Pancreatic Neuroendocrine Tumors—Diagnostic Pitfalls of Non-Diabetic Severe Hypoglycemia: Literature Review and Case Report
Abstract
1. Introduction
2. Materials and Methods
Aim
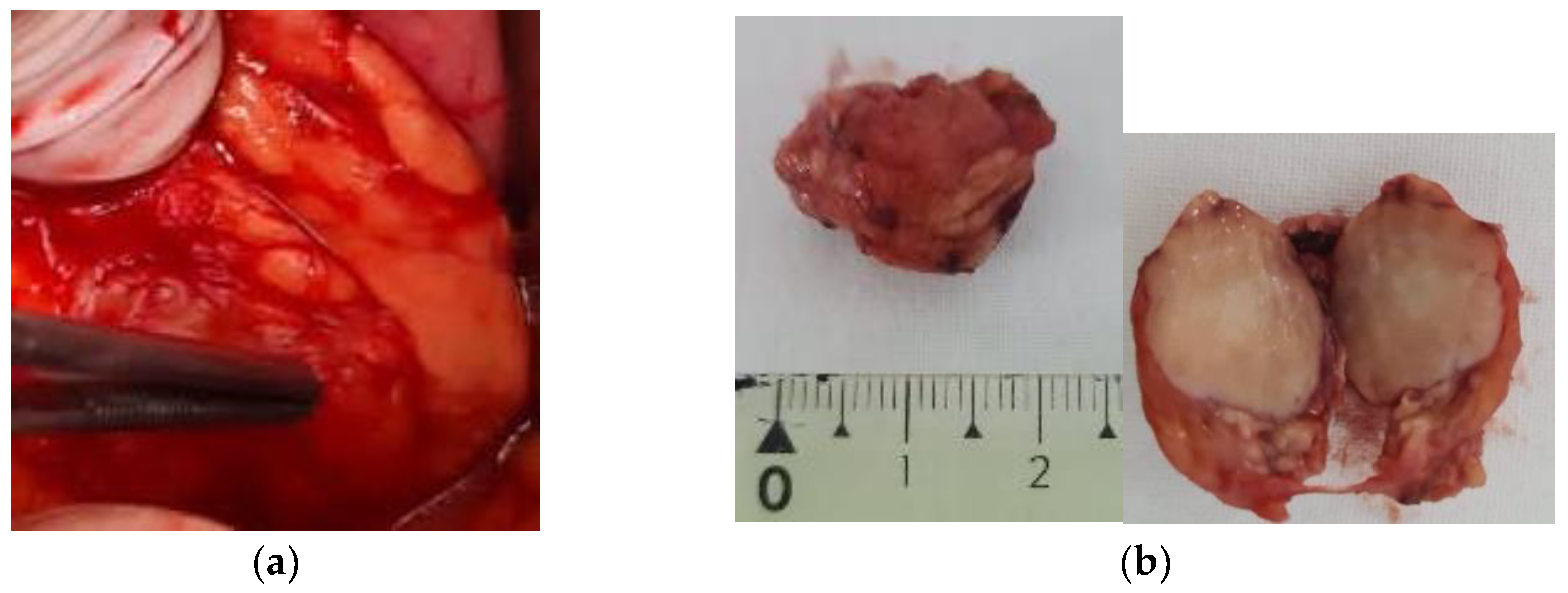
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Silveira, F.; Basile, M.L.; Kuga, F.S.; Próspero, J.D.; Paes, R.A.P.; Bernardi, F.D.C. Neuroendocrine tumors: An epidemiological study of 250 cases at a tertiary hospital. Rev. Assoc. Med. Bras. (1992) 2017, 63, 856–861. [Google Scholar] [CrossRef]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Sultana, Q.; Kar, J.; Verma, A.; Sanghvi, S.; Kaka, N.; Patel, N.; Sethi, Y.; Chopra, H.; Kamal, M.A.; Greig, N.H. A Comprehensive Review on Neuroendocrine Neoplasms: Presentation, Pathophysiology and Management. J. Clin. Med. 2023, 12, 5138. [Google Scholar] [CrossRef]
- Ahmed, F.W.; Majeed, M.S.; Kirresh, O. Non-Diabetic Hypoglycemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK573079/ (accessed on 10 January 2024).
- Mete, T.; Cesur, M. Non-diabetic hypoglycemia. Intercont. J. Int. Med. 2023, 1, 94–105. [Google Scholar] [CrossRef]
- Elghobashy, M.; Gama, R.; Sulaiman, R.A. Investigation and Causes of Spontaneous (Non-Diabetic) Hypoglycaemia in Adults: Pitfalls to Avoid. Diagnostics 2023, 13, 3275. [Google Scholar] [CrossRef]
- Vezzosi, D.; Bennet, A.; Fauvel, J.; Caron, P. Insulin, C-peptide and proinsulin for the biochemical diagnosis of hypoglycaemia related to endogenous hyperinsulinism. Eur. J. Endocrinol. 2007, 157, 75–83. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Kloeppel, G. Pancreatic neuroendocrine neoplasias. In The WHO Classification of Endocrine Tumors; IARC Press: Lyon, France, 2017. [Google Scholar]
- WHO Classification of Tumours Editorial Board. Digestive System Tumours, WHO Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2019. [Google Scholar]
- de Herder, W.W.; Niederle, B.; Scoazec, J.Y.; Pauwels, S.; Kloppel, G.; Falconi, M.; Kwekkeboom, D.J.; Oberg, K.; Eriksson, B.; Wiedenmann, B.; et al. Well-differentiated pancreatic tumor/carcinoma: Insulinoma. Neuroendocrinology 2006, 84, 183–188. [Google Scholar] [CrossRef]
- Cryer, P.E.; Axelrod, L.; Grossman, A.B.; Heller, S.R.; Montori, V.M.; Seaquist, E.R.; Service, F.J.; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2009, 94, 709–728. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Gama, R.; Teale, J.D.; Marks, V. Best practice No 173: Clinical and laboratory investigation of adult spontaneous hypoglycaemia. J. Clin. Pathol. 2003, 56, 641–646. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Brandi, M.L. Genetic disorders and insulinoma/glucagonoma. Endocr. Relat. Cancer 2024, 31, e230245. [Google Scholar] [CrossRef]
- Martens, P.; Tits, J. Approach to the patient with spontaneous hypoglycemia. Eur. J. Intern. Med. 2014, 25, 415–421. [Google Scholar] [CrossRef]
- Zetu, C.; Popa, S.; Golli, A.L.; Condurache, A.; Munteanu, R. Long-term improvement of dyslipidaemia, hyperuricemia and metabolic syndrome in patients undergoing laparoscopic sleeve gastrectomy. Arch. Endocrinol. Metab. 2021, 64, 704–709. [Google Scholar] [CrossRef]
- Karamanolis, N.N.; Kounatidis, D.; Vallianou, N.G.; Alexandropoulos, K.; Kovlakidi, E.; Kaparou, P.; Karampela, I.; Stratigou, T.; Dalamaga, M. Paraneoplastic hypoglycemia: An overview for optimal clinical guidance. Metabol. Open 2024, 23, 100305. [Google Scholar] [CrossRef]
- Rayas, M.S.; Salehi, M. Non-Diabetic Hypoglycemia. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK355894/ (accessed on 10 January 2024).
- Guettier, J.M.; Lungu, A.; Goodling, A.; Cochran, C.; Gorden, P. The role of proinsulin and insulin in the diagnosis of insulinoma: A critical evaluation of the Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4752–4758. [Google Scholar] [CrossRef]
- Ouleghzal, H.; Ziadi, T.; Menfaa, M.; Safi, S. Association of Insulinoma and Type 2 Diabetes Mellitus. Int. J. Endocrinol. Metab. 2016, 15, e39439. [Google Scholar] [CrossRef]
- Popa, S.G.; Ungureanu, B.S.; Gheonea, I.A.; Mitrea, A.; Ardeleanu, C.M.; Ghiluşi, M.C.; Şurlin, V.; Georgescu, E.F.; Georgescu, I.; MoŢa, M.; et al. Pitfalls in diagnosing a pancreatic neuroendocrine tumor: A case report. Rom. J. Morphol. Embryol. 2015, 56, 1495–1502. [Google Scholar]
- Bisgaard Bengtsen, M.; Møller, N. Experimentally Induced Hypoglycemia-associated Autonomic Failure in Humans: Determinants, Designs, and Drawbacks. J. Endocr. Soc. 2022, 6, bvac123. [Google Scholar] [CrossRef] [PubMed]
- Singbo, J.; Locketz, M.; Ross, I.L. Challenge of coexisting type 2 diabetes mellitus and insulinoma: A case report. J. Med. Case Rep. 2021, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Queiroz Almeida, M.; Machado, M.C.; Correa-Giannella, M.L.; Giannella-Neto, D.; Albergaria Pereira, M.A. Endogenous hyperinsulinemic hypoglycemia: Diagnostic strategies, predictive features of malignancy and long-term survival. J. Endocrinol. Investig. 2006, 29, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ro, C.; Chai, W.; Yu, V.E.; Yu, R. Pancreatic neuroendocrine tumors: Biology, diagnosis, and treatment. Chin. J. Cancer 2013, 32, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K. Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors). Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 72–78. [Google Scholar] [CrossRef]
- Antonello, D.; Gobbo, S.; Corbo, V.; Sipos, B.; Lemoine, N.R.; Scarpa, A. Update on the molecular pathogenesis of pancreatic tumors other than common ductal adenocarcinoma. Pancreatology 2009, 9, 25–33. [Google Scholar] [CrossRef]
- Leoncini, E.; Carioli, G.; La Vecchia, C.; Boccia, S.; Rindi, G. Risk factors for neuroendocrine neoplasms: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 68–81. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int. J. Cancer 2008, 123, 867–873. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Family history of cancer and associated risk of developing neuroendocrine tumors: A case-control study. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 959–965. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Bamlet, W.R.; McWilliams, R.R.; Hobday, T.J.; Burch, P.A.; Rabe, K.G.; Petersen, G.M. Risk factors for pancreatic neuroendocrine tumors: A clinic-based case-control study. Pancreas 2014, 43, 1219–1222. [Google Scholar] [CrossRef]
- Cigrovski Berković, M.; Catela Ivković, T.; Marout, J.; Zjačić-Rotkvić, V.; Kapitanović, S. Interleukin 1β gene single-nucleotide polymorphisms and susceptibility to pancreatic neuroendocrine tumors. DNA Cell Biol. 2012, 31, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Karakaxas, D.; Gazouli, M.; Coker, A.; Agalianos, C.; Papanikolaou, I.S.; Patapis, P.; Liakakos, T.; Dervenis, C. Genetic polymorphisms of inflammatory response gene TNF-α and its influence on sporadic pancreatic neuroendocrine tumors predisposition risk. Med. Oncol. 2014, 31, 241. [Google Scholar] [CrossRef] [PubMed]
- Hiripi, E.; Bermejo, J.L.; Sundquist, J.; Hemminki, K. Familial gastrointestinal carcinoid tumours and associated cancers. Ann. Oncol. 2009, 20, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.X.; Cong, L.; Zhao, Y.P.; Zhang, T.P.; Chen, G. Risk factors for the occurrence of insulinoma: A case-control study. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 324–328. [Google Scholar] [CrossRef]
- Okabayashi, T.; Shima, Y.; Sumiyoshi, T.; Kozuki, A.; Ito, S.; Ogawa, Y.; Kobayashi, M.; Hanazaki, K. Diagnosis and management of insulinoma. World J. Gastroenterol. 2013, 19, 829–837. [Google Scholar] [CrossRef]
- Tonelli, F.; Giudici, F.; Nesi, G.; Batignani, G.; Brandi, M.L. Operation for insulinomas in multiple endocrine neoplasia type 1: When pancreatoduodenectomy is appropriate. Surgery 2017, 161, 727–734. [Google Scholar] [CrossRef]
- Garg, R.; Memon, S.; Patil, V.; Bandgar, T. Extrapancreatic insulinoma. World J. Nucl. Med. 2020, 19, 162–164. [Google Scholar] [CrossRef]
- Hennings, J.; Garske, U.; Botling, J.; Hellman, P. Malignant insulinoma in ectopic pancreatic tissue. Dig. Surg. 2005, 22, 377–379. [Google Scholar] [CrossRef]
- Diaz-Sangines, B.P.; Gonzalez-Cofrades, J.; Vazquez-Camacho, E.E.; Malfavon-Farias, M.; Garcia-Lima, L. Insulinoma Management in a Pregnant Woman: A Case Report. Cureus 2023, 15, e34239. [Google Scholar] [CrossRef]
- Dobrindt, E.M.; Mogl, M.; Goretzki, P.E.; Pratschke, J.; Dukaczewska, A.K. Insulinoma in pregnancy (a case presentation and systematic review of the literature). Rare Tumors 2021, 13, 2036361320986647. [Google Scholar] [CrossRef]
- Teixeira, D.; Tavares, R.F.; Rodrigues, A.; Moreira, P. Insulinoma in primary care: A case report. Int. Surg. J. 2023, 10, 1687–1689. [Google Scholar] [CrossRef]
- Nahmias, A.; Grozinsky-Glasberg, S.; Salmon, A.; Gross, D.J. Pancreatic neuroendocrine tumors with transformation to insulinoma: An unusual presentation of a rare disease. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 150032. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buddhavarapu, V.S.; Dhillon, G.; Grewal, H.S.; Soles, B.; Halbur, L.; Surani, S.; Kashyap, R. Transformation of pancreatic nonfunctioning neuroendocrine tumor into metastatic insulinoma: A rare case report. Clin. Case Rep. 2023, 11, e8152. [Google Scholar] [CrossRef] [PubMed]
- Tarris, G.; Rouland, A.; Guillen, K.; Loffroy, R.; Lariotte, A.C.; Rat, P.; Bouillet, B.; Andrianiaina, H.; Petit, J.M.; Martin, L. Case Report: Giant insulinoma, a very rare tumor causing hypoglycemia. Front. Endocrinol. 2023, 14, 1125772. [Google Scholar] [CrossRef]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers 2019, 11, 1113. [Google Scholar] [CrossRef]
- Bevere, M.; Masetto, F.; Carazzolo, M.E.; Bettega, A.; Gkountakos, A.; Scarpa, A.; Simbolo, M. An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics 2023, 13, 2820. [Google Scholar] [CrossRef]
- Tsoli, M.; Koumarianou, A.; Angelousi, A.; Kaltsas, G. Established and novel circulating neuroendocrine tumor biomarkers for diagnostic, predictive and prognostic use. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101785. [Google Scholar] [CrossRef]
- Matondang, S.; Suwita, B.M.; Budianto, T.; Krisnuhoni, E. Atypical CT and MR imaging of insulinoma: A case report. J. Clin. Transl. Endocrinol. Case Rep. 2021, 19, 100075. [Google Scholar] [CrossRef]
- Noone, T.C.; Hosey, J.; Firat, Z.; Semelka, R.C. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 195–211. [Google Scholar] [CrossRef]
- Uccella, S.; La Rosa, S. Looking into digestive mixed neuroendocrine—Nonneuroendocrine neoplasms: Subtypes, prognosis, and predictive factors. Histopathology 2020, 77, 700–717. [Google Scholar] [CrossRef]
- Sotoudehmanesh, R.; Hedayat, A.; Shirazian, N.; Shahraeeni, S.; Ainechi, S.; Zeinali, F.; Kolahdoozan, S. Endoscopic ultrasonography (EUS) in the localization of insulinoma. Endocrine 2007, 31, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ba, Y.; Xing, Q.; Du, J.L. Diagnostic value of endoscopic ultrasound for insulinoma localization: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0206099. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Hati, A. Endoscopic ultrasound: A very important tool in detecting small insulinomas. QJM 2022, 115, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, J.; Zhu, J. Diagnostic performance of noninvasive imaging modalities for localization of insulinoma: A meta-analysis. Eur. J. Radiol. 2021, 145, 110016. [Google Scholar] [CrossRef]
- Hackeng, W.M.; Schelhaas, W.; Morsink, F.H.M.; Heidsma, C.M.; van Eeden, S.; Valk, G.D.; Vriens, M.R.; Heaphy, C.M.; Nieveen van Dijkum, E.J.M.; Offerhaus, G.J.A.; et al. Alternative Lengthening of Telomeres and Differential Expression of Endocrine Transcription Factors Distinguish Metastatic and Non-metastatic Insulinomas. Endocr. Pathol. 2020, 31, 108–118. [Google Scholar] [CrossRef]
- Gambella, A.; Falco, E.C.; Metovic, J.; Maletta, F.; De Angelis, C.; Maragliano, R.; Uccella, S.; Pacchioni, D.; Papotti, M. Amyloid-Rich Pancreatic Neuroendocrine Tumors: A Potential Diagnostic Pitfall in Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology (EUS-FNAC). Endocr. Pathol. 2021, 32, 318–325. [Google Scholar] [CrossRef]
- Graham, R.P.; Shrestha, B.; Caron, B.L.; Smyrk, T.C.; Grogg, K.L.; Lloyd, R.V.; Zhang, L. Islet-1 is a sensitive but not entirely specific marker for pancreatic neuroendocrine neoplasms and their metastases. Am. J. Surg. Pathol. 2013, 37, 399–405. [Google Scholar] [CrossRef]
- Hofland, J.; Refardt, J.C.; Feelders, R.A.; Christ, E.; de Herder, W.W. Approach to the Patient: Insulinoma. J. Clin. Endocrinol. Metab. 2024, 109, 1109–1118. [Google Scholar] [CrossRef]
- Aida, A.; Noto, H. Diagnosis and Treatment Course of Insulinoma Presenting as Hypoglycemia Unawareness Using a Factory-Calibrated Continuous Glucose Monitoring System. Am. J. Case Rep. 2022, 23, e936723. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Yamada, E.; Matsumoto, S.; Nakajima, Y.; Nobusawa, S.; Yokoo, H.; Sekiguchi, S.; Yoshino, S.; Horiguchi, K.; Ishida, E.; et al. A case of insulinoma-induced hypoglycemia managed by Dexcom G4 Platinum. Neuro Endocrinol. Lett. 2022, 43, 161–166. [Google Scholar]
- Yuan, T.; Liu, S.; Zhu, C.; Dong, Y.; Zhu, H.; Wu, X.; Tang, Y.; Zhao, W. Continuous Glucose Monitoring in Patients With Insulinoma Treated by Endoscopic Ultrasound-Guided Ethanol Injection. Pancreas 2021, 50, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Suzuki, K.; Miyamoto, M.; Miyamoto, T.; Yanagi, K.; Shimizu, M.; Hirata, K. Disruptive nocturnal behavior due to insulinoma revealed by continuous glucose monitoring. Eur. J. Neurol. 2014, 21, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Watkin, D.; Evans, J.; Yip, V.; Cuthbertson, D.J. Multidisciplinary management of refractory insulinomas. Clin. Endocrinol. 2018, 88, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Altszuler, N.; Moraru, E.; Hampshire, J. On the mechanism of diazoxide-induced hyperglycemia. Diabetes 1977, 26, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.M.; Topliss, D.J.; Hamblin, P.S. Successful medical management of insulinoma with diazoxide for 27 years. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 20-0132. [Google Scholar] [CrossRef]
- Gill, G.V.; Rauf, O.; MacFarlane, I.A. Diazoxide treatment for insulinoma: A national UK survey. Postgrad. Med. J. 1997, 73, 640–641. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.A.; Grozinsky-Glasberg, S. Emerging therapies for advanced insulinomas and glucagonomas. Endocr. Relat. Cancer 2023, 30, e230020. [Google Scholar] [CrossRef]
- Blum, I.; Aderka, D.; Doron, M.; Laron, Z. Suppression of hypoglycemia by DL-propranolol in malignant insulinoma. N. Engl. J. Med. 1978, 299, 487. [Google Scholar] [CrossRef]
- Hofland, J.; Falconi, M.; Christ, E.; Castaño, J.P.; Faggiano, A.; Lamarca, A.; Perren, A.; Petrucci, S.; Prasad, V.; Ruszniewski, P.; et al. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023, 35, e13318. [Google Scholar] [CrossRef]
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Hofland, J.; Brabander, T.; Verburg, F.A.; Feelders, R.A.; de Herder, W.W. Peptide Receptor Radionuclide Therapy. J. Clin. Endocrinol. Metab. 2022, 107, 3199–3208. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Rinke, A.; Valle, J.W.; Fazio, N.; Caplin, M.; Gorbounova, V.; OConnor, J.; Eriksson, B.; Sorbye, H.; Kulke, M.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2: Chemotherapy. Neuroendocrinology 2017, 105, 281–294. [Google Scholar] [CrossRef]
- Castellano, D.; Bajetta, E.; Panneerselvam, A.; Saletan, S.; Kocha, W.; O’Dorisio, T.; Anthony, L.B.; Hobday, T.; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: A subgroup analysis of the phase III RADIANT-2 study. Oncologist 2013, 18, 46–53. [Google Scholar] [CrossRef]
- Howe, J.R.; Merchant, N.B.; Conrad, C.; Keutgen, X.M.; Hallet, J.; Drebin, J.A.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Sada, A.; McKenzie, T.J.; Vella, A.; Levy, M.J.; Halfdanarson, T.R. Interventional vs surgical procedures in localized/nonmetastatic insulinomas (ablation vs surgery). Endocr. Relat. Cancer 2023, 30, e220362. [Google Scholar] [CrossRef]
- Habibollahi, P.; Bai, H.X.; Sanampudi, S.; Soulen, M.C.; Dagli, M. Effectiveness of Liver-Directed Therapy for the Management of Intractable Hypoglycemia in Metastatic Insulinoma. Pancreas 2020, 49, 763–767. [Google Scholar] [CrossRef]
- Sakin, A.; Tambas, M.; Secmeler, S.; Can, O.; Arici, S.; Yasar, N.; Geredeli, C.; Demir, C.; Cihan, S. Factors Affecting Survival in Neuroendocrine Tumors: A 15-Year Single Center Experience. Asian Pac. J. Cancer Prev. 2018, 19, 3597–3603. [Google Scholar] [CrossRef]
- Hoskovec, D.; Krška, Z.; Škrha, J.; Klobušický, P.; Dytrych, P. Diagnosis and Surgical Management of Insulinomas-A 23-Year Single-Center Experience. Medicina 2023, 59, 1423. [Google Scholar] [CrossRef]
- Warner, R.P.R.; Martin, A.J.; Kim, M.K. Neuroendocrine Tumors. In Mount Sinai Expert Guides: Oncology, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, S.G.; Golli, A.L.; Matei, C.F.; Sonei, A.N.; Vere, C.; Cimpeanu, R.; Munteanu, M.; Munteanu, A. Pancreatic Neuroendocrine Tumors—Diagnostic Pitfalls of Non-Diabetic Severe Hypoglycemia: Literature Review and Case Report. Diagnostics 2025, 15, 337. https://doi.org/10.3390/diagnostics15030337
Popa SG, Golli AL, Matei CF, Sonei AN, Vere C, Cimpeanu R, Munteanu M, Munteanu A. Pancreatic Neuroendocrine Tumors—Diagnostic Pitfalls of Non-Diabetic Severe Hypoglycemia: Literature Review and Case Report. Diagnostics. 2025; 15(3):337. https://doi.org/10.3390/diagnostics15030337
Chicago/Turabian StylePopa, Simona Georgiana, Andreea Loredana Golli, Cristina Florentina Matei, Alexandra Nicoleta Sonei, Cristin Vere, Radu Cimpeanu, Marian Munteanu, and Alexandru Munteanu. 2025. "Pancreatic Neuroendocrine Tumors—Diagnostic Pitfalls of Non-Diabetic Severe Hypoglycemia: Literature Review and Case Report" Diagnostics 15, no. 3: 337. https://doi.org/10.3390/diagnostics15030337
APA StylePopa, S. G., Golli, A. L., Matei, C. F., Sonei, A. N., Vere, C., Cimpeanu, R., Munteanu, M., & Munteanu, A. (2025). Pancreatic Neuroendocrine Tumors—Diagnostic Pitfalls of Non-Diabetic Severe Hypoglycemia: Literature Review and Case Report. Diagnostics, 15(3), 337. https://doi.org/10.3390/diagnostics15030337





