Evaluating Carotid Plaque Stiffness with Ultrasound 2D Shear-Wave Elastography in Patients Undergoing Coronary Artery Bypass Grafting
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Ultrasound Assessment and Data Acquisition
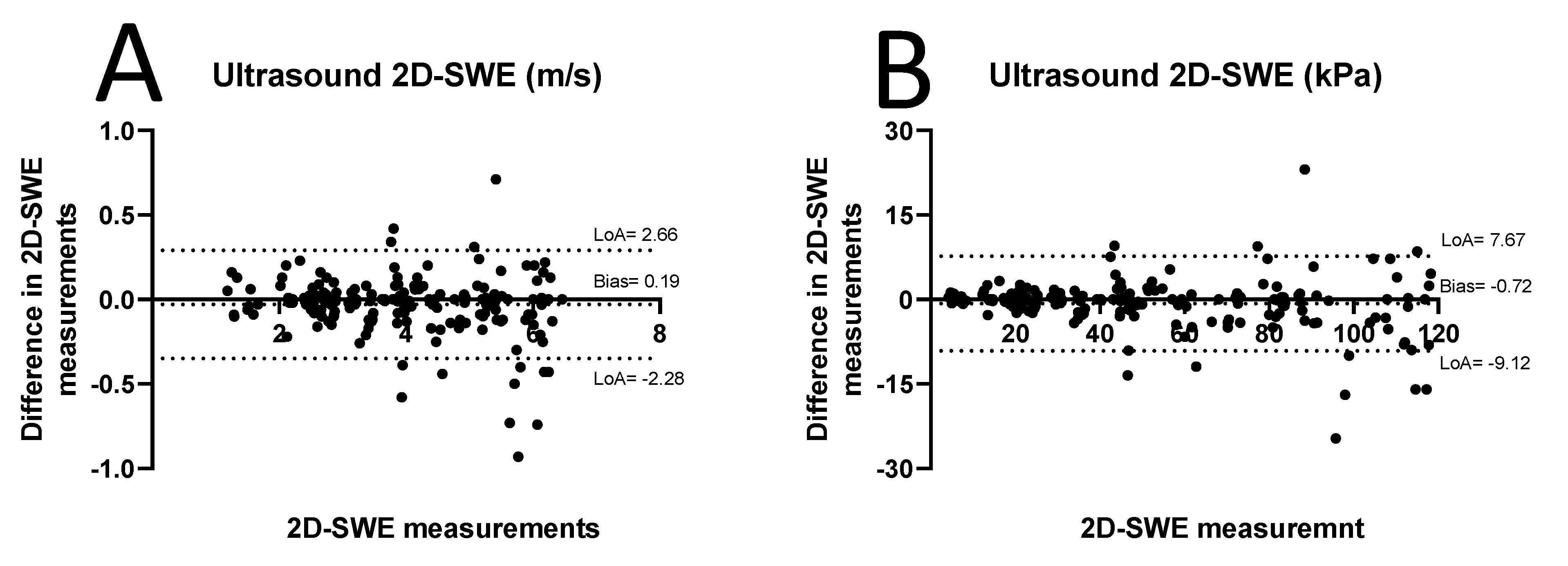
2.3. Statistical Analysis
3. Results
3.1. Patient Clinical Details and Carotid Plaque Characteristics
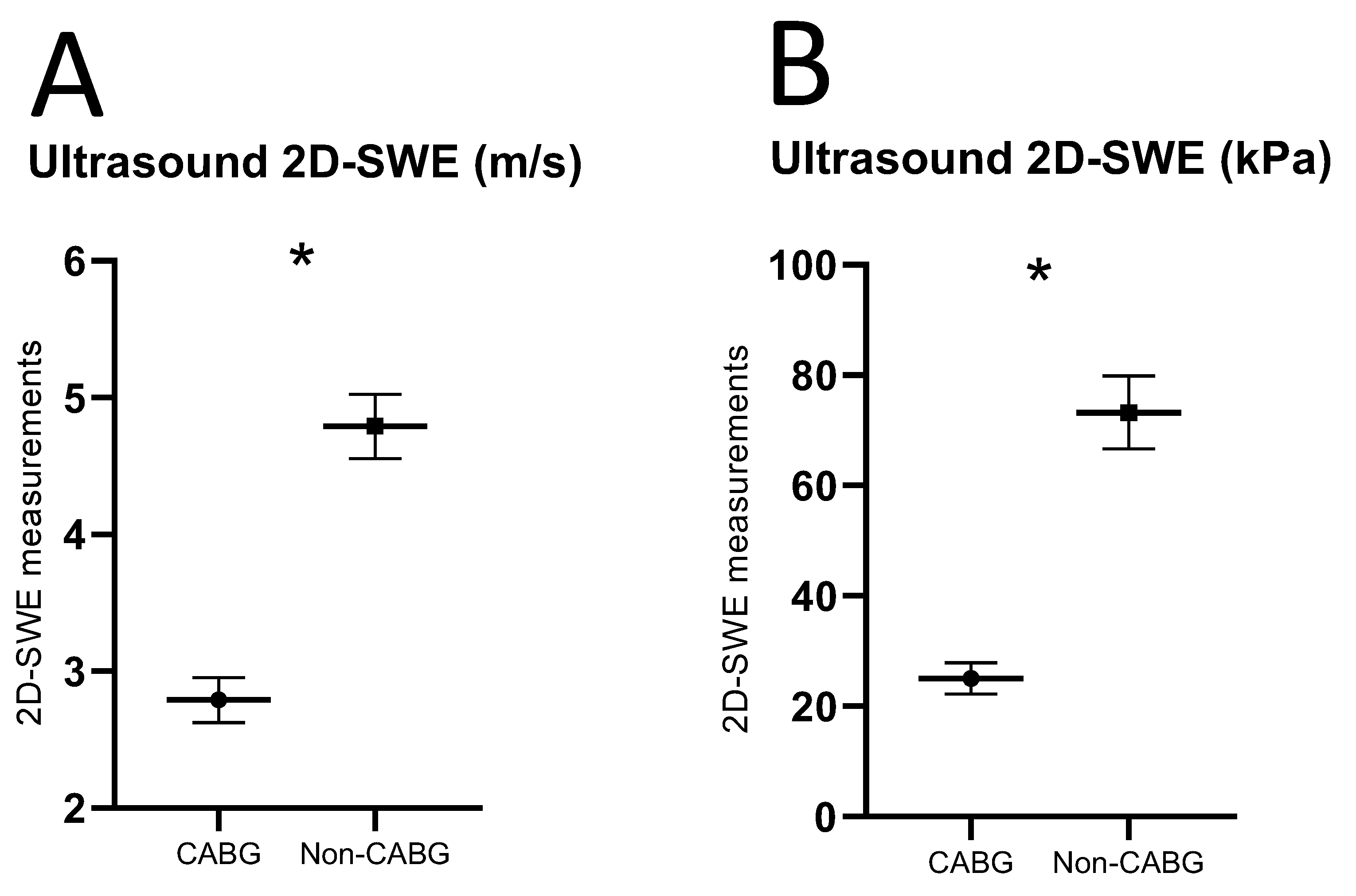
3.2. D-SWE of Carotid Plaques
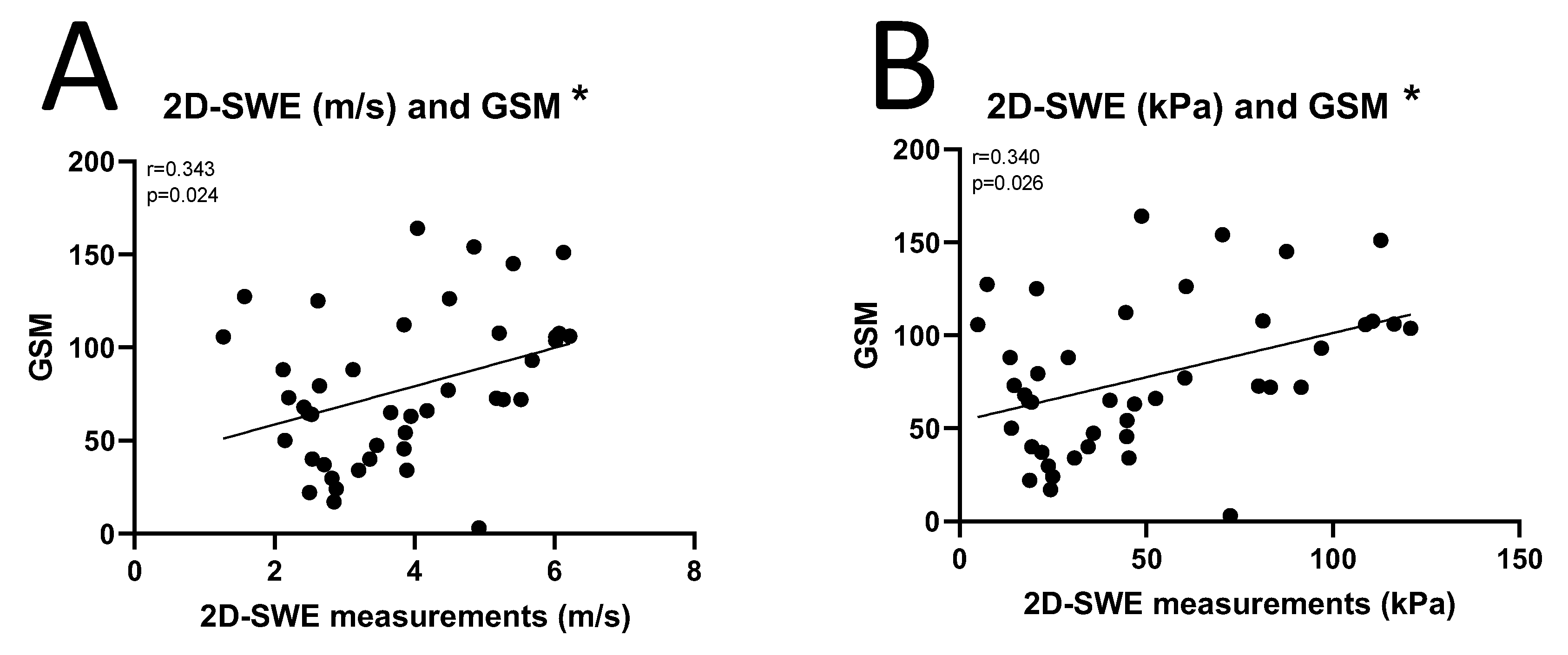
3.3. Correlation Between 2D-SWE and GSM of Carotid Plaques
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Rooy, M.-J.; Pretorius, E. Obesity, hypertension and hypercholesterolemia as risk factors for atherosclerosis leading to ischemic events. Curr. Med. Chem. 2014, 21, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.I.; Benziger, P.C.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Li, Z.; Lin, L.; Wu, H.; Yan, L.; Wang, H.; Yang, H.; Li, H. Global, Regional, and National Death, and Disability-Adjusted Life-Years (DALYs) for Cardiovascular Disease in 2017 and Trends and Risk Analysis From 1990 to 2017 Using the Global Burden of Disease Study and Implications for Prevention. Front. Public Healthy 2021, 9, 559751. [Google Scholar] [CrossRef] [PubMed]
- Jashari, F.; Ibrahimi, P.; Nicoll, R.; Bajraktari, G.; Wester, P.; Henein, M.Y. Coronary and carotid atherosclerosis: Similarities and differences. Atherosclerosis 2013, 227, 193–200. [Google Scholar] [CrossRef]
- Achim, A.; Péter, O.Á.; Cocoi, M.; Serban, A.; Mot, S.; Dadarlat-Pop, A.; Nemes, A.; Ruzsa, Z. Correlation between Coronary Artery Disease with Other Arterial Systems: Similar, Albeit Separate, Underlying Pathophysiologic Mechanisms. J. Cardiovasc. Dev. Dis. 2023, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, F.; Balestrieri, A.; Cau, R.; Punzo, B.; Cavaliere, C.; Maffei, E.; Saba, L. Insight from imaging on plaque vulnerability: Similarities and differences between coronary and carotid arteries—Implications for systemic therapies. Cardiovasc. Diagn. Ther. 2020, 10, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Shenouda, R.; Wester, P.; Henein, M.Y. Carotid Atherosclerosis in Predicting Coronary Artery Disease: A Systematic Review and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, E224–E237. [Google Scholar] [CrossRef] [PubMed]
- Hertzer, N.R.; Young, J.R.; Beven, E.G.; Graor, R.A.; O’hara, P.J.; Ruschhaupt, W.F.; deWolfe, V.G.; Maljovec, L.C. Coronary Angiography in 506 Patients With Extracranial Cerebrovascular Disease. Arch. Intern. Med. 1985, 145, 849–852. [Google Scholar] [CrossRef]
- Graor, R.A.; Hetzer, N.R. Management of coexistent carotid artery and coronary artery disease. Stroke 1988, 19, 1441–1444. [Google Scholar] [CrossRef]
- Chimowitz, M.I.; Frank Lafranchise, E.; Furlan, A.J.; Dorosti, K.; Paranandi, L.; Beck, G.J. Evaluation of coexistent carotid and coronary disease by combined angiography. J. Stroke Cerebrovasc. Dis. 1991, 1, 89–93. [Google Scholar] [CrossRef]
- Jahangiri, M.; Rees, G.M.; Edmondson, S.J.; Lumley, J.; Uppal, R. A surgical approach to coexistent coronary and carotid artery disease. Heart 1997, 77, 164–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vitali, E.; Lanfranconi, M.; Bruschi, G.; Colombo, T.; Russo, C. Combined surgical approach to coexistent carotid and coronary artery disease: Early and late results. Cardiovasc. Surg. 2003, 11, 113–119. [Google Scholar] [CrossRef]
- Al-Mubarak, N.; Roubin, G.S.; Liu, M.W.; Dean, L.S.; Gomez, C.R.; Iyer, S.S.; Vitek, J.J. Early results of percutaneous intervention for severe coexisting carotid and coronary artery disease. Am. J. Cardiol. 1999, 84, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Wall, M.J.; Soltero, E.R. Treatment of combined coronary and carotid artery disease. Curr. Opin. Cardiol. 2003, 18, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Jannati, M. Risk factors for stroke post coronary artery bypass graft surgery: A review of literature. Med. Clínica Práctica. 2024, 7, 100405. [Google Scholar] [CrossRef]
- Sultan, S.R.; Khayat, M.; Almutairi, B.; Marzouq, A.; Albngali, A.; Abdeen, R.; Alahmadi, A.A.S.; Toonsi, F. B-mode ultrasound characteristics of carotid plaques in symptomatic and asymptomatic patients with low-grade stenosis. PLoS ONE 2023, 18, e0291450. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.N.E.; Lovett, J.K.; Gallagher, P.J.; Rothwell, P.M. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The Oxford plaque study. Circulation 2006, 113, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.N.E.; Lovett, J.K.; Rothwell, P.M. Histological features of symptomatic carotid plaques in relation to age and smoking: The oxford plaque study. Stroke 2010, 41, 2288–2294. [Google Scholar] [CrossRef]
- Grønholdt, M.L.M.; Nordestgaard, B.G.; Schroeder, T.V.; Vorstrup, S.; Sillesen, H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation 2001, 104, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Tzorbatzoglou, I.; Lazari, P.; Maras, D.; Giannoukas, A.D. Carotid artery plaque echomorphology and its association with histopathologic characteristics. J. Vasc. Surg. 2018, 68, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Grazhdani, H.; Aliotta, L.; Gavazzi, L.M.; Foti, P.V.; Palmucci, S.; Inì, C.; Tiralongo, F.; Castiglione, D.; Renda, M.; et al. Imaging of Carotid Stenosis: Where Are We Standing? Comparison of Multiparametric Ultrasound, CT Angiography, and MRI Angiography, with Recent Developments. Diagnostics 2024, 14, 1708. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.-M.; D'Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas AEl Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Widman, E.; Maksuti, E.; Amador, C.; Urban, M.W.; Caidahl, K.; Larsson, M. Shear Wave Elastography Quantifies Stiffness in Ex Vivo Porcine Artery with Stiffened Arterial Region. Ultrasound Med. Biol. 2016, 42, 2423–2435. [Google Scholar] [CrossRef] [PubMed]
- Marlevi, D.; Maksuti, E.; Urban, M.W.; Winter, R.; Larsson, M. Plaque characterization using shear wave elastography—Evaluation of differentiability and accuracy using a combined ex vivo and in vitro setup. Phys. Med. Biol. 2018, 63, 235008. [Google Scholar] [CrossRef] [PubMed]
- Amzar, D.; Stoian, D.; Oglat, A.A.; Abukhalil, T. Ultrasound Elastography: Methods, Clinical Applications, and Limitations: A Review Article. Appl. Sci. 2024, 14, 4308. [Google Scholar]
- Herrmann, E.; de Lédinghen, V.; Cassinotto, C.; Chu, W.C.W.; Leung, V.Y.F.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Wang, X.; Xing, P.; Yang, Z.; Wu, C. Application of 3D and 2D quantitative shear wave elastography (SWE) to differentiate between benign and malignant breast masses. Sci. Rep. 2017, 7, 41216. [Google Scholar] [CrossRef] [PubMed]
- Filho, R.H.C.; Pereira, F.L.; Iared, W. Diagnostic Accuracy Evaluation of Two-Dimensional Shear Wave Elastography in the Differentiation Between Benign and Malignant Thyroid Nodules: Systematic Review and Meta-analysis. J. Ultrasound Med. 2020, 39, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Mbroh, J.; Tünnerhoff, J.; Poli, K.; Bender, B.; Schwarz, P.; Mengel, A.; Gomez-Exposito, A.; Kowarik, M.; Feil, K.; Wisslicen, M.; et al. Shear Wave Elastography for the Assessment of Carotid Plaque Vulnerability: A Systematic Review. Acta Neurol. Scand. 2023, 2023, 5084699. [Google Scholar] [CrossRef]
- Garrard, J.W.; Ummur, P.; Nduwayo, S.; Kanber, B.; Hartshorne, T.C.; West, K.P.; Moore, D.; Robinson, T.G.; Ramnarine, K.V. Shear Wave Elastography May Be Superior to Greyscale Median for the Identification of Carotid Plaque Vulnerability: A Comparison with Histology. Ultraschall Med. 2015, 36, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, N.; Venturini, L.; de Soccio, V.; Forte, V.; Lucchetti, P.; Cerone, G.; Alagna, G.; Caratozzolo, M.; Messineo, D.; Di Gioia, D.; et al. Multiparametric ultrasound evaluation with CEUS and shear wave elastography for carotid plaque risk stratification. J. Ultrasound 2018, 21, 293–300. [Google Scholar]
- Školoudík, D.; Kešnerová, P.; Vomáčka, J.; Hrbáč, T.; Netuka, D.; Forostyak, S.; Roubec, M.; Herzig, R.; Belšan, T.; ANTIQUE Trial Group. Shear-Wave Elastography Enables Identification of Unstable Carotid Plaque. Ultrasound Med. Biol. 2021, 47, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Warlow, C.; Farrell, B.; Fraser, A.; Sandercock, P.; Slattery, J. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar]
- Sultan, S.R. Ultrasound diagnostic potential of carotid plaques in symptomatic and asymptomatic patients: Insights from quantitative gray-scale analysis. J. Radiat. Res. Appl. Sci. 2024, 17, 100776. [Google Scholar] [CrossRef]
- Elatrozy, T.; Nicolaides, A.; Tegos, T.; Zarka, A.Z.; Griffin, M.; Sabetai, M. The effect of B-mode ultrasonic image standardisation on the echodensity of symptomatic and asymptomatic carotid bifurcation plaques. Int. Angiol. 1998, 17, 179–186. Available online: https://europepmc.org/article/med/9821032 (accessed on 17 April 2023).
- Sabetai, M.M.; Tegos, T.J.; Nicolaides, A.N.; Dhanjil, S.; Pare, G.J.; Stevens, J.M. Reproducibility of computer-quantified carotid plaque echogenicity: Can we overcome the subjectivity? Stroke 2000, 31, 2189–2196. [Google Scholar]
- Brinjikji, W.; Rabinstein, A.; Lanzino, G.; Murad, M.; Williamson, E.; DeMarco, J.; Huston, J. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2015, 40, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Yang, J.; Tang, L.; Jin, Y.; Zhang, J.; Liu, C.; Li, Q. Shear Wave Elastography Imaging for the Features of Symptomatic Carotid Plaques: A Feasibility Study. J. Ultrasound Med. 2017, 36, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Ramnarine, K.V.; Garrard, J.W.; Kanber, B.; Nduwayo, S.; Hartshorne, T.C.; Robinson, T.G. Shear wave elastography imaging of carotid plaques: Feasible, reproducible and of clinical potential. Cardiovasc. Ultrasound 2014, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Pruijssen, J.T.; de Korte, C.L.; Voss, I.; Hansen, H.H.G. Vascular Shear Wave Elastography in Atherosclerotic Arteries: A Systematic Review. Ultrasound Med. Biol. 2020, 46, 2145–2163. [Google Scholar] [CrossRef] [PubMed]
- Maksuti, E.; Widman, E.; Larsson, D.; Urban, M.W.; Larsson, M.; Bjällmark, A. Arterial Stiffness Estimation by Shear Wave Elastography: Validation in Phantoms with Mechanical Testing. Ultrasound Med. Biol. 2016, 42, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Ramnarine, K.V.; Garrard, J.W.; Dexter, K.; Nduwayo, S.; Panerai, R.B.; Robinson, T.G. Shear wave elastography assessment of carotid plaque stiffness: Invitro reproducibility study. Ultrasound Med. Biol. 2014, 40, 200–209. [Google Scholar] [CrossRef]
- Goudot, G.; Sitruk, J.; Jimenez, A.; Julia, P.; Khider, L.; Alsac, J.M.; El Batti, S.; Bruneval, P.; Amemyia, K.; Pedreira, O.; et al. Carotid Plaque Vulnerability Assessed by Combined Shear Wave Elastography and Ultrafast Doppler Compared to Histology. Transl. Stroke Res. 2022, 13, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Marais, L.; Pernot, M.; Khettab, H.; Tanter, M.; Messas, E.; Zidi, M.; Laurent, S.; Boutouyrie, P. Arterial Stiffness Assessment by Shear Wave Elastography and Ultrafast Pulse Wave Imaging: Comparison with Reference Techniques in Normotensives and Hypertensives. Ultrasound Med. Biol. 2019, 45, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.C.; Stein, J.H.; Cook, T.D.; Salamat, S.; Wang, X.; Varghese, T.; Jackson, D.C.; Garcia, C.S.; Wilbrand, S.M.; Robert J Dempsey, R.J. Histopathologic Validation of Grayscale Carotid Plaque Characteristics Related to Plaque Vulnerability. Ultrasound Med. Biol. 2017, 43, 129–137. [Google Scholar] [CrossRef]
- Doonan, R.J.; Gorgui, J.; Veinot, J.P.; Lai, C.; Kyriacou, E.; Corriveau, M.M.; Steinmetz, O.K.; Daskalopoulou, S.S. Plaque echodensity and textural features are associated with histologic carotid plaque instability. J. Vasc. Surg. 2016, 64, 671–677.e8. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Qiang, Y.; Pu, T.; Jie, L. Quantitative assessment of carotid atherosclerotic plaque: Initial clinical results using ShearWave TM Elastography. Int. J. Clin. Exp. Med. 2016, 9, 9347–9355. [Google Scholar]
- Shang, J.; Wang, W.; Feng, J.; Luo, G.G.; Dang, Y.; Sun, J.; Yang, Y.; Ruan, L. Carotid Plaque Stiffness Measured with Supersonic Shear Imaging and Its Correlation with Serum Homocysteine Level in Ischemic Stroke Patients. Korean J. Radiol. 2018, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, J.; Finet, G.; Le Floc’h, S.; Cloutier, G.; Gharib, A.M.; Heroux, J.; Pettigrew, R.I. Biomechanics of atherosclerotic coronary plaque: Site, stability and in vivo elasticity modeling. Ann. Biomed. Eng. 2014, 42, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Milzi, A.; Lemma, E.D.; Dettori, R.; Burgmaier, K.; Marx, N.; Reith, S.; Burgmaier, M. Coronary plaque composition influences biomechanical stress and predicts plaque rupture in a morpho-mechanic OCT analysis. Elife 2021, 10, e64020. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, X.Q.; Balu, N.; Neradilek, M.B.; Isquith, D.A.; Yamada, K.; Cantón, G.; Crouse, J.R., III; Anderson, T.J.; Huston, J., III; et al. Magnetic Resonance Imaging-Detected Carotid Plaque Lipid Content and Fibrous Cap Status Predict Systemic Cardiovascular Outcomes: The MRI Sub-study in AIM-HIGH. JACC Cardiovasc. Imaging 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, R.; Vancheri, S.; Maria Bassi, E.; Nicoll, R.; Sobhi, M.; El Sharkawy, E.; Wester, P.; Vancheri, F.; Henein, M.Y. The relationship between carotid and coronary calcification in patients with coronary artery disease. Clin. Physiol. Funct. Imaging 2021, 41, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.I.; Aboufakher, R.; Bess, R.; Frank, J.; Othman, M.; Doan, D.; Mesiha, N.; Rosman, H.S.; Szpunar, S. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc. Imaging 2013, 6, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Thim, T.; Hagensen, M.K.; Bentzon, J.F.; Falk, E. From vulnerable plaque to atherothrombosis. J. Intern. Med. 2008, 263, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- Sondore, D.; Trušinskis, K.; Linde, M.; Briede, I.; Narbute, I.; Jēgere, S.; Griķis, K.; Štrenge, K.; Ērglis, A. Association between carotid and coronary atherosclerotic plaque morphology: A virtual histology intravascular ultrasound study. J. Clin. Transl. Res. 2023, 9, 253. [Google Scholar] [PubMed]
- Chen, S.; Mi, C.; Zhang, S.; Li, Y.; Yun, Y.; Zhang, X.; Chen, J.; Li, Y.; Zhang, H.; Gao, T.; et al. The role of carotid artery stenosis in predicting stroke after coronary artery bypass grafting in a Chinese cohort study. Sci. Rep. 2023, 13, 21536. [Google Scholar] [CrossRef]
- Xodo, A.; Gregio, A.; Pilon, F.; Milite, D.; Danesi, T.H.; Badalamenti, G.; Lepidi, S.; D'Oria, M. Carotid Interventions in Patients Undergoing Coronary Artery Bypass Grafting: A Narrative Review. J. Clin. Med. 2024, 13, 3019. [Google Scholar] [CrossRef] [PubMed]
- Haywood, N.S.; Ratcliffe, S.J.; Zheng, X.; Mao, J.; Farivar, B.S.; Tracci, M.C.; Malas, M.B.; Goodney, P.P.; Clouse, W.D. Operative and long-term outcomes of combined and staged carotid endarterectomy and coronary bypass. J. Vasc. Surg. 2023, 77, 1424–1433.e1. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, R.; Tarighatnia, A.; Sharifipour, E.; Nourizadeh, E.; Parvizi, R.; Applegate, C.T.; Nader, N.D. Carotid artery stenting prior to coronary artery bypass grafting in patients with carotid stenosis: Clinical outcomes. Interv. Neuroradiol. 2023, 29, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Modugno, P.; Picone, V.; Centritto, E.M.; Calvo, E.; Canosa, C.; Piancone, F.; Testa, N.; Camposarcone, N.; Castellano, G.; Astore, P.; et al. Combined Treatment With Carotid Endoarterectomy and Coronary Artery Bypass Grafting: A Single-Institutional Experience in 222 Patients. Vasc. Endovasc. Surg. 2022, 56, 566. [Google Scholar] [CrossRef]

| Total | CABG | Control | p-Value | |
|---|---|---|---|---|
| Patient, n [%] | 32 [100] | 17 [53.13] | 15 [46.88] | - |
| Male, n [%] | 21 [65.63] | 12 [70.59] | 9 [60] | - |
| Age, median [IQR, years] | 67 [9.25] | 68 [12] | 67 [13] | 0.77 |
| Height, median [IQR, cm] | 160 [8.75] | 161 [10] | 160 [11] | 0.79 |
| Weight, median [IQR, kg] | 71 [12.25] | 70 [14] | 71 [9] | 0.82 |
| BMI, median [IQR, kg/m2] | 27.05 [7.31] | 26.95 [4.43] | 30 [7.85] | 0.79 |
| Hypertension, n [%] | 25 [78.13] | 14 [82.35] | 11 [73.33] | - |
| Diabetes, n [%] | 24 [75.00] | 12 [70.59] | 12 [80.00] | - |
| Smoking, n [%] | 11 [34.38] | 4 [23.53] | 7 [46.67] | - |
| Plaque, n [%] | 43 [100] | 21 [48.83] | 22 [51.16] | - |
| DoS, median [IQR, %] | 37.97 [19.12] | 34.78 [18.10] | 39.28 [20.94] | 0.24 |
| Plaque length, median [IQR, cm] | 1.12 [0.67] | 1.17 [0.56] | 0.97 [0.70] | 0.17 |
| Plaque thickness, median [IQR, cm] | 0.31 [0.11] | 0.31 [0.11] | 0.32 [0.11] | 0.98 |
| Plaque area, median [IQR, cm] | 0.27 [0.25] | 0.30 [0.24] | 0.25 [0.26] | 0.26 |
| 2D-SWE, median [IQR, m/s] | 3.85 [2.54] | 3.64 [1.02] | 4.91 [1.69] | <0.001 |
| 2D-SWE, median [IQR, kPa] | 44.53 [59.48] | 20.96 [17.30] | 72.54 [48.39] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, A.; Alharbi, A.A.; Alharbi, A.K.; Alkhaldi, A.; Filimban, A.Z.; Alfatni, A.; Kaifi, R.; Albngali, A.; Alkharaiji, M.; Alserihy, O.; et al. Evaluating Carotid Plaque Stiffness with Ultrasound 2D Shear-Wave Elastography in Patients Undergoing Coronary Artery Bypass Grafting. Diagnostics 2025, 15, 338. https://doi.org/10.3390/diagnostics15030338
Alzahrani A, Alharbi AA, Alharbi AK, Alkhaldi A, Filimban AZ, Alfatni A, Kaifi R, Albngali A, Alkharaiji M, Alserihy O, et al. Evaluating Carotid Plaque Stiffness with Ultrasound 2D Shear-Wave Elastography in Patients Undergoing Coronary Artery Bypass Grafting. Diagnostics. 2025; 15(3):338. https://doi.org/10.3390/diagnostics15030338
Chicago/Turabian StyleAlzahrani, Adel, Amjad Ali Alharbi, Amjad Khalid Alharbi, Asma Alkhaldi, Asseel Z. Filimban, Abrar Alfatni, Reham Kaifi, Ahmad Albngali, Mohammed Alkharaiji, Omar Alserihy, and et al. 2025. "Evaluating Carotid Plaque Stiffness with Ultrasound 2D Shear-Wave Elastography in Patients Undergoing Coronary Artery Bypass Grafting" Diagnostics 15, no. 3: 338. https://doi.org/10.3390/diagnostics15030338
APA StyleAlzahrani, A., Alharbi, A. A., Alharbi, A. K., Alkhaldi, A., Filimban, A. Z., Alfatni, A., Kaifi, R., Albngali, A., Alkharaiji, M., Alserihy, O., & Sultan, S. R. (2025). Evaluating Carotid Plaque Stiffness with Ultrasound 2D Shear-Wave Elastography in Patients Undergoing Coronary Artery Bypass Grafting. Diagnostics, 15(3), 338. https://doi.org/10.3390/diagnostics15030338





