Targeting Ventricular Arrhythmias in Non-Ischemic Patients: Advances in Diagnosis and Treatment
Abstract
:1. Introduction
2. Defining Non-Ischemic Cardiomyopathies
2.1. Dilated Cardiomyopathy (DCM)
2.1.1. SCN5A Mutations
2.1.2. Laminopathies
2.1.3. Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) with LV Involvement
2.2. Hypertrophic Cardiomyopathy (HCM)
2.3. Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
2.4. Non-Dilated Left Ventricular Cardiomyopathy
2.4.1. Left Ventricular Non-Compaction (LVNC)
2.4.2. Chagas Disease
2.5. Restrictive Cardiomyopathy (RCM)
Cardiac Amyloidosis
3. Prevalence of Ventricular Tachycardia in NICM Subtypes
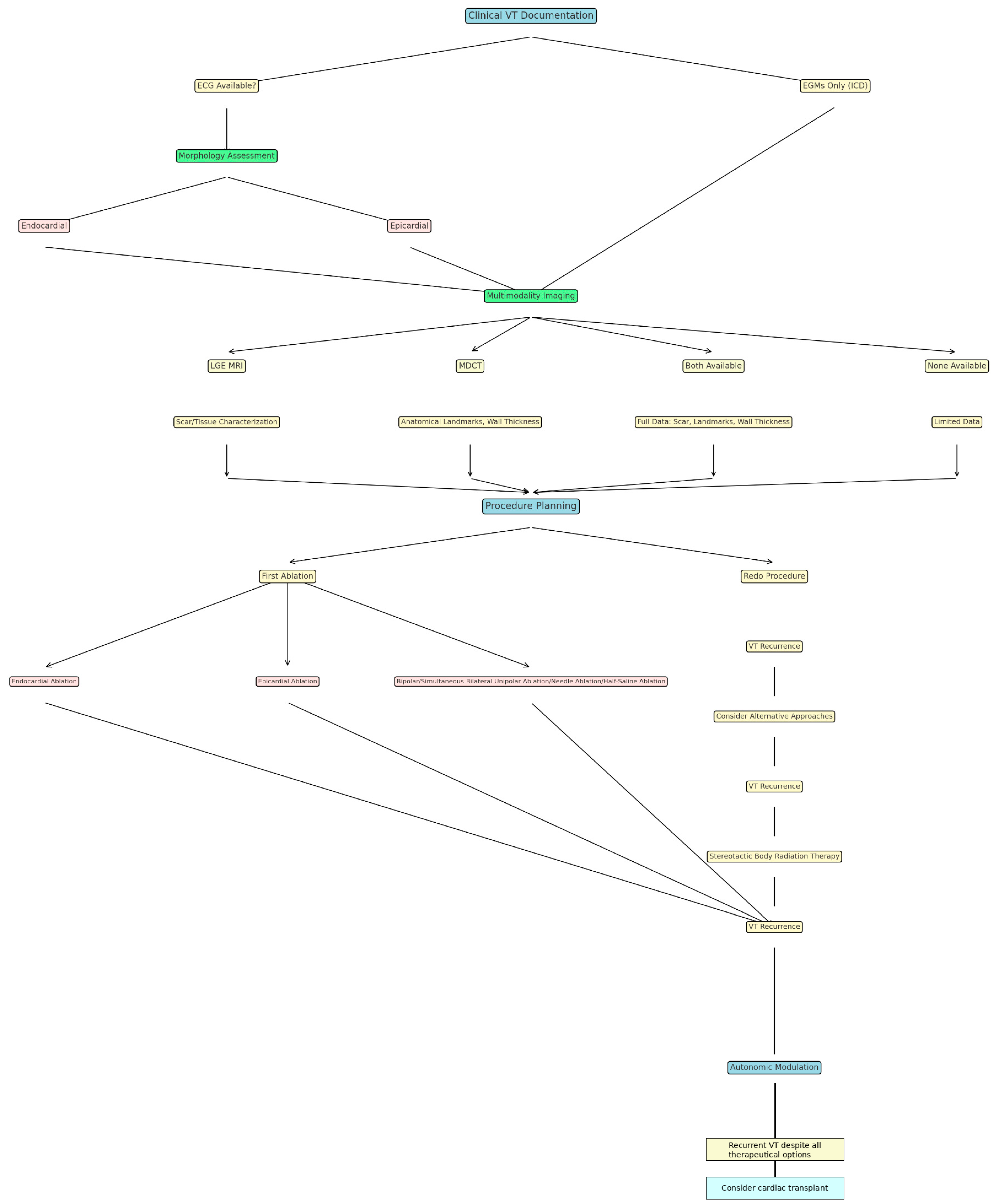
4. Pre-Procedural Imaging: VT Substrate Assessment Through Multimodality Imaging Techniques
4.1. Late Gadolinium Enhancement Magnetic Resonance Imaging (LGE-MRI)
4.2. Multi-Detector Computed Tomography (MDCT)
4.3. Echocardiography
4.4. Positron Emission Tomography (PET)
5. Ablative Techniques
5.1. Endocardial Ablation
5.2. Epicardial Ablation
5.3. Bipolar Ablation
5.4. Half-Saline-Infused Ablation
5.5. Surgical Ablation
5.6. Needle Ablation
5.7. Transarterial Coronary Ethanol Ablation and Retrograde Coronary Venous Ethanol Ablation
5.8. Ultra-Low-Temperature Cryoablation
5.9. Remote Magnetic Navigation
5.10. Novel and Emerging Approaches
5.10.1. Stereotactic Body Radiation Therapy (SBRT)
5.10.2. Neuraxial Modulation
5.10.3. Pulsed Field Ablation
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, M.; Wijnmaalen, A.P.; de Riva, M.; Trines, S.A.; Androulakis, A.F.A.; Glashan, C.A.; Schalij, M.J.; Peter van Tintelen, J.; Jongbloed, J.D.H.; Zeppenfeld, K. Prevalence and Prognostic Impact of Pathogenic Variants in Patients With Dilated Cardiomyopathy Referred for Ventricular Tachycardia Ablation. ACC Clin. Electrophysiol. 2020, 6, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Yagihara, N.; Watanabe, H.; Barnett, P.; Duboscq-Bidot, L.; Thomas, A.C.; Yang, P.; Ohno, S.; Hasegawa, K.; Kuwano, R.; Chatel, S.; et al. Variants in the SCN5A Promoter Associated With Various Arrhythmia Phenotypes. J. Am. Heart Assoc. 2016, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Bai, R.; Bahu, M.; Morris, M.F.; Weiss, J.P.; Tung, R. Localized intramural reentry confined within the ventricular septum in lamin cardiomyopathy. HeartRhythm Case Rep. 2022, 8, 840–844. [Google Scholar] [CrossRef]
- Kumar, S.; Androulakis, A.F.; Sellal, J.M.; Maury, P.; Gandjbakhch, E.; Waintraub, X.; Rollin, A.; Richard, P.; Charron, P.; Baldinger, S.H.; et al. Multicenter Experience With Catheter Ablation for Ventricular Tachycardia in Lamin A/C Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2016, 9, e004357. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Hypertrophic Cardiomyopathy With Left Ventricular Systolic Dysfunction: Insights From the SHaRe Registry. Circulation 2020, 41, 1371–1383. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef]
- Corrado, D.; Zorzi, A.; Cipriani, A.; Bauce, B.; Bariani, R.; Beffagna, G.; De Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; et al. Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021987. [Google Scholar] [CrossRef]
- Corrado, D.; Anastasakis, A.; Basso, C.; Bauce, B.; Blomström-Lundqvist, C.; Bucciarelli-Ducci, C.; Cipriani, A.; De Asmundis, C.; Gandjbakhch, E.; Jiménez-Jáimez, J.; et al. Proposed diagnostic criteria for arrhythmogenic cardiomyopathy: European Task Force consensus report. Int. J. Cardiol. 2024, 395, 131447. [Google Scholar] [CrossRef] [PubMed]
- te Riele, A.S.; Tandri, H.; Bluemke, D.A. Arrhythmogenic right ventricular cardiomyopathy (ARVC): Cardiovascular magnetic resonance update. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2014, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; A Blom, N.; A de Boer, R.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Weidemann, F.; Hall, J.L. Left ventricular noncompaction: A distinct cardiomyopathy or a trait shared by different cardiac diseases? J. Am. Coll. Cardiol. 2014, 64, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Velasco, A.; Pisani, C.F.; Alviz, I.; Briceno, D.; Díaz, J.C.; Della Rocca, D.G.; Natale, A.; Higuchi, M.d.L.; Scanavacca, M.; et al. Advanced Therapies for Ventricular Arrhythmias in Patients With Chagasic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1225–1242. [Google Scholar] [CrossRef]
- Ammash, N.M.; Seward, J.B.; Bailey, K.R.; Edwards, W.D.; Tajik, A.J. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation 2000, 101, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- de Marneffe, N.; Dulgheru, R.; Ancion, A.; Moonen, M.; Lancellotti, P. Cardiac amyloidosis: A review of the literature. Acta Cardiol. 2022, 77, 683–692. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Kimura, Y.; Ebert, M. Mapping and Ablation of Ventricular Tachycardia in Inherited Left Ventricular Cardiomyopathies. JACC Clin. Electrophysiol. 2024, 10, 585–603. [Google Scholar] [CrossRef]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. Hypertrophic Cardiomyopathy Outcomes Investigators. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Arsenos, P.; Gatzoulis, K.A.; Tsiachris, D.; Dilaveris, P.; Sideris, S.; Sotiropoulos, I.; Archontakis, S.; Antoniou, C.-K.; Kordalis, A.; Skiadas, I.; et al. Arrhythmic risk stratification in ischemic, non-ischemic and hypertrophic cardiomyopathy: A two-step multifactorial, electrophysiology study inclusive approach. World J. Cardiol. 2022, 14, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadawi, M.; Aslam, F.; Tao, M.; Fan, R.; Singh, A.; Rashba, E. Association of late gadolinium enhancement in cardiac magnetic resonance with mortality, ventricular arrhythmias, and heart failure in patients with nonischemic cardiomyopathy: A systematic review and meta-analysis. Heart Rhythm O2 2023, 4, 241–250. [Google Scholar] [CrossRef]
- Whitaker, J.; Rajani, R.; Ismail, T.F.; Wright, M.; Zei, C.P. The Use of Pre- and Peri-Procedural Imaging During VT Ablation. Curr. Treat. Options Cardio Med. 2024, 26, 13–28. [Google Scholar] [CrossRef]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: Executive summary. Heart Rhythm. 2020, 17, e155–e205. [Google Scholar] [CrossRef] [PubMed]
- Koruth, J.S.; Dukkipati, S.; Miller, M.A.; Neuzil, P.; D’Avila, A.; Reddy, V.Y. Bipolar irrigated radiofrequency ablation: A therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm. 2012, 9, 1932–1941. [Google Scholar] [CrossRef]
- Enriquez, A.; Hanson, M.; Nazer, B.; Gibson, D.N.; Cano, O.; Tokioka, S.; Fukamizu, S.; Millan, P.S.; Hoyos, C.; Matos, C.; et al. Bipolar ablation involving coronary venous system for refractory left ventricular summit arrhythmias. Heart Rhythm O2 2023, 5, 24–33. [Google Scholar] [CrossRef]
- Hanson, M.; Enriquez, A.; Garcia, F. Intramural Ventricular Arrhythmias: How to Crack a Hard Nut. Curr. Cardiol. Rep. 2024, 26, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Futyma, P.; Sultan, A.; Zarębski, Ł.; Imnadze, G.; Maslova, V.; Bordignon, S.; Kousta, M.; Knecht, S.; Pavlović, N.; Peichl, P.; et al. Bipolar radiofrequency ablation of refractory ventricular arrhythmias: Results from a multicentre network. Europace 2024, 26, euae248. [Google Scholar] [CrossRef]
- Yamada, T.; McElderry, H.T.; Doppalapudi, H.; Okada, T.; Murakami, Y.; Yoshida, Y.; Yoshida, N.; Inden, Y.; Murohara, T.; Plumb, V.J.; et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: Anatomic concepts relevant to ablation. Circ. Arrhythm. Electrophysiol. 2010, 3, 616–623. [Google Scholar] [CrossRef]
- Futyma, P.; Sauer, W.H. Bipolar Radiofrequency Catheter Ablation of Left Ventricular Summit Arrhythmias. Card. Electrophysiol. Clin. 2023, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.-P.; Lin, C.-Y.; Shirai, Y.; Futyma, P.; Santangeli, P.; Lin, Y.-J.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Chang, H.-Y.; et al. Outcomes of catheter ablation of ventricular arrhythmia originating from the left ventricular summit: A multicenter study. Heart Rhythm. 2020, 17, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Kuniewicz, M.; Baszko, A.; Ali, D.; Karkowski, G.; Loukas, M.; Walocha, J.A.; Hołda, M.K. Left Ventricular Summit-Concept, Anatomical Structure and Clinical Significance. Diagnostics 2021, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Nehoff, H.L.; Melton, I.; Crozier, I.G. A case report of a rare complication of an iatrogenic ventricular septal defect secondary to radiofrequency ablation. HeartRhythm Case Rep. 2023, 9, 542–544. [Google Scholar] [CrossRef]
- Schönbauer, R.; Sommer, P.; Misfeld, M.; Dinov, B.; Fiedler, L.; Huo, Y.; Gaspar, T.; Breithardt, O.-A.; Hindricks, G.; Arya, A. Relevant ventricular septal defect caused by steam pop during ablation of premature ventricular contraction. Circulation 2013, 127, e843–e844. [Google Scholar] [CrossRef] [PubMed]
- Darma, A.; Bertagnolli, L.; Torri, F.; Paetsch, I.; Jahnke, C.; Dinov, B.; Hindricks, G.; Arya, A. LV Pseudoaneurysm With Concomitant Mitral Valve Defect After LV Summit Ablation: A Rare Late Complication. JACC Case Rep. 2021, 3, 1756–1759. [Google Scholar] [CrossRef]
- Benhayon, D.; Nof, E.; Chik, W.W.; Marchlinski, F. Catheter Ablation in the Right Ventricular Outflow Tract Associated With Occlusion of Left Anterior Descending Coronary Artery. J. Cardiovasc. Electrophysiol. 2017, 28, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Zheng, L.; Zipse, M.M.; Borne, R.T.; Tzou, W.S.; Fleeman, B.; Sauer, W.H. Bipolar radiofrequency ablation creates different lesion characteristics compared to simultaneous unipolar ablation. J. Cardiovasc. Electrophysiol. 2019, 30, 2960–2967. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, A.; Berjano, E. Comparative Analysis of Different Methods of Modeling the Thermal Effect of Circulating Blood Flow During RF Cardiac Ablation. IEEE Trans. Biomed. Eng. 2016, 63, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Futyma, P.; Głuszczyk, R.; Futyma, M.; Kułakowski, P. Right atrial position of a return electrode for bipolar ablation of the left posterosuperior process ventricular tachycardia. Pacing Clin. Electrophysiol. 2019, 42, 474–477. [Google Scholar] [CrossRef]
- Futyma, P.; Ciąpała, K.; Głuszczyk, R.; Sander, J.; Futyma, M.; Kułakowski, P. Bipolar ablation of refractory atrial and ventricular arrhythmias: Importance of temperature values of intracardiac return electrodes. J. Cardiovasc. Electrophysiol. 2019, 30, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Yavin, H.D.; Higuchi, K.; Zilberman, I.; Sroubek, J.; Tchou, P.; Bubar, Z.P.; Barkagan, M.; Leshem, E.; Shapira-Daniels, A.; et al. Increasing Lesion Dimensions of Bipolar Ablation by Modulating the Surface Area of the Return Electrode. JACC Clin. Electrophysiol. 2022, 8, 498–510. [Google Scholar] [CrossRef]
- Tonko, J.B.; Lambiase, P. Exploring the Full Potential of Radiofrequency Technology: A Practical Guide to Advanced Radiofrequency Ablation for Complex Ventricular Arrhythmias. Curr. Cardiol. Rep. 2024, 26, 269–282. [Google Scholar] [CrossRef]
- Hasegawa, K.; Yoneda, Z.T.; Powers, E.M.; Tokutake, K.; Kurata, M.; Richardson, T.D.; A Montgomery, J.; Shen, S.; Estrada, J.C.; Saavedra, P.J.; et al. Safety of ventricular arrhythmia radiofrequency ablation with half-normal saline irrigation. Europace 2024, 26, euae018. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Vicera, J.J.B.; Lin, Y.; Chang, S.; Lo, L.; Hu, Y.; Lin, C.; Tuan, T.; Chao, T.; Liao, J.; et al. Clinical efficacy of open-irrigated electrode cooled with half-normal saline for initially failed radiofrequency ablation of idiopathic outflow tract ventricular arrhythmias. J. Cardiovasc. Electrophysiol. 2019, 30, 1508–1516. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, H.; Ma, K.; Ling, Z.; Zhao, D.; Wang, Y.; Zhang, Z.; Shao, M.; Song, H.; Jiang, W.; et al. Half versus normal saline irrigation during catheter ablation of outflow tract ventricular arrhythmias (HALF): A multi-center, parallel, open-label, randomized controlled study. J. Interv. Card. Electrophysiol. 2023, 66, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Anter, E.; Hutchinson, M.D.; Deo, R.; Haqqani, H.M.; Callans, D.J.; Gerstenfeld, E.P.; Garcia, F.C.; Bala, R.; Lin, D.; Riley, M.P.; et al. Surgical ablation of refractory ventricular tachycardia in patients with nonischemic cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2011, 4, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, M.; Rothstein, P.; Sauer, P.; Zipse, M.M.; Sandhu, A.; Tumolo, A.Z.; Borne, R.T.; Aleong, R.G.; Cleveland, J.C.; Fullerton, D.; et al. Open surgical ablation of ventricular tachycardia: Utility and feasibility of contemporary mapping and ablation tools. Heart Rhythm O2 2021, 2, 271–279. [Google Scholar] [CrossRef]
- Liang, J.J.; Canterbury, A.; Kancharla, K.; Santangeli, P. Catheter and surgical ablation for ventricular tachycardia in patients with left ventricular assist devices. Heart Rhythm. 2023, 20, 927–932. [Google Scholar] [CrossRef]
- Sapp, J.L. Needle Ablation for Ventricular Tachycardia: From Bench to Bedside. Can. J. Cardiol. 2022, 38, 1150–1152. [Google Scholar] [CrossRef]
- Stevenson, W.G.; Tedrow, U.B.; Reddy, V.; AbdelWahab, A.; Dukkipati, S.; John, R.M.; Fujii, A.; Schaeffer, B.; Tanigawa, S.; Elsokkari, I.; et al. Infusion Needle Radiofrequency Ablation for Treatment of Refractory Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2019, 73, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Dukkipati, S.R.; Nakamura, T.; Nakajima, I.; Oates, C.; Narui, R.; Tanigawa, S.; Sljapic, T.; Whang, W.; Koruth, J.S.; Choudry, S.; et al. Intramural Needle Ablation for Refractory Premature Ventricular Contractions. Circ. Arrhythm. Electrophysiol. 2022, 15, e010020. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Somonte, P.; Dyrda, K.; Nault, I.; Rivard, L.; Verma, A. Evaluation of Saline-Enhanced Radiofrequency Needle-Tip Ablation for Ventricular Tachycardia (SERF VT CANADA Trial). Can. J. Cardiol. 2022, 38, 1277–1285. [Google Scholar] [CrossRef]
- Inoue, H.; Waller, B.F.; Zipes, D.P. Intracoronary ethyl alcohol or phenol injection ablates aconitine-induced ventricular tachycardia in dogs. J. Am. Coll. Cardiol. 1987, 10, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Brugada, P.; de Swart, H.; Smeets, J.L.; Wellens, H.J. Transcoronary chemical ablation of ventricular tachycardia. Circulation 1989, 79, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Brugada, P.; de Swart, H.; Smeets, J.L.; Bär, F.W.; Wellens, H.J. Termination of tachycardias by interrupting blood flow to the arrhythmogenic area. Am. J. Cardiol. 1988, 62, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lador, A.; Da-Wariboko, A.; Tavares, L.; Valderrábano, M. Alcohol Ablation for Ventricular Tachycardia. Methodist. Debakey Cardiovasc. J. 2021, 17, 19–23. [Google Scholar] [CrossRef]
- Da-Wariboko, A.; Lador, A.; Tavares, L.; Dave, A.S.; Schurmann, P.A.; Peichl, P.; Kautzner, J.; Papiashvili, G.; Valderrábano, M. Double-balloon technique for retrograde venous ethanol ablation of ventricular arrhythmias in the absence of suitable intramural veins. Heart Rhythm. 2020, 17, 2126–2134. [Google Scholar] [CrossRef]
- Verma, A.; Essebag, V.; Neuzil, P.; Dyrda, K.; Balt, J.; Dinov, B.; Darma, A.; Arya, A.; Sacher, F.; Reddy, V.Y.; et al. Cryocure-VT: The safety and effectiveness of ultra-low-temperature cryoablation of monomorphic ventricular tachycardia in patients with ischaemic and non-ischaemic cardiomyopathies. Europace 2024, 26, euae076. [Google Scholar] [CrossRef]
- Jubran, N. Cryoablation for Monomorphic Ventricular Tachycardia (FULCRUM-VT). Available online: https://clinicaltrials.gov/study/NCT05675865 (accessed on 7 January 2025).
- Daniels, C.S.; Rubinsky, B. Cryosurgery with pulsed electric fields. PLoS ONE 2011, 6, e26219. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Feld, G.K.; Cox, J.L.; Dewland, T.A.; Babkin, A.; De Potter, T.; Raju, N.; Haissaguerre, M. Combined pulsed field ablation with ultra-low temperature cryoablation: A preclinical experience. J. Cardiovasc. Electrophysiol. 2023, 34, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Blandino, A.; Bianchi, F.; Masi, A.S.; Mazzanti, A.; D’Ascenzo, F.; Grossi, S.; Musumeci, G. Outcomes of manual versus remote magnetic navigation for catheter ablation of ventricular tachycardia: A systematic review and updated meta-analysis. Pacing Clin. Electrophysiol. 2021, 44, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Noten, A.M.E.; Szili-Torok, T.; Ernst, S.; Burkhardt, D.; Cavaco, D.; Chen, X.; Cheung, J.W.; de Chillou, C.; Crystal, E.; Cooper, D.H.; et al. Best practices in robotic magnetic navigation-guided catheter ablation of cardiac arrhythmias, a position paper of the Society for Cardiac Robotic Navigation. Front. Cardiovasc. Med. 2024, 11, 1431396. [Google Scholar] [CrossRef] [PubMed]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N. Engl. J. Med. 2017, 377, 2325–2336. [Google Scholar] [CrossRef]
- Lloyd, M.S.; Wight, J.; Schneider, F.; Hoskins, M.; Attia, T.; Escott, C.; Lerakis, S.; Higgins, K.A. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm. 2020, 17, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Krug, D.; Blanck, O.; Demming, T.; Dottermusch, M.; Koch, K.; Hirt, M.; Kotzott, L.; Zaman, A.; Eidinger, L.; Siebert, F.-A.; et al. Stereotactic body radiotherapy for ventricular tachycardia (cardiac radiosurgery): First-in-patient treatment in Germany. Strahlenther. Onkol. 2020, 196, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Arkles, J.; Markman, T.; Trevillian, R.; Yegya-Raman, N.; Garg, L.; Nazarian, S.; Santangeli, P.; Garcia, F.; Callans, D.; Frankel, D.S.; et al. One-year outcomes after stereotactic body radiotherapy for refractory ventricular tachycardia. Heart Rhythm. 2024, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ninni, S.; Gallot-Lavallée, T.; Klein, C.; Longère, B.; Brigadeau, F.; Potelle, C.; Crop, F.; Rault, E.; Decoene, C.; Lacornerie, T.; et al. Stereotactic Radioablation for Ventricular Tachycardia in the Setting of Electrical Storm. Circ. Arrhythm. Electrophysiol. 2022, 15, e010955. [Google Scholar] [CrossRef]
- Tung, R.; Shivkumar, K. Neuraxial modulation for treatment of VT storm. J. Biomed. Res. 2015, 29, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, M.; Barwad, P.; Corrales, F.J.M.; Tandri, H.; Mathuria, N.; Shah, R.; Sorg, J.M.; Gima, J.; Mandal, K.; Morales, L.C.S.; et al. Cardiac Sympathetic Denervation for Refractory Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2017, 69, 3070–3080. [Google Scholar] [CrossRef]
- Stavrakis, S.; Kulkarni, K.; Singh, J.P.; Katritsis, D.G.; Armoundas, A.A. Autonomic Modulation of Cardiac Arrhythmias: Methods to Assess Treatment and Outcomes. JACC Clin. Electrophysiol. 2020, 6, 467–483. [Google Scholar] [CrossRef]
- Hadaya, J.; Ardell, J.L. Autonomic Modulation for Cardiovascular Disease. Front. Physiol. 2020, 11, 617459. [Google Scholar] [CrossRef] [PubMed]
- van Weperen, V.Y.H.; Vos, M.A.; Ajijola, O.A. Autonomic modulation of ventricular electrical activity: Recent developments and clinical implications. Clin. Auton. Res. 2021, 31, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Chen, S.; Tohoku, S.; Bordignon, S.; Bologna, F.; Chun, K.R.J. Single shot electroporation of premature ventricular contractions from the right ventricular outflow tract. Europace 2022, 24, 597. [Google Scholar] [CrossRef] [PubMed]
- Katrapati, P.; Weiss, J.P.; Baning, D.; Zawaneh, M.; Su, W.; Tung, R. Pulsed field ablation for incessant scar-related ventricular tachycardia: First US report. Heart Rhythm. 2024, 21, 1236–1239. [Google Scholar] [CrossRef]
- Lozano-Granero, C.; Hirokami, J.; Franco, E.; Tohoku, S.; Matía-Francés, R.; Schmidt, B.; Hernández-Madrid, A.; Gómez, J.L.Z.; Moreno, J.; Chun, J. Case Series of Ventricular Tachycardia Ablation With Pulsed-Field Ablation: Pushing Technology Further (Into the Ventricle). JACC Clin. Electrophysiol. 2023, 9, 1990–1994. [Google Scholar] [CrossRef]
- Koruth, J.S.; Kuroki, K.; Iwasawa, J.; Viswanathan, R.; Brose, R.; Buck, E.D.; Donskoy, E.; Dukkipati, S.R.; Reddy, V.Y. Endocardial ventricular pulsed field ablation: A proof-of-concept preclinical evaluation. Europace 2020, 22, 434–439. [Google Scholar] [CrossRef]
- Askarinejad, A.; Kohansal, E.; Sabahizadeh, A.; Hesami, H.; Adimi, S.; Haghjoo, M. Pulsed-Field Ablation in Management of Ventricular Tachycardia: A Systematic Review of Case Reports and Clinical Outcomes. Clin. Cardiol. 2024, 47, e70018. [Google Scholar] [CrossRef]
- Sapp, J.L.; Wells, G.A.; Parkash, R.; Stevenson, W.G.; Blier, L.; Sarrazin, J.-F.; Thibault, B.; Rivard, L.; Gula, L.; Leong-Sit, P.; et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N. Engl. J. Med. 2016, 375, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K. Ventricular Tachycardia Ablation in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2018, 4, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.D.; Marchlinski, F.E. Epicardial Ablation of VT in Patients with Nonischemic LV Cardiomyopathy. Card. Electrophysiol. Clin. 2010, 2, 93–103. [Google Scholar] [CrossRef]
- Bhaskaran, A.; De Silva, K.; Rao, K.; Campbell, T.; Trivic, I.; Bennett, R.G.; Kizana, E.; Kumar, S. Ventricular Tachycardia Ablation in Non-ischemic Cardiomyopathy. Korean Circ. J. 2020, 50, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Sacher, F.; Sobieszczyk, P.; Tedrow, U.; Eisenhauer, A.C.; Field, M.E.; Selwyn, A.; Raymond, J.-M.; Koplan, B.; Epstein, L.M.; Stevenson, W.G. Transcoronary ethanol ventricular tachycardia ablation in the modern electrophysiology era. Heart Rhythm. 2008, 5, 62–68. [Google Scholar] [CrossRef]
- Subramanian, M.; Atreya, A.R.; Saggu, D.K.; Yalagudri, S.; Calambur, N. Catheter ablation of ventricular tachycardia: Strategies to improve outcomes. Front. Cardiovasc. Med. 2023, 10, 966634. [Google Scholar] [CrossRef]
- Stanciulescu, L.A.; Vatasescu, R. Ventricular Tachycardia Catheter Ablation: Retrospective Analysis and Prospective Outlooks—A Comprehensive Review. Biomedicines 2024, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Missel, R.; Gyawali, P.K.; Murkute, J.V.; Li, Z.; Zhou, S.; AbdelWahab, A.; Davis, J.; Warren, J.; Sapp, J.L.; Wang, L. A hybrid machine learning approach to localizing the origin of ventricular tachycardia using 12-lead electrocardiograms. Comput. Biol. Med. 2020, 126, 104013. [Google Scholar] [CrossRef] [PubMed]
- Kabra, R.; Israni, S.; Vijay, B.; Baru, C.; Mendu, R.; Fellman, M.; Sridhar, A.; Mason, P.; Cheung, J.W.; DiBiase, L.; et al. Emerging role of artificial intelligence in cardiac electrophysiology. Cardiovasc. Digit. Health J. 2022, 3, 263–275. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Marti-Gutierrez, N.; Park, S.-W.; Wu, J.; Lee, Y.; Suzuki, K.; Koski, A.; Ji, D.; Hayama, T.; Ahmed, R.; et al. Correction of a pathogenic gene mutation in human embryos. Nature 2017, 548, 413–419. [Google Scholar] [CrossRef]
- Kinnear, C.; Said, A.; Meng, G.; Zhao, Y.; Wang, E.Y.; Rafatian, N.; Parmar, N.; Wei, W.; Billia, F.; Simmons, C.A.; et al. Myosin inhibitor reverses hypertrophic cardiomyopathy in genotypically diverse pediatric iPSC-cardiomyocytes to mirror variant correction. Cell Rep. Med. 2024, 5, 101520. [Google Scholar] [CrossRef]
- Mundisugih, J.; Ravindran, D.; Kizana, E. Exploring the Therapeutic Potential of Gene Therapy in Arrhythmogenic Right Ventricular Cardiomyopathy. Biomedicines 2024, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.; Zeng, A.; Greer-Short, A.; Aycinena, J.A.; Tefera, A.E.; Shenwai, R.; Farshidfar, F.; Van Pell, M.; Xu, E.; Reid, C.; et al. AAV9:PKP2 improves heart function and survival in a Pkp2-deficient mouse model of arrhythmogenic right ventricular cardiomyopathy. Commun. Med. 2024, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, E.; Versteeg, D.; de Ruiter, H.; Perini, I.; Seibertz, F.; Döring, Y.; Zentilin, L.; Tsui, H.; van Kampen, S.J.; Tiburcy, M.; et al. Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy. Nat. Cardiovasc. Res. 2023, 2, 1262–1276. [Google Scholar] [CrossRef]
- Bradford, W.H.; Zhang, J.; Gutierrez-Lara, E.J.; Liang, Y.; Do, A.; Wang, T.-M.; Nguyen, L.; Mataraarachchi, N.; Wang, J.; Gu, Y.; et al. Plakophilin 2 gene therapy prevents and rescues arrhythmogenic right ventricular cardiomyopathy in a mouse model harboring patient genetics. Nat. Cardiovasc. Res. 2023, 2, 1246–1261. [Google Scholar] [CrossRef] [PubMed]
- van Opbergen, C.J.; Narayanan, B.; Sacramento, C.B.; Stiles, K.M.; Mishra, V.; Frenk, E.; Ricks, D.; Chen, G.; Zhang, M.; Yarabe, P.; et al. AAV-Mediated Delivery of Plakophilin-2a Arrests Progression of Arrhythmogenic Right Ventricular Cardiomyopathy in Murine Hearts: Preclinical Evidence Supporting Gene Therapy in Humans. Circ. Genom. Precis. Med. 2024, 17, e004305. [Google Scholar] [CrossRef] [PubMed]

| NICM Subtype | Typical Fibrosis Distribution | Most Common VT Exit Points |
|---|---|---|
| Dilated Cardiomyopathy (DCM) | Two fibrosis distribution patterns: a septal one (usually with an extension towards the anterior and the inferior segments) and one involving the basal LV lateral wall. The septal pattern has an overall worse prognosis and oftentimes requires a more complex ablative approach (bilateral unipolar approach, bipolar ablation, needle techniques, saline infused ablation, transcoronary ethanol ablation and surgical ablation). | Interventricular septum and the basal LV lateral wall |
| SCN5A Mutations | Fibrosis in the conduction system and adjacent myocardial tissue. | His-Purkinje system, interventricular septum |
| Laminopathies | Mid-myocardial or subepicardial, often basal LV anterior wall and septum, but can sometimes be confined to the inferior wall or the subaortic mitral continuity. | Septal and basal LV anterior wall, basal inferior wall and the mitroaortic continuity |
| Left Ventricular Non-Compaction (LVNC) | Subendocardial fibrosis with trabeculated myocardial layers, particularly in the apex and mid-ventricle. | Apical and mid-ventricular regions |
| Hypertrophic Cardiomyopathy (HCM) | Patchy fibrosis, predominantly in hypertrophied regions, septal involvement common; apical in cases where apical aneurysm is associated. | Septal regions, left ventricular outflow tract (LVOT), LV apex if an apical aneurysm is present |
| Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) | Subepicardial, predominantly in the right ventricular free wall (especially in peritricuspidian inflow area but also the outflow tract). | Right ventricular free wall, subtricuspidian, outflow tract |
| Cardiac Sarcoidosis | Focal and patchy, frequently in the basal septum and lateral walls. | Basal septum, inferolateral wall |
| Cardiac Amyloidosis | Diffuse interstitial fibrosis, commonly affecting the entire myocardium with no specific predilection. The first affected area is the subendocardial area, but its substrate is more diffuse than seen in ischemic heart disease. | Diffuse VTs, multiple sites due to diffuse myocardial involvement, initially subendocardially |
| Chagas Disease | No specific LGE pattern—the scar can be subendocardial, transmural (most common), subepicardial or localized within the midwall. The scar is frequently localized in the interventricular septum, as well as the inferior, lateral and inferolateral LV walls. | Inferior interventricular septum, inferior, lateral and inferolateral LV walls |
| Myocarditis | Patchy and diffuse, often involving the lateral wall and septum. | Commonly involving the lateral wall and interventricular septum |
| Ablation Technique | Definition | Clinical Trials | Pros | Cons | Limitations | References |
|---|---|---|---|---|---|---|
| Endocardial Ablation | Ablation performed from the inner surface of the heart chambers. | VANISH Trial: Compared catheter ablation with escalated antiarrhythmic drug therapy in patients with ischemic cardiomyopathy and VT. | Minimally invasive; effective for subendocardial substrates. | Limited efficacy for epicardial or intramural substrates. | May not reach deeper or epicardial arrhythmogenic foci. | [76,77] |
| Epicardial Ablation | Ablation performed from the outer surface of the heart. | No specific large RCTs; observational studies suggest efficacy in non-ischemic cardiomyopathies. | Accesses arrhythmogenic substrates inaccessible endocardially. | Invasive; risk of complications like coronary artery or phrenic nerve injury. | Requires pericardial access; potential for procedural complications. | [77,78,79] |
| Bipolar Ablation | Ablation using two electrodes to create a circuit, allowing deeper lesion formation. | Limited clinical trial data; primarily case series and observational studies. | Creates deeper lesions with lower power; potentially more effective for intramural substrates. | Technically challenging; requires precise electrode positioning. | Limited availability; lack of standardized criteria for selecting the individuals suitable for bipolar ablation; requires further extensive clinical trial validation. | [25] |
| Half-Saline Infused Ablation | Ablation with half-normal saline irrigation to cool the electrode, allowing higher power delivery and deeper lesions. | Limited RCTs available (the HALF study); supported by small prospective studies and retrospective analyses. | Enables the creation of larger, deeper lesions. When the power is guided by impedance drop (and, if possible, assisted by ICE) it has a high acute success rate and a safety profile similar to NS ablation. | Risk of steam pops; potential for tissue overheating if not calibrated correctly. | Limited by catheter design and operator experience. | [40,41,42] |
| Surgical Ablation | Open-heart surgery to remove or isolate arrhythmogenic tissue. | Historical studies; largely supplanted by catheter-based techniques. | Direct visualization; effective for extensive substrates. | Highly invasive; significant morbidity and recovery time. | Reserved for patients undergoing cardiac surgery for other indications or those with refractory VAs and an extensive substrate inaccessible with standard approaches. | [43,44] |
| Needle Ablation | Percutaneous needle delivery of energy directly into myocardial tissue. | Emerging technique; limited clinical data available (the SERF VT study). | Targets deep intramural substrates; precise lesion placement. | Invasive; potential for myocardial perforation. | Experimental; not widely adopted in clinical practice. | [47,48,49] |
| Transarterial Coronary Ethanol Ablation (TCEA) | Injection of ethanol into coronary arteries supplying arrhythmogenic tissue to induce necrosis. | Limited to case reports and small series. | Effective for select cases with well-defined arterial supply to VT focus. | Risk of coronary artery damage; myocardial infarction. | Highly selective patient selection required; not widely practiced. | [80] |
| Retrograde Coronary Venous Ethanol Ablation (RCVEA) | Injection of ethanol into coronary veins to target arrhythmogenic tissue. | Sparse clinical data; limited to small case series. | Higher ethanol dilution due to retrograde flow, low risk of myocardial injury and potential arterial cannulation; lower complication rate than the transarterial approach. | Risk of venous thrombosis; limited efficacy data. | Experimental; lack of standardized protocols. | [53] |
| Ultra-Low-Temperature Cryoablation (ULTCA) | Near-critical nitrogen refrigerant near its boiling temperature of −196 °C continuously injected through small lumen catheters to penetrate deep scar areas and reach intramural substrates. | Limited clinical data; one clinical trial published to date (Cryocure-VT). One pivotal clinical trial currently under development (FULCRUM-VT). | Ability to create deeper lesions, reach intramural substrates and penetrate chronic scar tissue with potentially less complications than high-energy RF. | Requires specialized equipment; steep learning curve. | Early stages; published trials with short follow-up period; not widely adopted in clinical practice; limited by operator experience and lack of standardized protocols; requires further extensive clinical trial validation. | [55] |
| Remote Magnetic Navigation (RMN) | Use of magnetic fields to remotely steer ablation catheters within the heart. | Studies indicate safety and efficacy in various arrhythmias, including VT. | Enhanced catheter stability; reduced radiation exposure. | Requires specialized equipment; steep learning curve. | Limited availability; high procedural costs. | [59] |
| Pulsed Field Ablation (PFA) | Application of ultrashort, high-voltage electrical pulses to create microscopic pores in cell membranes and induce apoptosis. | Already an established therapy for AF; an emerging technique for VT. Limited to case reports and small series. | Able to target areas with poor catheter stability, fast applications with larger lesions in a shorter timeframe (may be useful in patients who cannot hemodynamically tolerate the inducible VTs). | Lesions may not be completely transmural; has not proven effective on epicardial substrate. | May not reach deeper or epicardial arrhythmogenic foci. Further research is required. | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciulescu, L.A.; Dorobantu, M.; Vatasescu, R. Targeting Ventricular Arrhythmias in Non-Ischemic Patients: Advances in Diagnosis and Treatment. Diagnostics 2025, 15, 420. https://doi.org/10.3390/diagnostics15040420
Stanciulescu LA, Dorobantu M, Vatasescu R. Targeting Ventricular Arrhythmias in Non-Ischemic Patients: Advances in Diagnosis and Treatment. Diagnostics. 2025; 15(4):420. https://doi.org/10.3390/diagnostics15040420
Chicago/Turabian StyleStanciulescu, Laura Adina, Maria Dorobantu, and Radu Vatasescu. 2025. "Targeting Ventricular Arrhythmias in Non-Ischemic Patients: Advances in Diagnosis and Treatment" Diagnostics 15, no. 4: 420. https://doi.org/10.3390/diagnostics15040420
APA StyleStanciulescu, L. A., Dorobantu, M., & Vatasescu, R. (2025). Targeting Ventricular Arrhythmias in Non-Ischemic Patients: Advances in Diagnosis and Treatment. Diagnostics, 15(4), 420. https://doi.org/10.3390/diagnostics15040420







