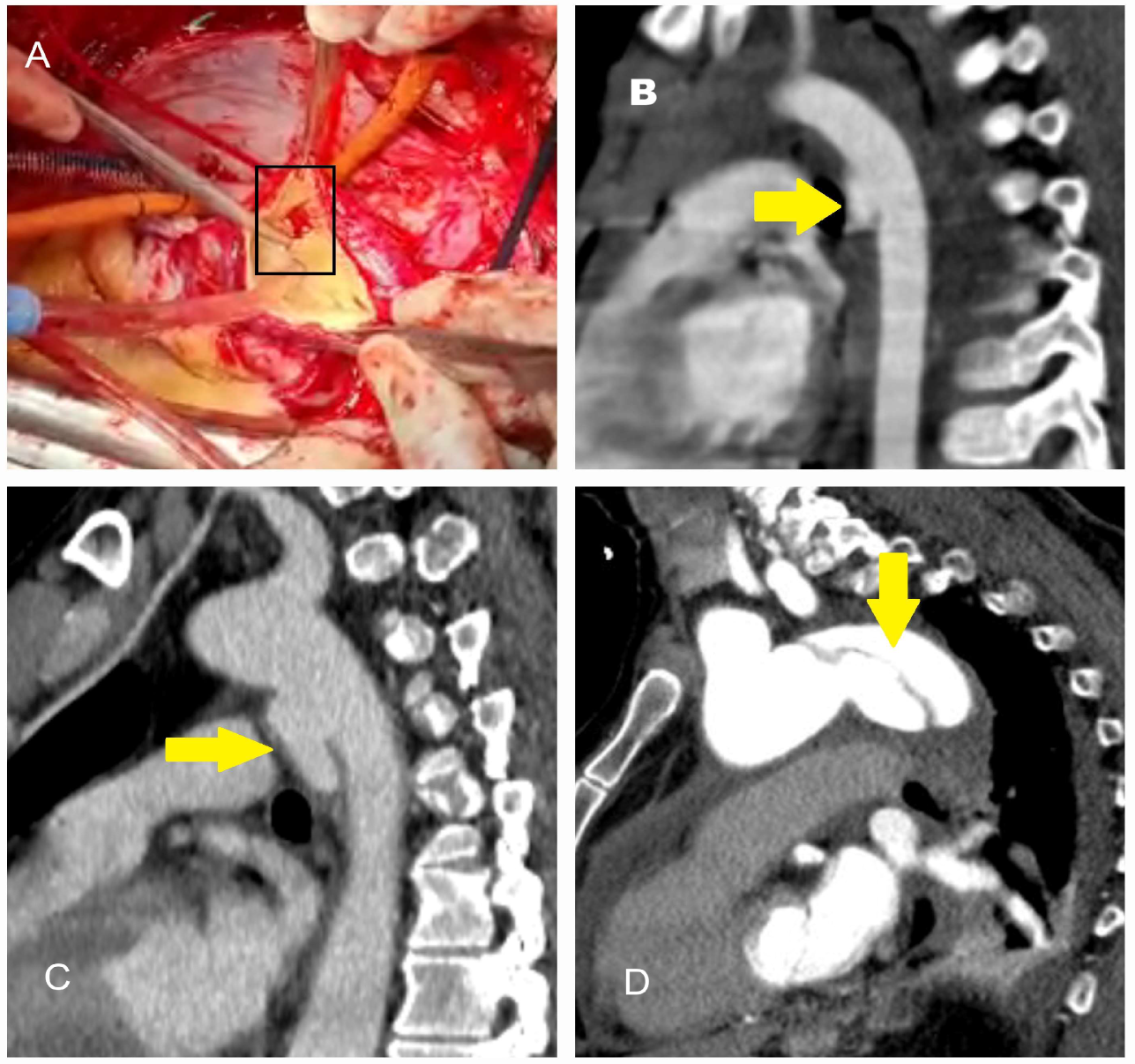
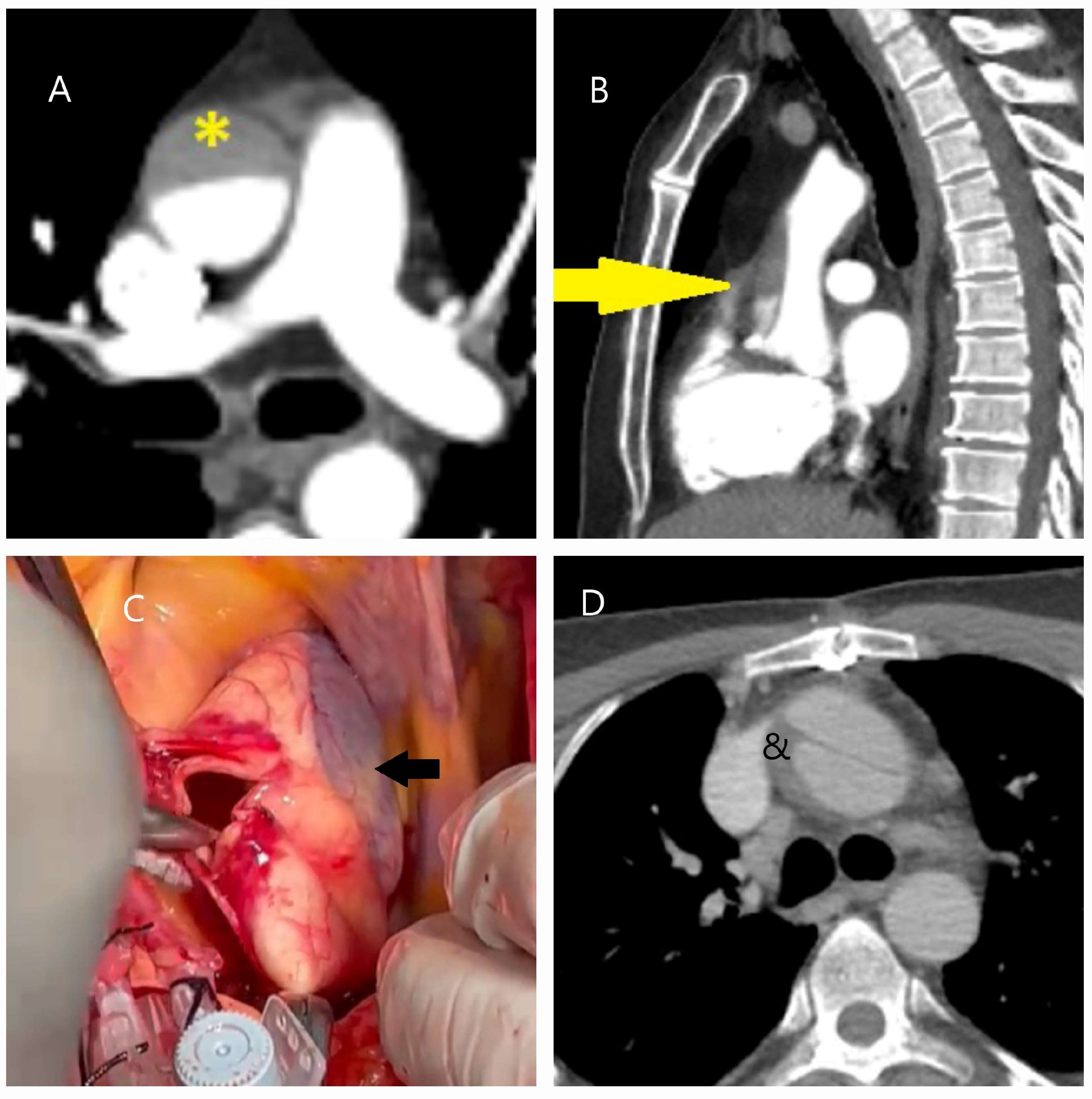
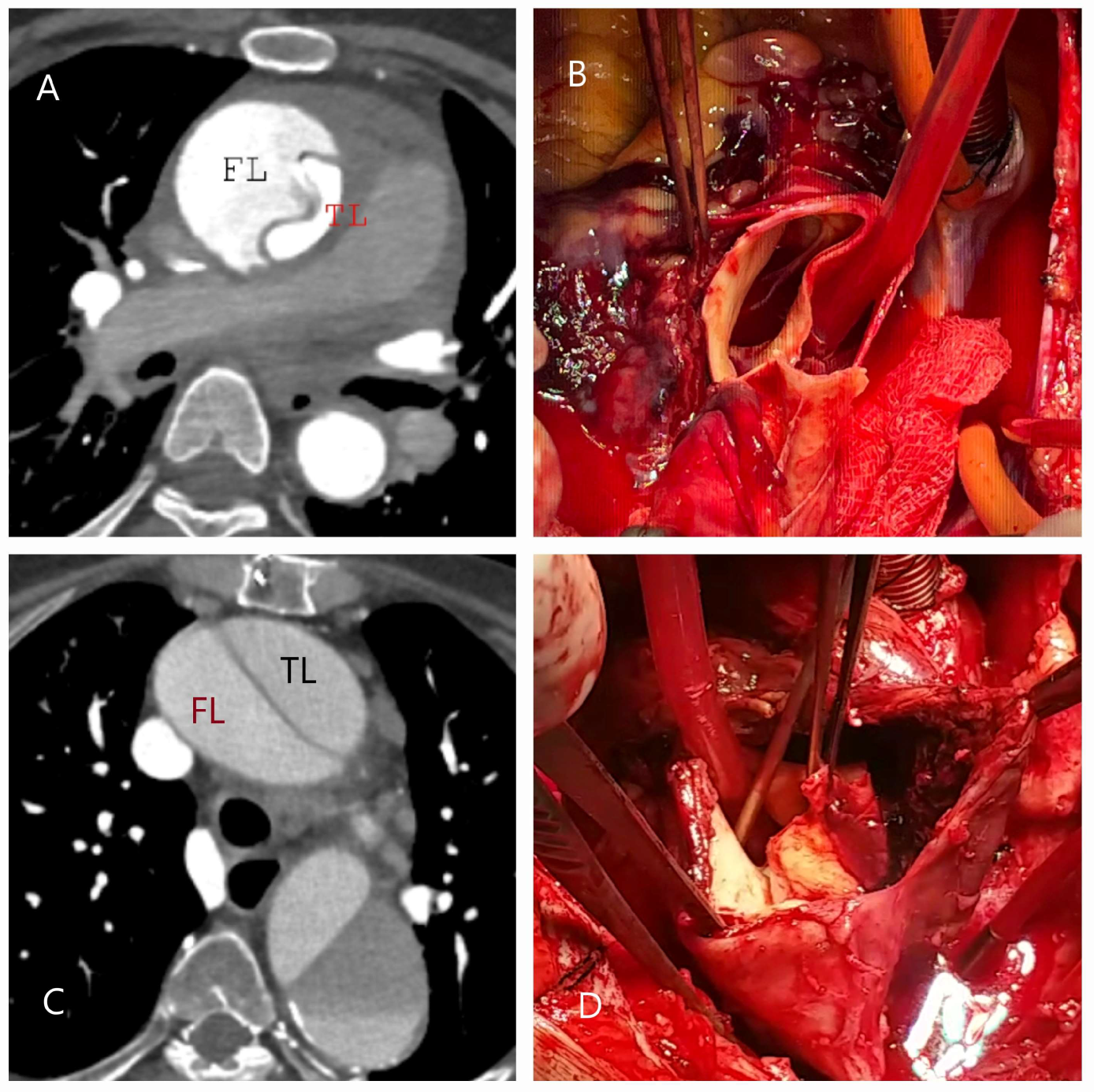

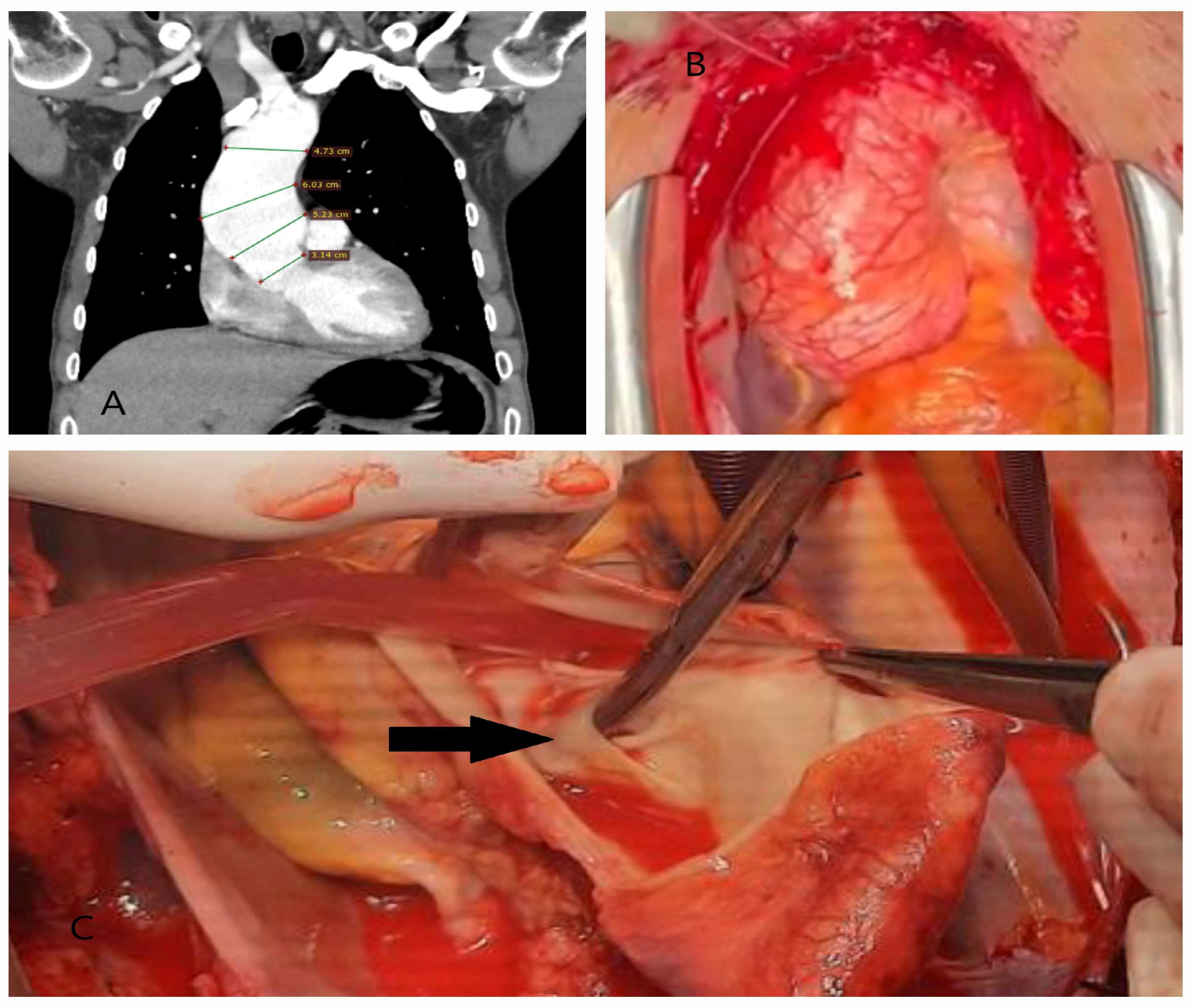
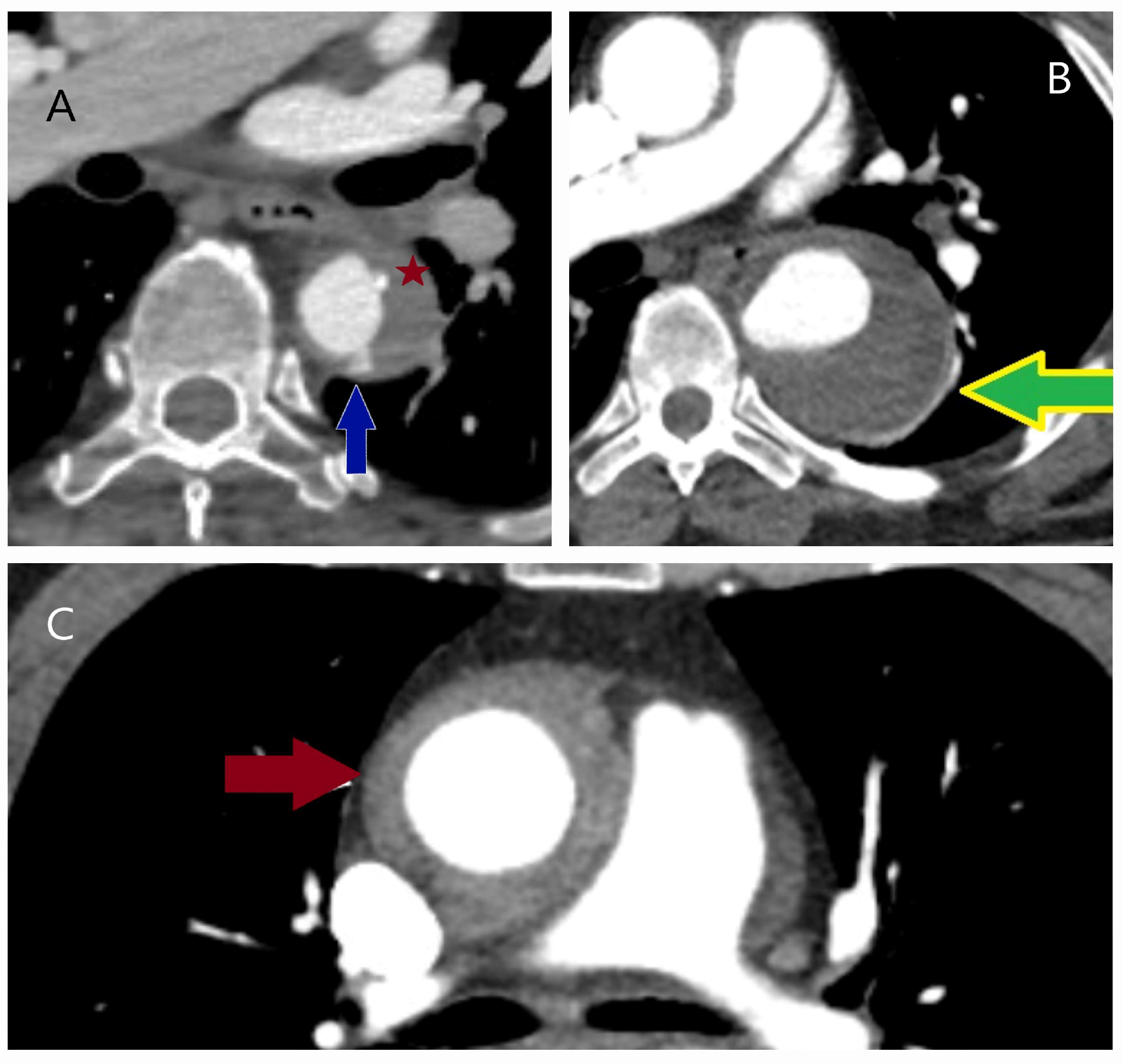
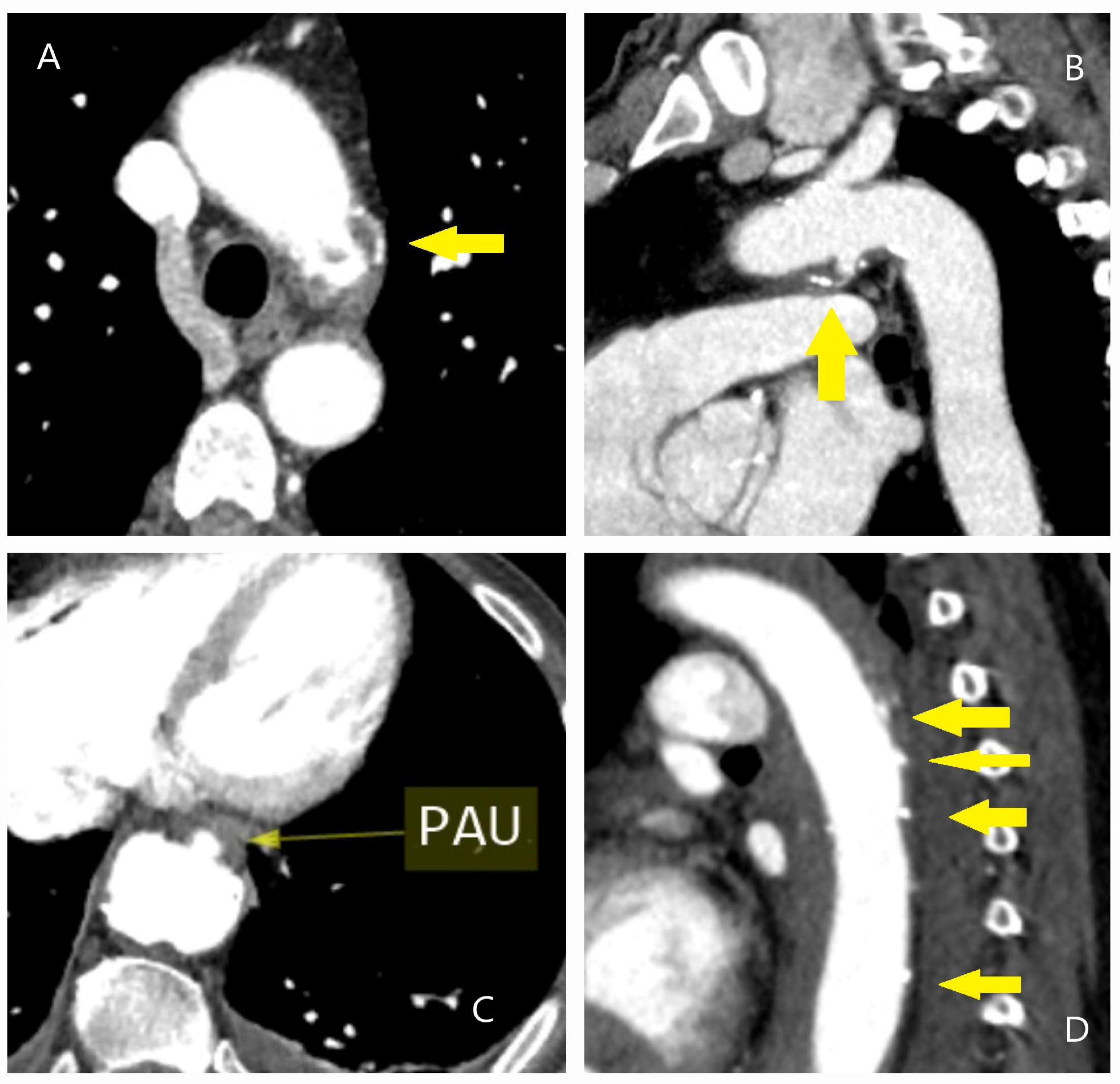
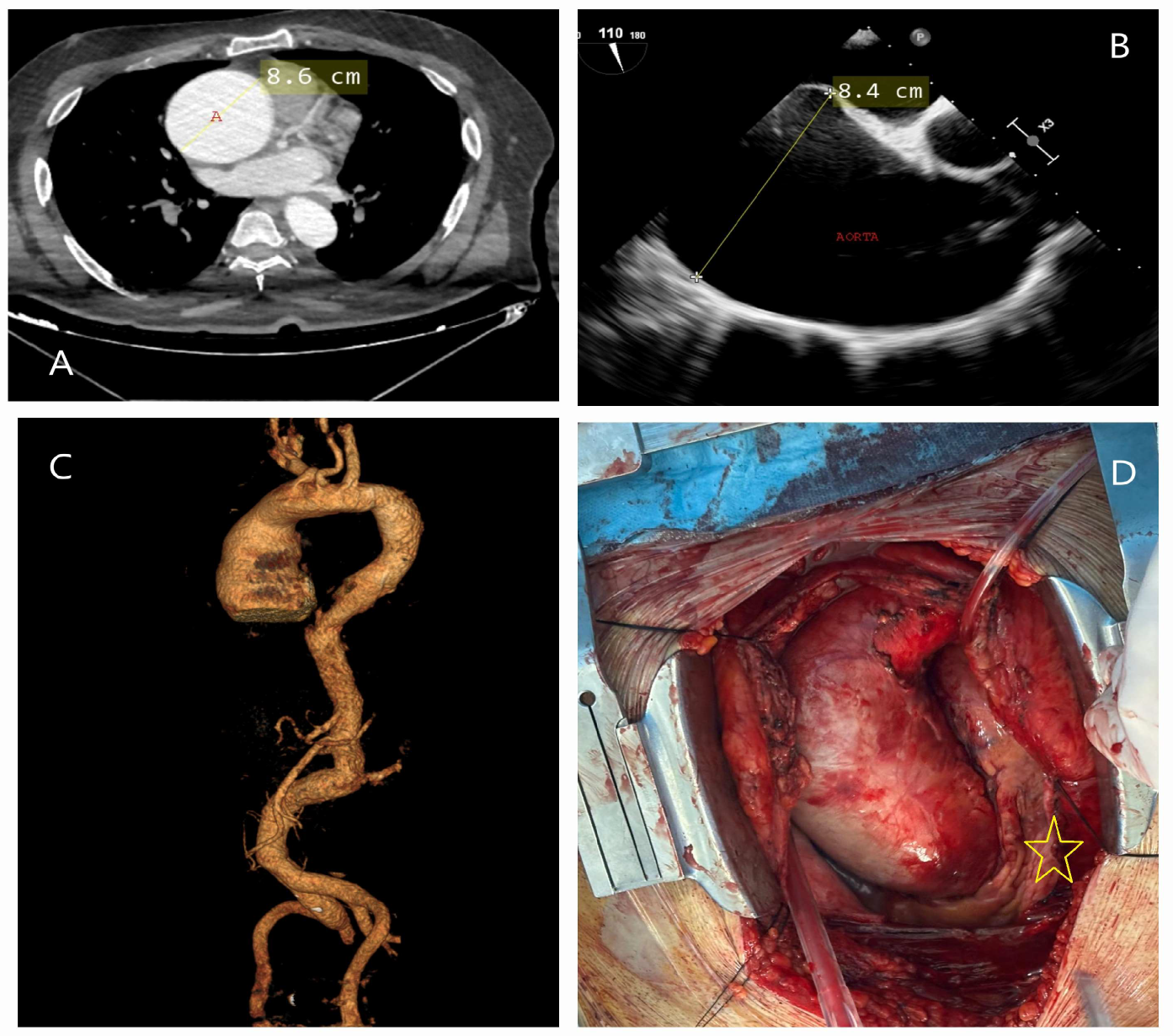

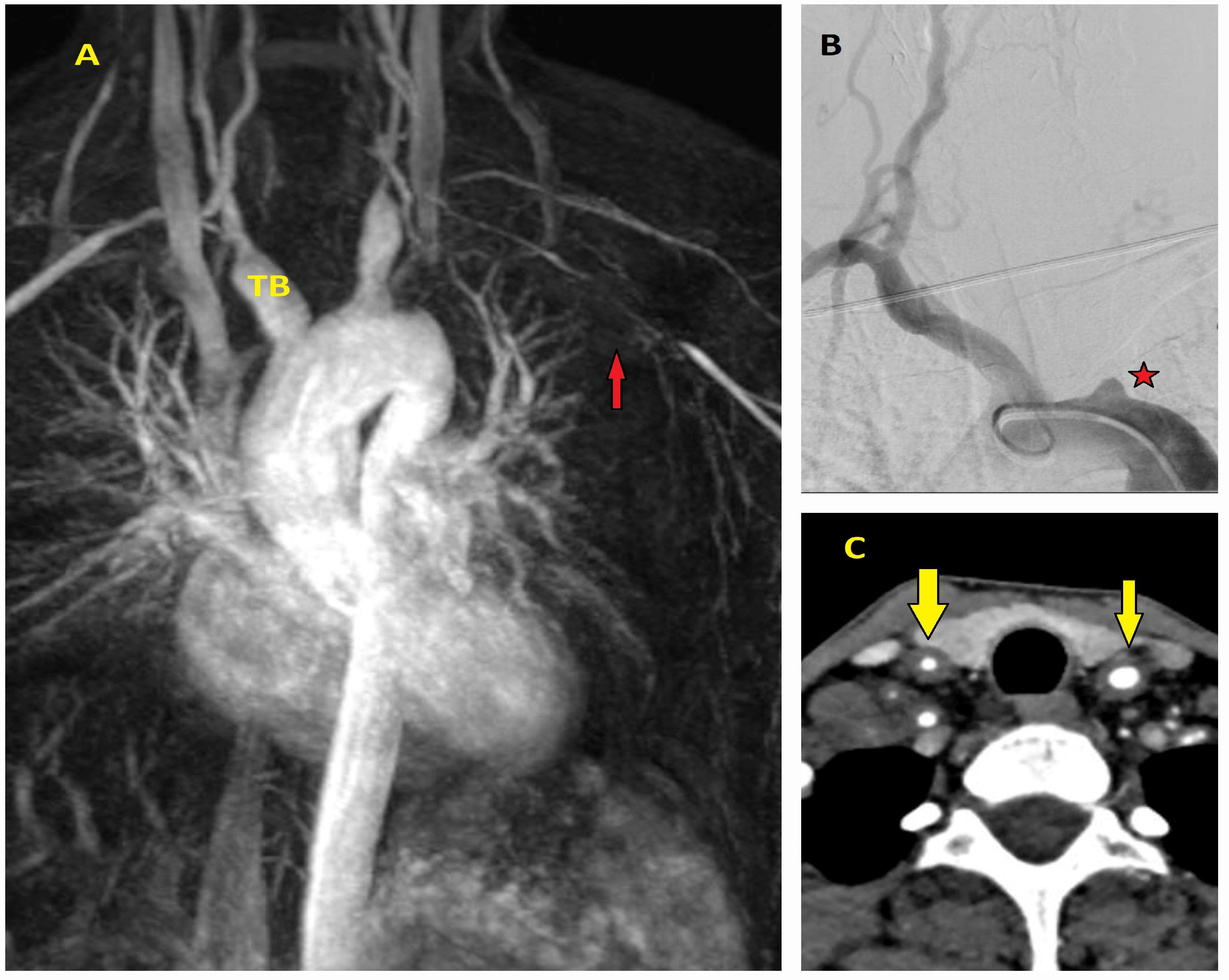




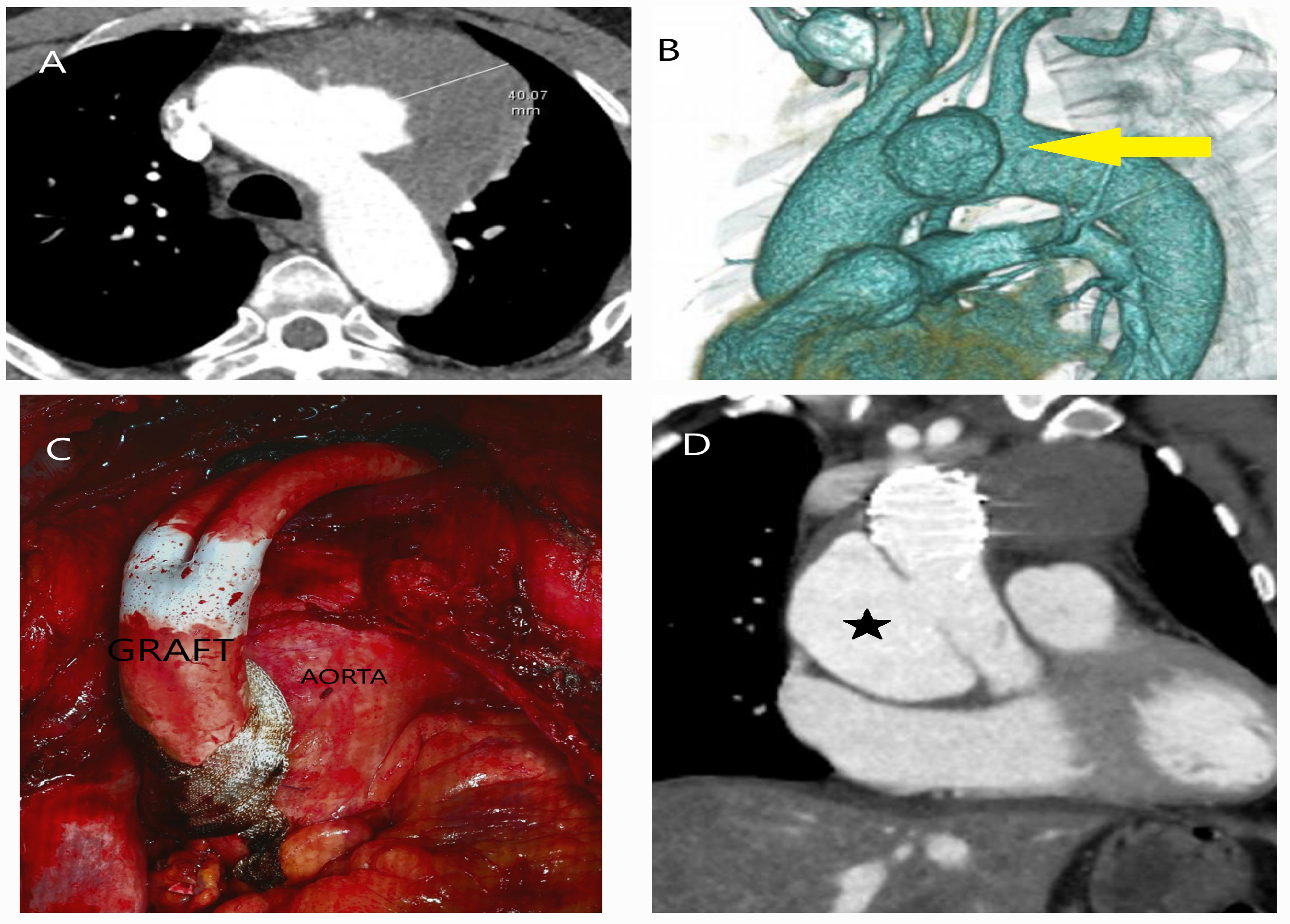

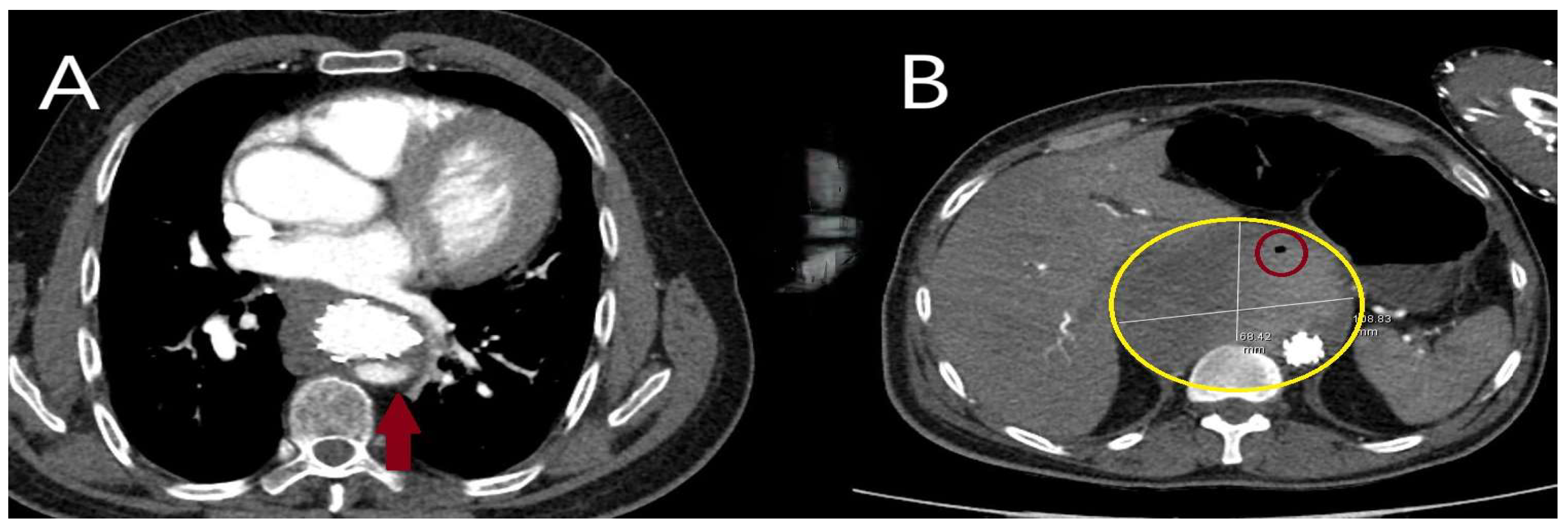

| Classification | Description |
|---|---|
| Type (T) | Type A, type B, non-A, non-B |
| Entry Tear (E) | E0: No entry tear E1: Entry tear in the ascending aorta (the area from the aortic valve to the proximal brachiocephalic trunk) E2: Entry tear in the aortic arch (the area from the brachiocephalic trunk to the left subclavian artery) E3: The aorta distal to the left subclavian artery |
| Malperfusion (M) | M0: No radiological or clinical evidence of malperfusion M1: Cardiac malperfusion (M1+: with ischemic changes, M1-: without ischemic changes) M2: Supra-aortic malperfusion (with or not cerebral or upper extremity) M3: Visceral or lower extremity malperfusion; dissection or false lumen origin of at least one visceral, renal, or iliac artery, or collapse of the aortic true lumen leading to functional closure of at least one branch of the visceral, renal, or iliac arteries |
| Biomarker | Study (Year/Design) | Participant | Cutoff Value | Type (Diagnostic/Prognostic) | Remarks |
|---|---|---|---|---|---|
| Homocysteine (μmol/L) | Giusti et al. [46] (2003) Prospective | 107 Marfan 189 healthy | 13.5 | Both | In hyperhomocysteinemic Marfan patients, the risk of aortic dissection was found to be approximately 4.5 times higher (OR 4.46, 95% CI 1.30–15.27, p = 0.017) |
| Sbarouni et al. [47] (2013) Prospective | 31 acute AD 30 AsAA 20 healthy | 22 | Diagnostic | The values in cases of acute dissection were 22 ± 14 compared to 17 ± 5 in chronic aortic aneurysm (p = 0.05) | |
| Golledge et al. [48] (2023) Prospective | 471 with AbAA (30 to 54 mm) | 15 | Prognostic | In patients with small abdominal aortic aneurysms, the risk of major adverse cardiovascular events (MACE) is higher, with each 5.6 μM increase in homocysteine associated with a 29% increase in MACE risk (OR: 1.29, 95% CI: 1.13–1.48, p < 0.001) | |
| IL-6 (pg/mL) | Wen et al. [49] (2012) Prospective | 64 acute AD 42 chronic AD 98 hypertension 96 healthy | 10.9 | Diagnostic | 3.3-fold increase in acute dissection compared to healthy individuals (p < 0.05) |
| Wu et al. [50] (2020) Retrospective | 141 type A AD | >108 | Prognostic | a marker of early poor postoperative prognosis (area under the curve 0.901, 95% CI; 0.839–0.963), especially in combination with high D-dimer levels | |
| Chen et al. [51] (2022) Retrospective | 331 type A AD | >259 | Prognostic | Peak IL-6 > 259 pg/mL was identified as an independent risk factor for 30-day mortality; however, it was not predictive of renal injury | |
| Creatine kinase-BB isozyme (IU/L) | Suzuki et al. [52] (1997) Prospective | 10 acute AD 20 control | 3.4 | Diagnostic | It was measured at higher concentrations in aortic dissections (a 7.8-fold), and peak concentrations were observed 6-12 h after the onset of the disease |
| Smooth muscle myosin heavy chain (µg/L) | Suzuki et al. [53] (2020) Cross-Sectional | 95 acute AD 48 AMI 131 healthy | 2.5 | Diagnostic | High in patients with acute AD presenting within 3 h, 25-fold increase compared to healthy individuals with 90.9% sensitivity. The test showed 98% specificity and 96% accuracy at a 2.5 mg/L threshold for aortic dissection diagnosis |
| Calponin (smooth muscle troponin-like protein; ng/mL) | Suzuki et al. [54] (2008) Prospective | 59 acute AD 158 control | 2,8 (acidic) 159 (basic) | Diagnostic | Optimal acidic calponin values showed 50% sensitivity and 87% specificity at 2.8 ng/mL, while basic calponin values showed 63% sensitivity and 73% specificity at 159 ng/mL (for 6 h). Acidic and basic calponins increased over two- and three-fold, respectively, within the first 6 h in AD |
| Lian et al. [55] (2023) Retrospective | 49 AAS 130 non-AAS | 6.96 (acidic) | Diagnostic | In AAS, a two-fold increase compared to non-AAS was observed, with a cutoff value of 6.96 ng/mL (under the ROC curve: 88.9%) | |
| Matrix metalloproteinases (ng/mL) | Koullias et al. [56] (2004) Histopathologic analysis | 30 thoracic aneurysm 17 dissection 7 young cadavers | Diagnostic | The aortic walls in patients with dissection exhibit significantly higher levels of MMP2 and MMP9 expression compared to those with non-dissecting aneurysms | |
| Wen et al. [49] (2012) Prospective | 64 acute AD 42 chronic AD 98 hypertension 96 healthy | 37.7 55.7 107.2 | Both | MMP9 concentrations are higher in patients with chronic AD (55.7) than acute AD (37.7) and increase immediately after surgical treatment or stenting. Among treated patients who died, the level was notably high (107.2) | |
| Giachino et al. [57] (2013) Prospective | 52 acute AD 74 non-AD | 3.6 (MMP8) 20 (MMP9) | Diagnostic | In acute AD, MMP8 levels were 2.75 times higher, and MMP9 levels were 2 times higher. At a cutoff value of 11.0 ng/mL, the negative predictive value of MMP8 reaches 100% when used in combination with D-dimer | |
| Proietta et al. [58] (2014) Prospective | 23 AD 21 CAS 21 CVRF 10 healthy | 20.4 | Diagnostic | MMP12 shows a sixfold increase in AD patients compared to healthy individuals and is a potential biomarker for AD, especially in those without genetic predisposition | |
| Zhang et al. [59] (2014) Case–control | 25 DTAA 17 organ-donor | Diagnostic | Dissection tissue exhibits increased levels of total MMP1 and MMP9, decreased MMP2, and immunostaining revealing higher expression of MMP-1, -3, -9, -12, and -13 in the media of the false lumen’s outer aortic wall compared to control tissue | ||
| Vianello et al. [60] (2016) Prospective | 22 type A AD 11 type B AD 30 healthy | 1.5–2 | Diagnostic | In acute AD, MMP1 levels are elevated, particularly in type A and within the first 24 h, whereas MMP2 and MMP9 do not show significant increases compared to controls | |
| Zhang et al. [61] (2017) Retrospective | 72 AbAA 72 HT 72 healthy | 2.8 | Diagnostic | In hypertensive patients with AbAA patients, the blood level of MMP7 is 5.5 times higher than in healthy individuals and is highly expressed in diseased aortic tissue | |
| Li et al. [62] (2018) Case–control | 88 AD 88 healthy | 379.4 | Diagnostic | AUC for MMP9: 0.810 (sensitivity: 68.2%, specificity: 84.1%); elevated in AD, correlated with CRP, not with D-dimer | |
| Jia et al. [63] (2023) Prospective | 155 acute AD | 16.6 | Both | High MMP9 levels are both an independent risk factor for mortality and a significant positive correlation with aortic diameter and false lumen area | |
| Irqsusi et al. [64] (2024) Histopathologic analysis | 52 AD 52 AsAA 7 CAD | Diagnostic | Higher expression of MMP1 and MMP9 is observed in the adventitia, and elevated MMP9 levels in the media, particularly in patients with AD | ||
| Soluble suppression of tumorigenesis-2 (sST2); (IL)–1 receptor family member (ng/mL) | Wang et al. [65] (2018) Hybrid cohorts | 1027 retrospective 333 prospective | 34.6 | Diagnostic | High in acute dissection compared to AMI and PE; sensitivity of 99.1%, specificity of 84.9%. Using sST2 at around 35 ng/mL can exclude aortic dissection with a negative likelihood ratio of <0.1 and a negative predictive value of >90% |
| Morello et al. [66] (2020) Prospective | 88 AAS 209 non-AAS | Different levels | Diagnostic | It is elevated in AAS patients, but in ROC analysis, the AUC of sST2 was 0.63 (D-dimer: 0.82, p < 0.001), indicating modest accuracy, with sensitivity ranging from 35.2 to 95.5% and specificity from 10.8 to 85.1% across different cutoffs | |
| Zhu et al. [67] (2024) Retrospective | 90 type B AD 92 IMH 90 non-AAS | 27.54 | Diagnostic | sST2 levels increase with the onset of type B AD (sensitivity of 80.92% and specificity of 75.00%), but its combination with D-dimer demonstrates strong diagnostic performance in intramural aortic hematoma (sensitivity, 69.20%; specificity, 80.00%) |
| Imaging Modality | Sensitivity (%) | Specificity (%) | Advantages | Disadvantages |
|---|---|---|---|---|
| Computed Tomography (CT) | 95–100 | 98–99 | Rapid, widely available, high resolution. Provides detailed anatomical information for dissection and rupture. | Radiation exposure, contrast nephropathy, or anaphylaxis risk. Motion artifacts can affect image quality. |
| Magnetic Resonance Imaging (MRI) | 97–100 | 94–100 | No radiation exposure, excellent soft tissue contrast. Ideal for soft tissue abnormalities such as intramural hematomas. | Limited availability, time-consuming, and contraindicated in unstable patients. Expensive infrastructure requirements. |
| Transthoracic Echocardiography (TTE) | 60–90 (for ascending aorta) 30–60 (for descending aorta) | 80–96 60–80 | Non-invasive, widely available. Useful for initial evaluation and bedside use. | Limited accuracy for distal aorta, operator-dependent. Poor imaging in obese or chronic obstructive pulmonary disease patients. |
| Transesophageal Echocardiography (TEE) | 86–100 | 90–100 | High resolution for proximal aorta, accessible in critical settings. Effective for real-time blood flow assessment. | Semi-invasive, requires sedation or anesthesia. Cannot be used in patients with esophageal pathology. |
| Chest X-Ray (CXR) | 30–50 | 60–70 | Low-cost, quick, initial screening tool. Often used to rule out obvious abnormalities. | Low sensitivity and specificity, non-diagnostic in many cases. Limited ability to assess complex aortic pathology. |
| Positron Emission Tomography (PET) | 85–95 | 80–90 | Detects inflammatory and infectious processes, functional imaging. Useful for vasculitis and other systemic diseases and can detect inflammation in aortic syndromes. | Expensive, limited availability, requires radioactive tracers. Long preparation and imaging times. |
| Invasive Aortography | 85–95 | >95 | Gold standard for vascular imaging. Provides detailed lumen and vascular anatomy with high resolution. | Invasive procedure requiring arterial access. Associated with risks like bleeding, infection, and contrast nephropathy. Not provide any information on aortic wall thickness. |
| Metabolite | Effect | Potential Treatment | References |
|---|---|---|---|
| Hyperhomocysteinemia |
| Vitamin B6 Vitamin B12 Folic acid Antioxidants (vitamin E and C/polyphenols) Taurine Probucol | Balint et al. [114] Huang et al. [115] Van Hove et al. [116] Chen et al. [117] Li et al. [118] |
| Kynurenine–tryptophan pathway |
| Kynureninase Melatonin | Wang et al. [119] Xia et al. [120] |
| Impaired lipid metabolism |
| Statins Long-chain omega-3 polyunsaturated fatty acid Nitro-oleic acid | Kattoor et al. [121] Jovin et al. [122] Meital et al. [123] Nettersheim et al. [124] |
| Glycolytic activity |
| Glycolysis inhibitor 2-deoxyglucose (2-DG) Small interfering RNA (siRNA) | Tsuruda et al. [125] Kim et al. [126] |
| Amyloid |
| A treatment protocol is determined based on the location of involvement (e.g., colchicine, melphalan, steroids, etc.). | He et al. [127] Peng et al. [128] Merlini et al. [129] |
| Succinate |
| Dimethyl malonate | Cui et al. [130] Chouchani et al. [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, Ü.; Jalalzai, I. A Narrative Review of Biomarkers and Imaging in the Diagnosis of Acute Aortic Syndrome. Diagnostics 2025, 15, 183. https://doi.org/10.3390/diagnostics15020183
Arslan Ü, Jalalzai I. A Narrative Review of Biomarkers and Imaging in the Diagnosis of Acute Aortic Syndrome. Diagnostics. 2025; 15(2):183. https://doi.org/10.3390/diagnostics15020183
Chicago/Turabian StyleArslan, Ümit, and Izatullah Jalalzai. 2025. "A Narrative Review of Biomarkers and Imaging in the Diagnosis of Acute Aortic Syndrome" Diagnostics 15, no. 2: 183. https://doi.org/10.3390/diagnostics15020183
APA StyleArslan, Ü., & Jalalzai, I. (2025). A Narrative Review of Biomarkers and Imaging in the Diagnosis of Acute Aortic Syndrome. Diagnostics, 15(2), 183. https://doi.org/10.3390/diagnostics15020183