Sarcopenia as a Potential Risk Factor for Denosumab-Related Osteonecrosis of the Jaw in Asian Prostate Cancer Patients with Bone Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Population and Data Collection
2.3. CT-Based Body Composition Measurements
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Comparison Between Patients with and Without DRONJ
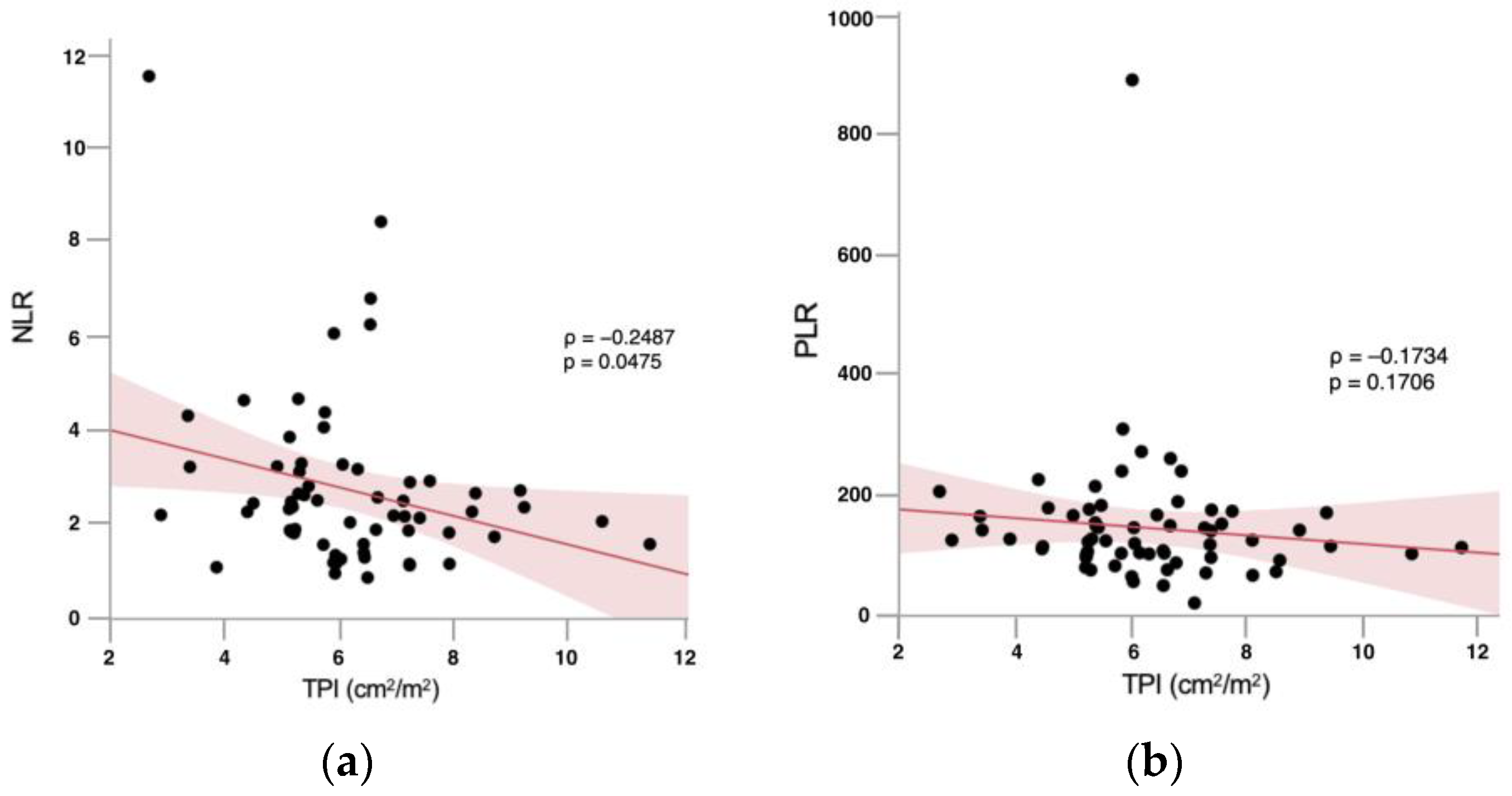
3.3. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClung, M. Role of RANKL inhibition in osteoporosis. Arthritis Res. Ther. 2007, 9, S3. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Felsenfeld, A.L.; Tetradis, S. Osteonecrosis of the jaw in a patient on denosumab. J. Oral Maxillofac. Surg. 2010, 68, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Taylor, K.H.; Middlefell, L.S.; Mizen, K.D. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br. J. Oral Maxillofac. Surg. 2010, 48, 221–223. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ position paper on medication-related osteonecrosis of the jaws—2022 update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Saia, G.; Blandamura, S.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Ferronato, G.; Nocini, P.F.; Bedogni, A. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J. Oral Maxillofac. Surg. 2010, 68, 797–804. [Google Scholar] [CrossRef]
- Laskou, F.; Fuggle, N.R.; Patel, H.P.; Jameson, K.; Cooper, C.; Dennison, E. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J. Cachexia Sarcopenia Muscle 2022, 13, 220–229. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Phu, S.; Bani-Hassan, E.; Brennan-Olsen, S.L.; Zanker, J.; Vogrin, S.; Conzade, R.; Kirk, B.; Al-Saedi, A.; Probst, V.; et al. The Joint Occurrence of Osteoporosis and Sarcopenia (Osteosarcopenia): Definitions and Characteristics. J. Am. Med. Dir. Assoc. 2020, 21, 220–225. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Lin, Y.; Zhong, X.; Lu, D.; Yao, W.; Zhou, J.; Wu, R.; Feng, F. Association of visceral and subcutaneous fat with bone mineral density in US adults: A cross-sectional study. Sci. Rep. 2023, 13, 10682. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Li, C.; Zhang, H.; Liu, Y.; Wei, J.M. Total Psoas Area Index is Valuable to Assess Sarcopenia, Sarcopenic Overweight/Obesity and Predict Outcomes in Patients Undergoing Open Pancreatoduodenectomy. Risk Manag. Healthc. Policy 2020, 13, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, W.; Hirota, Y.; Miyazaki, S.; Nakamura, T.; Ogawa, Y.; Shimomura, I.; Yamauchi, T.; Yokote, K.; Creation Committee for Guidelines for the Management of Obesity Disease 2022 by Japan Society for the Study of Obesity (JASSO). Definition, criteria, and core concepts of guidelines for the management of obesity disease in Japan. Endocr. J. 2024, 71, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.Y.; Ho, M.; Chau, P.H. The Prevalence of Sarcopenia in Chinese Older Adults: Meta-Analysis and Meta-Regression. Nutrients 2021, 13, 1441. [Google Scholar] [CrossRef]
- Yamashita, J.; McCauley, L.K. Antiresorptives and osteonecrosis of the jaw. J. Evid. Based Dent. Pract. 2012, 12 (Suppl. S3), 233–247. [Google Scholar] [CrossRef]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Fizazi, K.; Body, J.; Brown, J.E.; Carducci, M.; Diel, I.; Fujiwara, Y.; Martín, M.; Paterson, A.; Tonkin, K.; et al. Safety of long-term denosumab therapy: Results from the open-label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support. Care Cancer 2016, 24, 447–455. [Google Scholar]
- Bracchi, P.; Zecca, E.; Brunelli, C.; Miceli, R.; Tinè, G.; Maniezzo, M.; Lo Dico, S.; Caputo, M.; Shkodra, M.; Caraceni, A.T. A real-world study on the prevalence and risk factors of medication-related osteonecrosis of the jaw in cancer patients with bone metastases treated with Denosumab. Cancer Med. 2023, 12, 18317–18326. [Google Scholar] [CrossRef]
- Yoshimura, H.; Ohba, S.; Yoshida, H.; Saito, K.; Inui, K.; Yasui, R.; Ichikawa, D.; Aiki, M.; Kobayashi, J.; Matsuda, S.; et al. Denosumab-related osteonecrosis of the jaw in a patient with bone metastases of prostate cancer: A case report and literature review. Oncol. Lett. 2017, 14, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, Y.K.; Kim, T.Y.; Ha, Y.C.; Jang, S.; Kim, H.Y. Incidence of and risk for osteonecrosis of the jaw in Korean osteoporosis patients treated with bisphosphonates: A nationwide cohort-study. Bone 2021, 143, 115650. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.H.; Dodson, T.B.; Zavras, A.I. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: A case-control study. J. Oral Maxillofac. Surg. 2008, 66, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.J.M.; Pontes, J.C.X.; Figueiredo, L.S.; Araújo, R.D.S.; Sousa, M.C.P.; Aquino, J.S.; Castro, R.D.; Alves, A.F. Obesity influences the development of bisphosphonate-induced osteonecrosis in Wistar rats. J. Appl. Oral Sci. 2023, 31, e20230133. [Google Scholar] [CrossRef]
- Bulló, M.; García-Lorda, P.; Megias, I.; Salas-Salvadó, J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes. Res. 2003, 11, 525–531. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Kuo, Y.F.; Freeman, J.L.; Goodwin, J.S. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: A population-based analysis. J. Natl. Cancer Inst. 2007, 99, 1016–1024. [Google Scholar] [CrossRef]
- Molcho, S.; Peer, A.; Berg, T.; Futerman, B.; Khamaisi, M. Diabetes microvascular disease and the risk for bisphosphonate-related osteonecrosis of the jaw: A single center study. J. Clin. Endocrinol. Metab. 2013, 98, E1807–E1812. [Google Scholar] [CrossRef]
- Watters, A.L.; Hansen, H.J.; Williams, T.; Chou, J.F.; Riedel, E.; Halpern, J.; Tunick, S.; Bohle, G.; Huryn, J.M.; Estilo, C.L. Intravenous bisphosphonate-related osteonecrosis of the jaw: Long-term follow-up of 109 patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 192–200. [Google Scholar] [CrossRef]
- Kammerhofer, G.; Vegh, D.; Bányai, D.; Végh, Á.; Joob-Fancsaly, A.; Hermann, P.; Geczi, Z.; Hegedus, T.; Somogyi, K.S.; Bencze, B.; et al. Association between Hyperglycemia and Medication-Related Osteonecrosis of the Jaw (MRONJ). J. Clin. Med. 2023, 12, 2976. [Google Scholar] [CrossRef]
- Khamaisi, M.; Regev, E.; Yarom, N.; Avni, B.; Leitersdorf, E.; Raz, I.; Elad, S. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. J. Clin. Endocrinol. Metab. 2007, 92, 1172–1175. [Google Scholar] [CrossRef]
- Barasch, A.; Cunha-Cruz, J.; Curro, F.A.; Hujoel, P.; Sung, A.H.; Vena, D.; Voinea-Griffin, A.E.; CONDOR Collaborative Group; Beadnell, S.; Craig, R.G.; et al. Risk factors for osteonecrosis of the jaws: A case-control study from the CONDOR dental PBRN. J. Dent. Res. 2011, 90, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra-Pérez, M.S.; Vicente-Barrero, M.; Sosa-Henríquez, M.; Rodríguez-Bocanegra, E.; Limiñana-Cañal, J.M.; López-Márquez, A.; Pérez-Plasencia, D.; Ramos-Macías, A. Bone metabolism and clinical study of 44 patients with bisphosphonate-related osteonecrosis of the jaws. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e948–e955. [Google Scholar] [CrossRef]
- McCarthy, A.D.; Uemura, T.; Etcheverry, S.B.; Cortizo, A.M. Advanced glycation endproducts interfere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int. J. Biochem. Cell Biol. 2004, 36, 840–848. [Google Scholar] [CrossRef]
- Gangoiti, M.V.; Anbinder, P.S.; Cortizo, A.M.; McCarthy, A.D. Morphological changes induced by advanced glycation endproducts in osteoblastic cells: Effects of co-incubation with alendronate. Acta Histochem. 2013, 115, 649–657. [Google Scholar] [CrossRef]
- Nassar, H.; Kantarci, A.; van Dyke, T.E. Diabetic periodontitis: A model for activated innate immunity and impaired resolution of inflammation. Periodontol. 2000 2007, 43, 233–244. [Google Scholar] [CrossRef]
- Sarasquete, M.E.; García-Sanz, R.; Marín, L.; Alcoceba, M.; Chillón, M.C.; Balanzategui, A.; Santamaria, C.; Rosiñol, L.; de la Rubia, J.; Hernandez, M.T.; et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: A genome-wide single nucleotide polymorphism analysis. Blood 2008, 112, 2709–2712. [Google Scholar] [CrossRef]
| Variables | Overall Patients | |
|---|---|---|
| Total | No. (%) | 64 (100) |
| Age | Mean (range) | 75.1 (54–89) |
| Ethnicity | ||
| Japanese | No. (%) | 60 (93.7) |
| Chinese | No. (%) | 3 (4.7) |
| Korean | No. (%) | 1 (1.6) |
| Height (cm) | Median (IQR) | 162 (157−168) |
| Body weight (kg) | Median (IQR) | 56 (49.3−65.8) |
| BMI (kg/m2) | Median (IQR) | 21.7 (19.8−23.4) |
| Diabetes mellitus | No. (%) | 13 (20.3) |
| Hypertension | No. (%) | 26 (40.6) |
| Current smoking | No. (%) | 19 (30.0) |
| Gleason score | ||
| 6 | No. (%) | 1 (1.6) |
| 7 | No. (%) | 15 (23.4) |
| ≥8 | No. (%) | 48 (75.0) |
| PSA (ng/mL) | Median (IQR) | 247.9 (64.9−1127.5) |
| Neutrophils (103/mm3) | Mean ± SD | 4.34 ± 1.93 |
| Lymphocytes (103/mm3) | Mean ± SD | 1.9 ± 0.72 |
| Platelets (103/mm3) | Mean ± SD | 219.0 ± 65.2 |
| NLR | Mean ± SD | 2.7 ± 1.8 |
| PLR | Mean ± SD | 139.8 ± 110.0 |
| TPI (cm2/m2) | Mean ± SD | 6.2 ± 1.6 |
| Sarcopenia | No. (%) | 9 (14.1) |
| SFA (cm2) | Mean ± SD | 100.0 ± 59.5 |
| VFA (cm2) | Mean ± SD | 118.0 ± 64.8 |
| DRONJ | No. (%) | 12 (18.8) |
| Time to DRONJ onset (months) | Mean ± SD | 20.3 ± 8.5 |
| Group A (n = 12) | Group B (n = 52) | p | |
|---|---|---|---|
| Median age, years (IQR) | 76 (69.8–79.8) | 76.5 (71–80) | 0.6543 |
| Median BMI, kg/m2 (IQR) | 20.5 (19.5–21.7) | 22.0 (19.8–24.2) | 0.1196 |
| Diabetes mellitus, n (%) | 3 (25.0) | 10 (19.2) | 0.6543 |
| Hypertension, n (%) | 4 (33.3) | 22 (42.3) | 0.5683 |
| Current smoking, n (%) | 3 (25.0) | 16 (30.8) | 0.6934 |
| Sarcopenia, n (%) | 4/12 (33.3) | 5/52 (9.6) | 0.0331 |
| Median VFA, cm2 (IQR) | 95.8 (48.9–120.8) | 117.6 (73.1–170.4) | 0.2527 |
| Visceral obesity, n (%) | 5 (41.7) | 29 (55.8) | 0.3775 |
| Median SFA, cm2 (IQR) | 75.3 (50.2–133.8) | 96.1 (52.3–153.1) | 0.2527 |
| Median PSA, ng/mL (IQR) | 199.9 (43.9–1643) | 263.7 (73.5–1030.7) | 0.7634 |
| Median Gleason score (IQR) | 8 (7.3–9) | 8 (7.3–9) | 0.5122 |
| Median NLR, (IQR) | 2.7 (1.4–3.8) | 2.2 (1.7–2.8) | 0.3143 |
| Median PLR, (IQR) | 136.6 (110.1–165.2) | 113.4 (90.2–162.4) | 0.2901 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizushima, S.; Watanabe, D.; Yanagida, K.; Kawae, N.; Goto, K.; Takagi, T.; Kajihara, H.; Mizushima, A. Sarcopenia as a Potential Risk Factor for Denosumab-Related Osteonecrosis of the Jaw in Asian Prostate Cancer Patients with Bone Metastases. Diagnostics 2025, 15, 2635. https://doi.org/10.3390/diagnostics15202635
Mizushima S, Watanabe D, Yanagida K, Kawae N, Goto K, Takagi T, Kajihara H, Mizushima A. Sarcopenia as a Potential Risk Factor for Denosumab-Related Osteonecrosis of the Jaw in Asian Prostate Cancer Patients with Bone Metastases. Diagnostics. 2025; 15(20):2635. https://doi.org/10.3390/diagnostics15202635
Chicago/Turabian StyleMizushima, Shinobu, Daisuke Watanabe, Kazuki Yanagida, Norikazu Kawae, Kashia Goto, Tatsuya Takagi, Hajime Kajihara, and Akio Mizushima. 2025. "Sarcopenia as a Potential Risk Factor for Denosumab-Related Osteonecrosis of the Jaw in Asian Prostate Cancer Patients with Bone Metastases" Diagnostics 15, no. 20: 2635. https://doi.org/10.3390/diagnostics15202635
APA StyleMizushima, S., Watanabe, D., Yanagida, K., Kawae, N., Goto, K., Takagi, T., Kajihara, H., & Mizushima, A. (2025). Sarcopenia as a Potential Risk Factor for Denosumab-Related Osteonecrosis of the Jaw in Asian Prostate Cancer Patients with Bone Metastases. Diagnostics, 15(20), 2635. https://doi.org/10.3390/diagnostics15202635






