Prediction of the Cause of Fundus-Obscuring Vitreous Hemorrhage Using Machine Learning
Abstract
1. Introduction
2. Materials and Methods
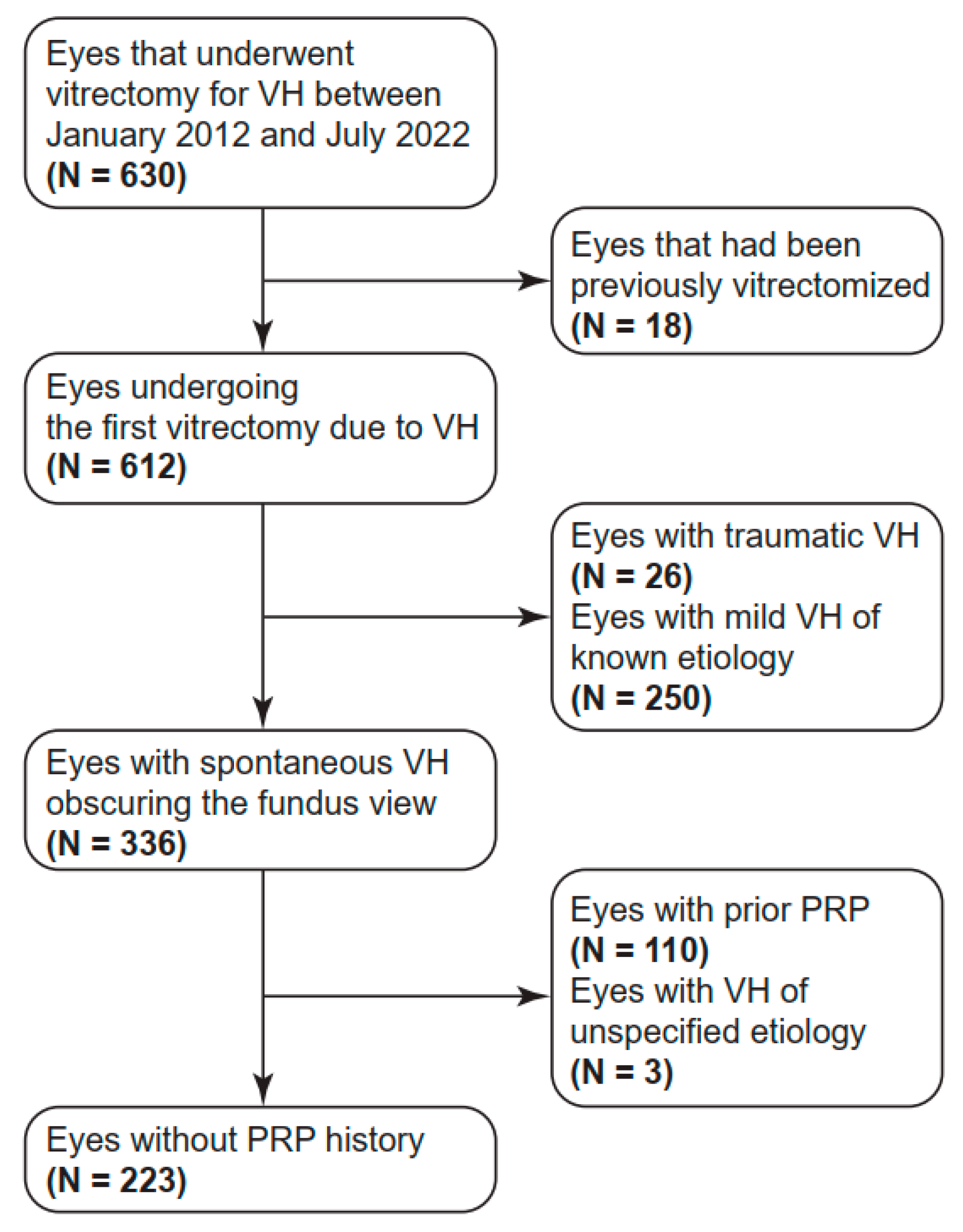
2.1. Subject Enrollment
2.2. Data Collection
2.3. Machine Learning
2.4. Statistical Analyses
3. Results
3.1. Clinical Characteristics of Patients
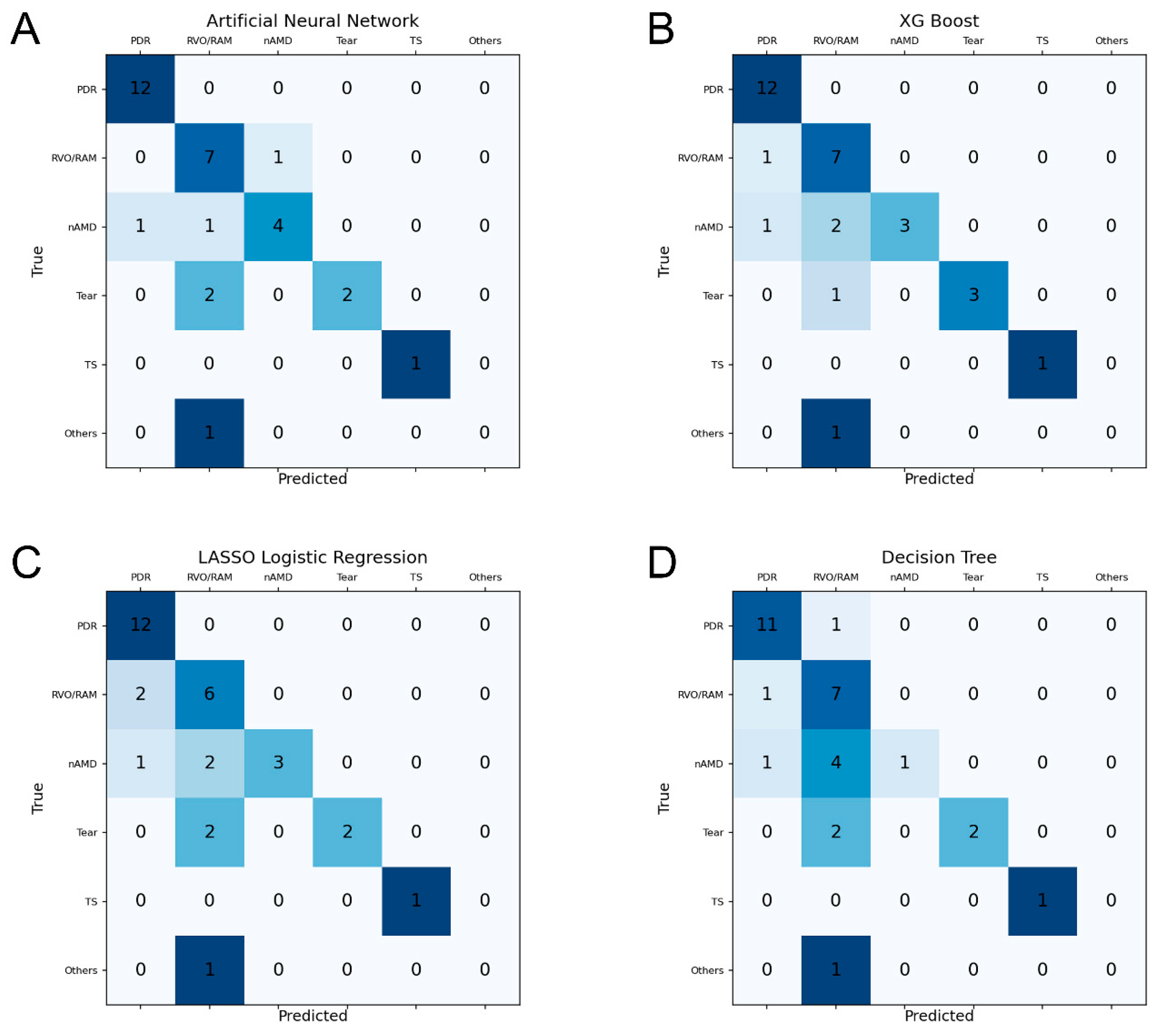
3.2. Prediction Performance of ML Models
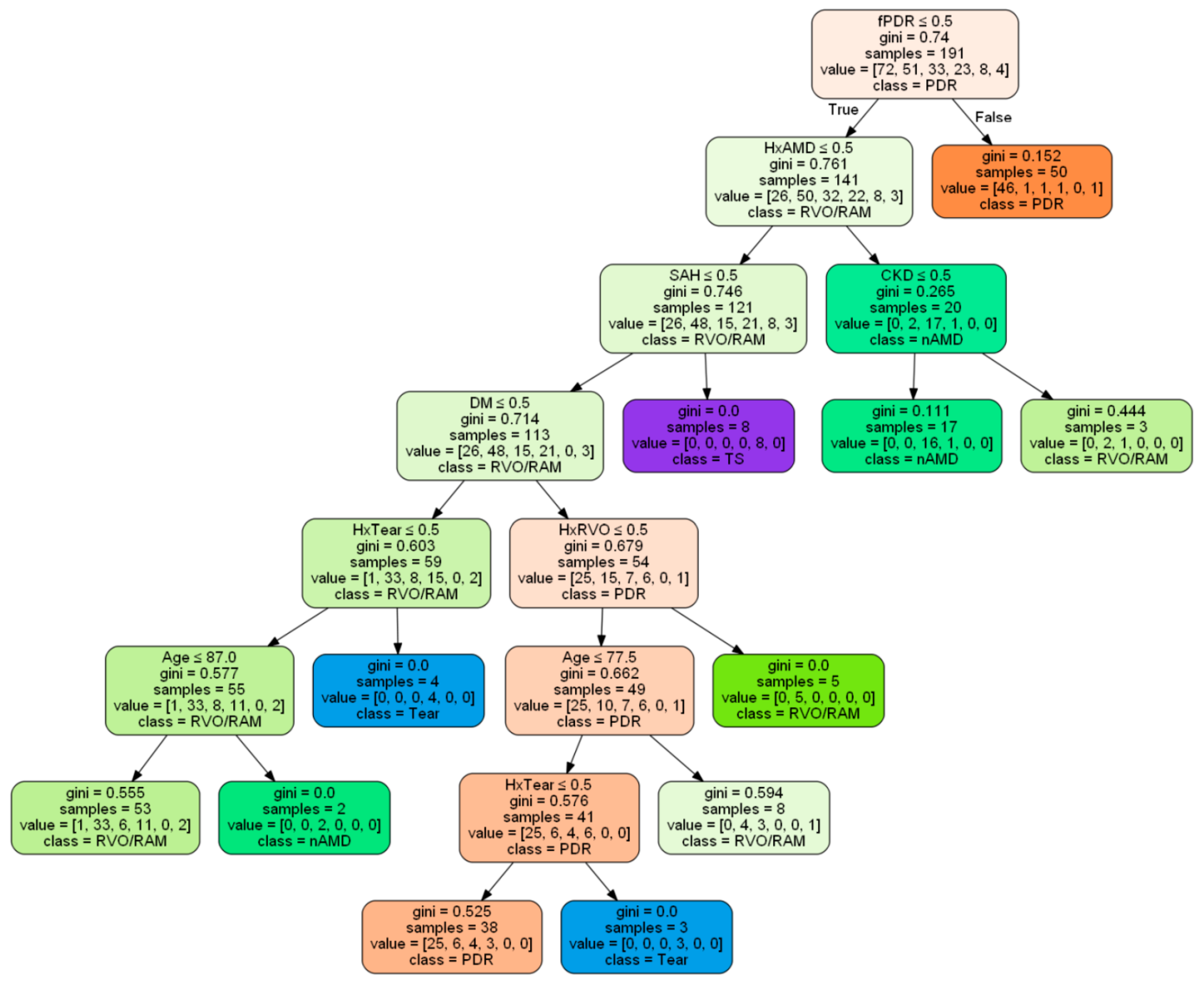
3.3. Common Predictive Factors and a Decision Tree
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANN | Artificial neural network |
| CAD | Coronary artery disease |
| CKD | Chronic kidney disease |
| CVA | Cerebrovascular accident |
| DM | Diabetes mellitus |
| FOVH | Fundus-obscuring vitreous hemorrhage |
| HTN | Hypertension |
| LASSO | Least absolute shrinkage and selection operator |
| ML | Machine learning |
| nAMD | Neovascular age-related macular degeneration |
| PDR | Proliferative diabetic retinopathy |
| PPV | Pars plana vitrectomy |
| PRP | Pan-retinal photocoagulation |
| RAM | Retinal arterial macroaneurysm |
| RD | Retinal detachment |
| RFE | Recursive feature elimination |
| RVO | Retinal vein occlusion |
| SAH | Subarachnoid hemorrhage |
| VH | Vitreous hemorrhage |
| XG-Boost | Extreme gradient boosting |
References
- Spraul, C.W.; Grossniklaus, H.E. Vitreous hemorrhage. Surv. Ophthalmol. 1997, 42, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Cheang, W.M.; Hwang, D.K.; Lin, C.H. Vitreous haemorrhage: A population-based study of the incidence and risk factors in Taiwan. Int. J. Ophthalmol. 2017, 10, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, G.; Sjödell, L.; Lindblom, B. A prospective study of dense spontaneous vitreous hemorrhage. Am. J. Ophthalmol. 1995, 119, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Srishti, R.; Khanum, A.; Thirumalesh, M.B.; Dave, V.; Arora, A.; Bansal, R.; Surve, A.; Azad, S.; Kumar, V. Vitreous hemorrhage—Causes, diagnosis, and management. Indian J. Ophthalmol. 2023, 71, 28–38. [Google Scholar] [CrossRef]
- Confalonieri, F.; Barone, G.; Ferraro, V.; Ambrosini, G.; Gaeta, A.; Petrovski, B.; Lumi, X.; Petrovski, G.; Di Maria, A. Early versus late pars plana vitrectomy in vitreous hemorrhage: A Systematic Review. J. Clin. Med. 2023, 12, 6652. [Google Scholar] [CrossRef]
- Sarrafizadeh, R.; Hassan, T.S.; Ruby, A.J.; Williams, G.A.; Garretson, B.R.; Capone, A., Jr.; Trese, M.T.; Margherio, R.R. Incidence of retinal detachment and visual outcome in eyes presenting with posterior vitreous separation and dense fundus-obscuring vitreous hemorrhage. Ophthalmology 2001, 108, 2273–2278. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef]
- Hashemian, H.; Peto, T.; Ambrósio, R., Jr.; Lengyel, I.; Kafieh, R.; Muhammed Noori, A.; Khorrami-Nejad, M. Application of artificial intelligence in ophthalmology: An updated comprehensive review. J. Ophthalmic Vis. Res. 2024, 19, 354–367. [Google Scholar] [CrossRef]
- Pitkänen, L.; Tommila, P.; Kaarniranta, K.; Jääskeläinen, J.E.; Kinnunen, K. Retinal arterial macroaneurysms. Acta Ophthalmol. 2014, 92, 101–104. [Google Scholar] [CrossRef]
- Cheung, N.; Klein, R.; Wang, J.J.; Cotch, M.F.; Islam, A.F.; Klein, B.E.; Cushman, M.; Wong, T.Y. Traditional and novel cardiovascular risk factors for retinal vein occlusion: The multiethnic study of atherosclerosis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4297–4302. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A scalable tree boosting system. In KDD ’16: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Published online: August 2016. Available online: https://dl.acm.org/doi/10.1145/2939672.2939785 (accessed on 15 August 2022).
- Cho, B.J.; Kim, K.M.; Bilegsaikhan, S.E.; Suh, Y.J. Machine learning improves the prediction of febrile neutropenia in Korean inpatients undergoing chemotherapy for breast cancer. Sci. Rep. 2020, 10, 14803. [Google Scholar] [CrossRef] [PubMed]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. Available online: https://www.jmlr.org/papers/v3/guyon03a.html (accessed on 22 April 2023).
- Santos, M.S.; Soares, J.P.; Abreu, P.H.; Araujo, H.; Santos, J. Cross-validation for imbalanced datasets: Avoiding overoptimistic and overfitting approaches. IEEE Comput. Intell. Mag. 2018, 13, 59–76. [Google Scholar] [CrossRef]
- Dana, M.R.; Werner, M.S.; Viana, M.A.; Shapiro, M.J. Spontaneous and traumatic vitreous hemorrhage. Ophthalmology 1993, 100, 1377–1383. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Sandinha, M.T.; Kotagiri, A.K.; Owen, R.I.; Geenen, C.; Steel, D.H. Accuracy of B-scan ultrasonography in acute fundus obscuring vitreous hemorrhage using a standardized scanning protocol and a dedicated ophthalmic ultrasonographer. Clin. Ophthalmol. 2017, 11, 1365–1370. [Google Scholar] [CrossRef]
- Rabinowitz, R.; Yagev, R.; Shoham, A.; Lifshitz, T. Comparison between clinical and ultrasound findings in patients with vitreous hemorrhage. Eye 2004, 18, 253–256. [Google Scholar] [CrossRef]
- Ko, F.; Knox, D.L. The ocular pathology of Terson’s syndrome. Ophthalmology 2010, 117, 1423–1429.e1422. [Google Scholar] [CrossRef]
- Hayashida, M.; Miki, A.; Imai, H.; Otsuka, K.; Azumi, A.; Nakamura, M. Impact of early vitrectomy for dense vitreous hemorrhage of unknown etiology. Ophthalmologica 2019, 242, 234–238. [Google Scholar] [CrossRef]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.S. Posterior vitreous detachment, retinal breaks, and lattice degeneration Preferred Practice Pattern®. Ophthalmology 2020, 127, P146–P181. [Google Scholar] [CrossRef]
- Speilburg, A.M.; Teitelbaum, B.; Pang, Y.; Ittiara, S. Symmetry of peripheral retinal nonperfusion in diabetic retinopathy by ischemic index. J. Optom. 2018, 11, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Riangwiwat, T.; Limpruttidham, N.; Rattanawong, P.; Rosen, R.B.; Deobhakta, A. Association of retinal vein occlusion with cardiovascular events and mortality: A systematic review and meta-analysis. Retina 2019, 39, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Chou, C.L.; Chen, J.S.; Kuo, C.H.; Wang, Y.C.; Lai, Y.H.; Lin, Y.L.; Wang, C.H.; Fang, T.C. Risk of mortality and of atherosclerotic events among patients who underwent hemodialysis and subsequently developed retinal vascular occlusion: A Taiwanese retrospective cohort study. JAMA Ophthalmol. 2016, 134, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Melamud, A.; Pham, H.; Stoumbos, Z. Early vitrectomy for spontaneous, fundus-obscuring vitreous hemorrhage. Am. J. Ophthalmol. 2015, 160, 1073–1077.e1071. [Google Scholar] [CrossRef]
- Antoszyk, A.N.; Glassman, A.R.; Beaulieu, W.T.; Jampol, L.M.; Jhaveri, C.D.; Punjabi, O.S.; Salehi-Had, H.; Wells, J.A.; Maguire, M.G.; Stockdale, C.R. Effect of intravitreous aflibercept vs vitrectomy with panretinal photocoagulation on visual acuity in patients with vitreous hemorrhage from proliferative diabetic retinopathy: A randomized clinical trial. JAMA 2020, 324, 2383–2395. [Google Scholar] [CrossRef]
- Russo, A.; Longo, A.; Avitabile, T.; Bonfiglio, V.; Fallico, M.; Boscia, F.; Furino, C.; Cillino, S.; Toro, M.; Rejdak, R. Incidence and risk factors for tractional macular detachment after anti-vascular endothelial growth factor agent pretreatment before vitrectomy for complicated proliferative diabetic retinopathy. J. Clin. Med. 2019, 8, 1960. [Google Scholar] [CrossRef]
- Gabrielle, P.H.; Delyfer, M.N.; Glacet-Bernard, A.; Conart, J.B.; Uzzan, J.; Kodjikian, L.; Arndt, C.; Tadayoni, R.; Soudry-Faure, A.; Creuzot Garcher, C.P. Surgery, tissue plasminogen activator, antiangiogenic agents, and age-related macular degeneration study: A randomized controlled trial for submacular hemorrhage secondary to age-related macular degeneration. Ophthalmology 2023, 130, 947–957. [Google Scholar] [CrossRef]
- Nazarali, S.; Kherani, I.; Hurley, B.; Williams, G.; Fielden, M.; Adatia, F.; Kherani, A. Outcomes of vitrectomy in Terson syndrome: A multicenter Canadian perspective. Retina 2020, 40, 1325–1330. [Google Scholar] [CrossRef]
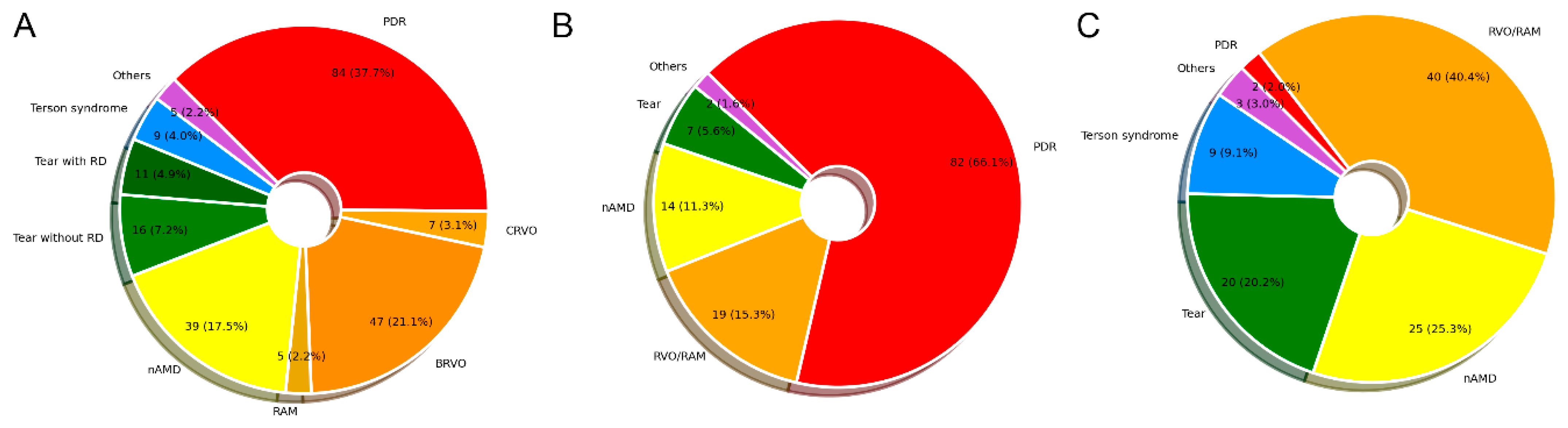
| All Patients (n = 204) | PDR (n = 72) | RVO/RAM (n = 57) | nAMD (n = 38) | Tear (n = 26) | Terson Syndrome (n = 6) | Others (n = 5) | p Value | |
|---|---|---|---|---|---|---|---|---|
| No. of eyes | 223 | 84 | 59 | 39 | 27 | 9 | 5 | |
| Age, year | 64.0 ± 12.8 | 58.7 ± 12.3 | 68.6 ± 11.2 | 73.1 ± 11.7 | 61.7 ± 7.6 | 50.0 ± 7.3 | 65.4 ± 13.6 | <0.001 a |
| Male | 125 (56.1) | 57 (67.9) | 25 (42.4) | 22 (56.4) | 16 (59.3) | 3 (33.3) | 2 (40.0) | 0.032 b |
| Right eye | 114 (51.1) | 43 (51.2) | 29 (49.2) | 21 (53.8) | 13 (48.1) | 5 (55.6) | 3 (60.0) | 0.993 b |
| Systemic disease | ||||||||
| DM | 124 (55.6) | 82 (97.6) | 19 (32.2) | 14 (35.9) | 7 (25.9) | 0 (0) | 2 (40.3) | <0.001 b |
| HTN | 131 (58.7) | 47 (56.0) | 43 (72.9) | 23 (59.0) | 16 (59.3) | 0 (0) | 2 (40.0) | 0.001 b |
| CKD | 37 (16.6) | 22 (26.2) | 8 (13.6) | 5 (12.8) | 1 (3.7) | 0 (0) | 1 (20.0) | 0.044 b |
| CVA | 15 (6.7) | 7 (8.3) | 1 (1.7) | 5 (12.8) | 1 (3.7) | 0 (0) | 1 (20.0) | 0.142 b |
| CAD | 19 (8.5) | 7 (8.3) | 8 (13.6) | 2 (5.1) | 1 (3.7) | 0 (0) | 1 (20.0) | 0.430 b |
| SAH | 9 (4.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (100.0) | 0 (0) | <0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Han, B.S.; Ha, J.E.; Park, M.S.; Kwon, S.; Cho, B.-J. Prediction of the Cause of Fundus-Obscuring Vitreous Hemorrhage Using Machine Learning. Diagnostics 2025, 15, 371. https://doi.org/10.3390/diagnostics15030371
Kim J, Han BS, Ha JE, Park MS, Kwon S, Cho B-J. Prediction of the Cause of Fundus-Obscuring Vitreous Hemorrhage Using Machine Learning. Diagnostics. 2025; 15(3):371. https://doi.org/10.3390/diagnostics15030371
Chicago/Turabian StyleKim, Jinsoo, Bo Sook Han, Joo Eun Ha, Min Seon Park, Soonil Kwon, and Bum-Joo Cho. 2025. "Prediction of the Cause of Fundus-Obscuring Vitreous Hemorrhage Using Machine Learning" Diagnostics 15, no. 3: 371. https://doi.org/10.3390/diagnostics15030371
APA StyleKim, J., Han, B. S., Ha, J. E., Park, M. S., Kwon, S., & Cho, B.-J. (2025). Prediction of the Cause of Fundus-Obscuring Vitreous Hemorrhage Using Machine Learning. Diagnostics, 15(3), 371. https://doi.org/10.3390/diagnostics15030371






