Image Quality in Adaptive Optics Optical Coherence Tomography of Diabetic Patients
Abstract
:1. Introduction
2. Materials and Methods
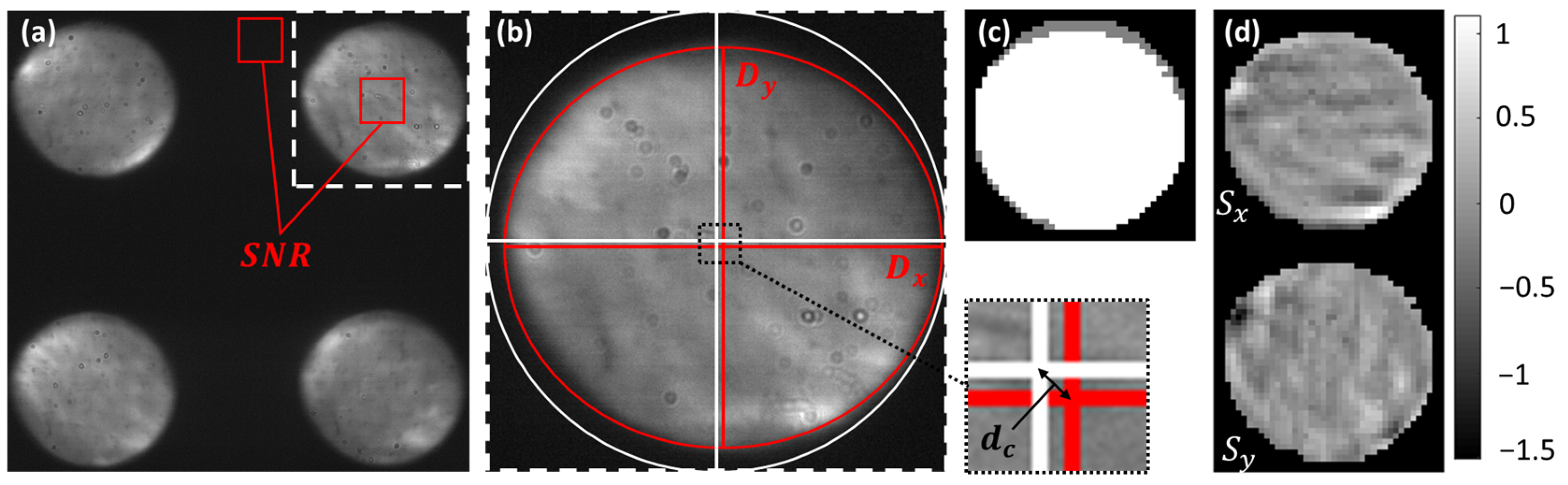
2.1. Assessment of AO-OCT Image Quality
2.2. Evaluation of Pyramid Wavefront Sensor Data
3. Results
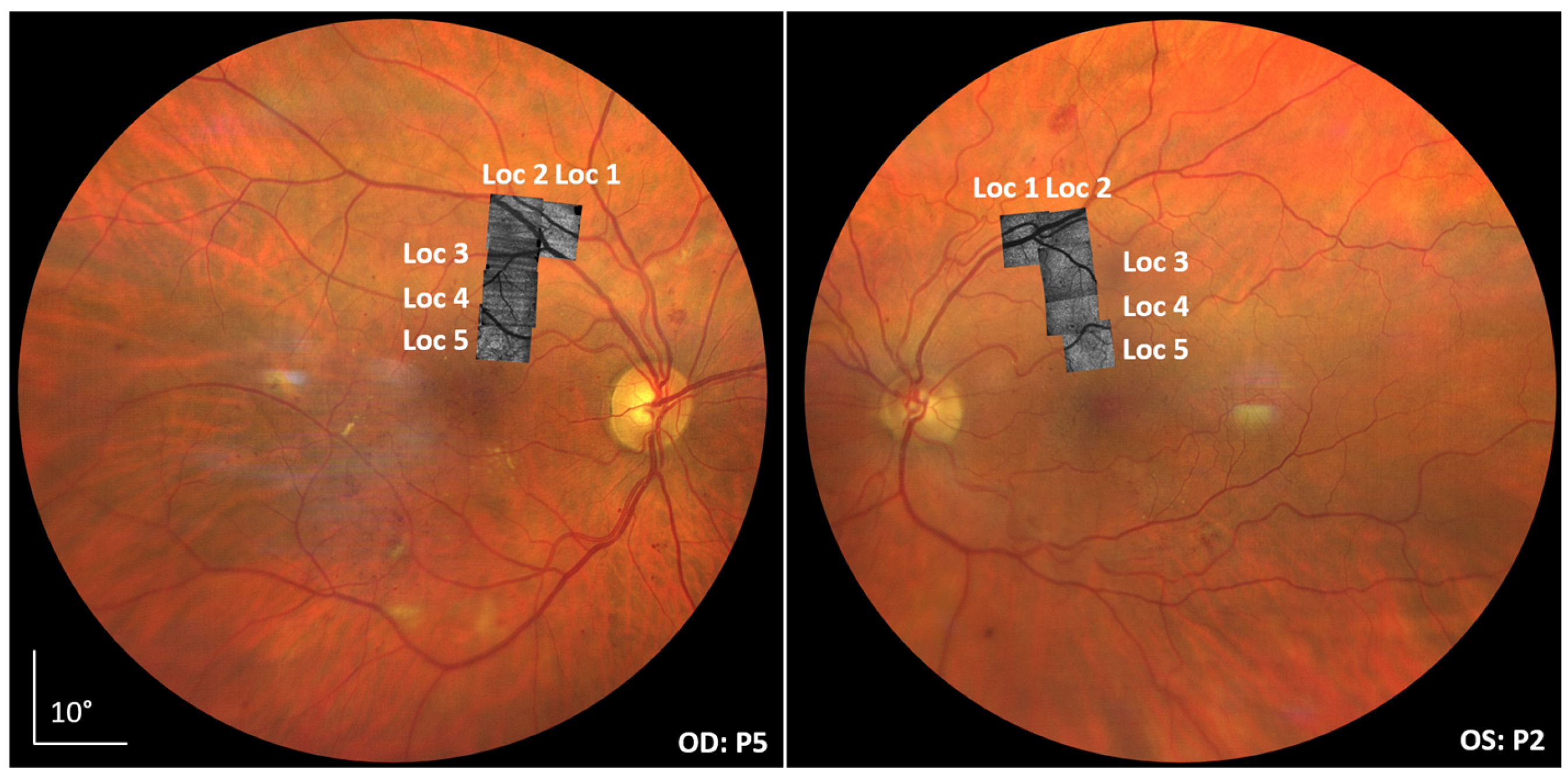
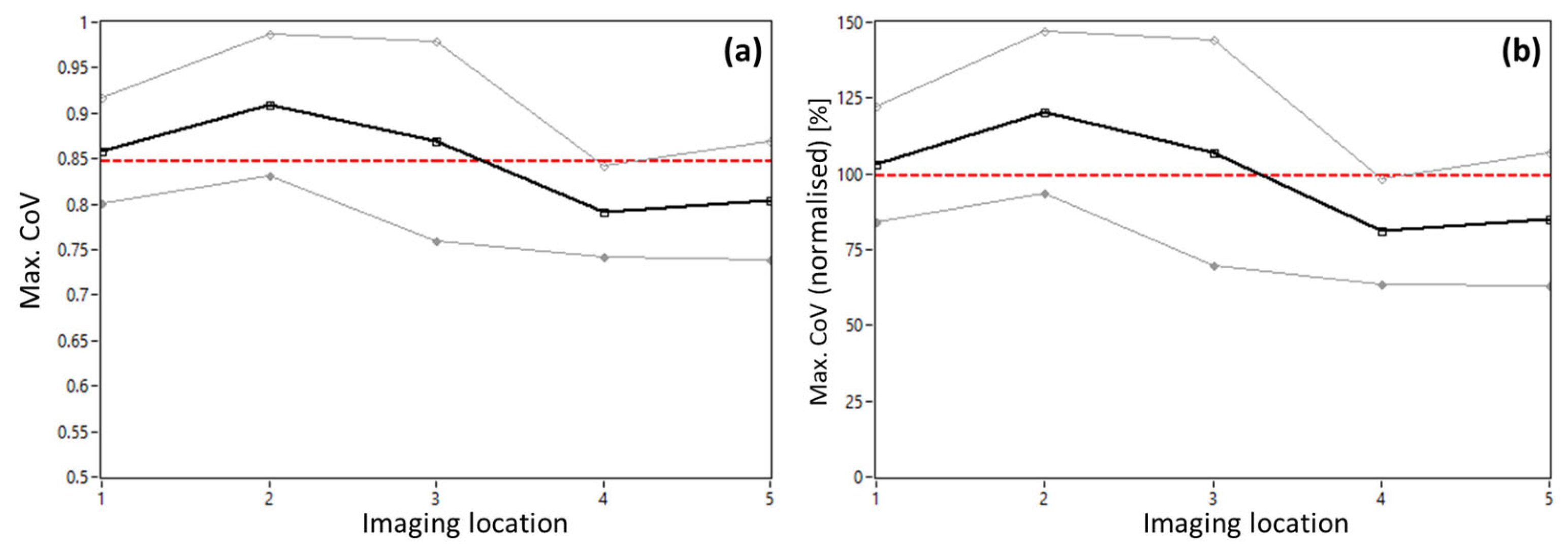
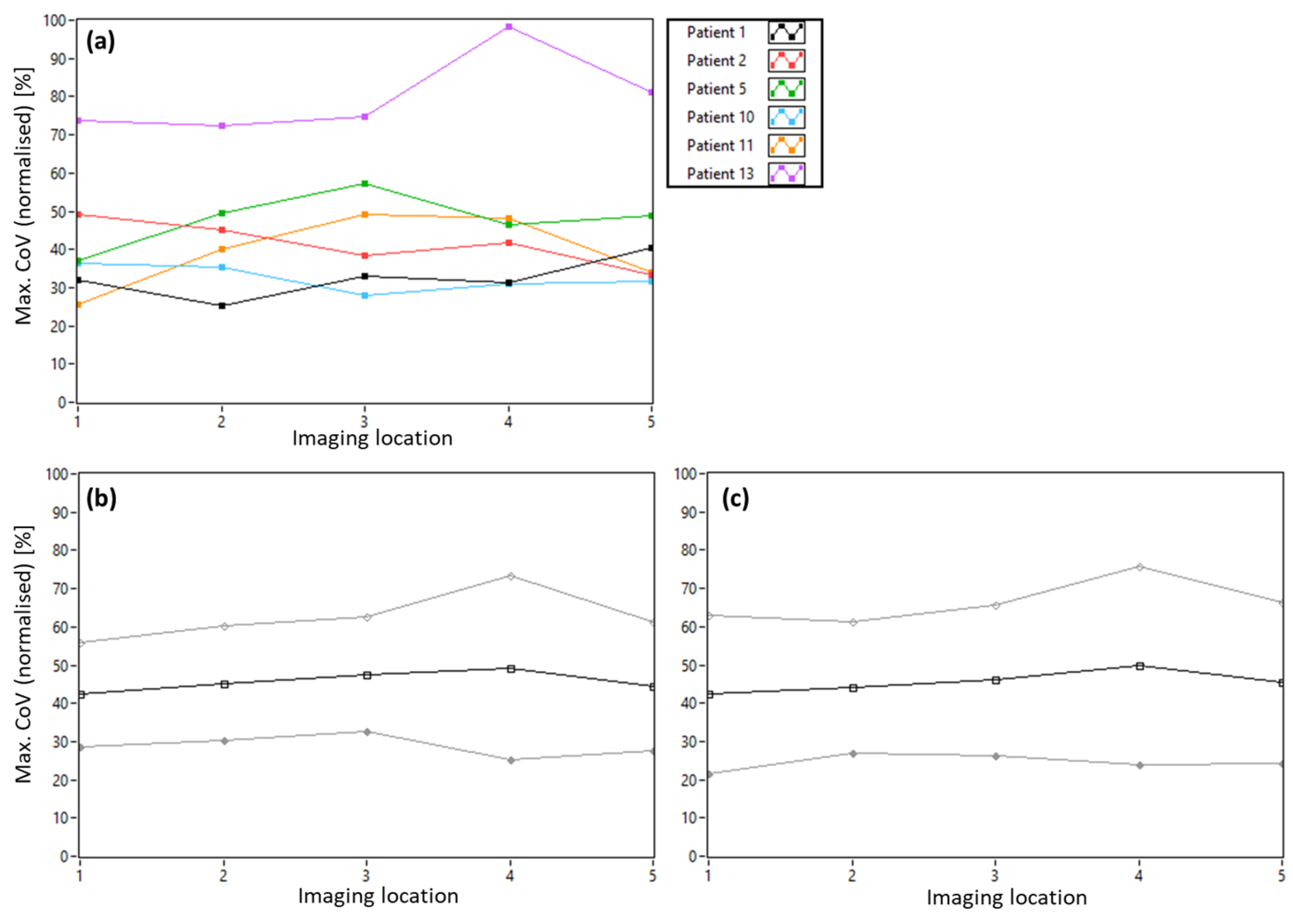
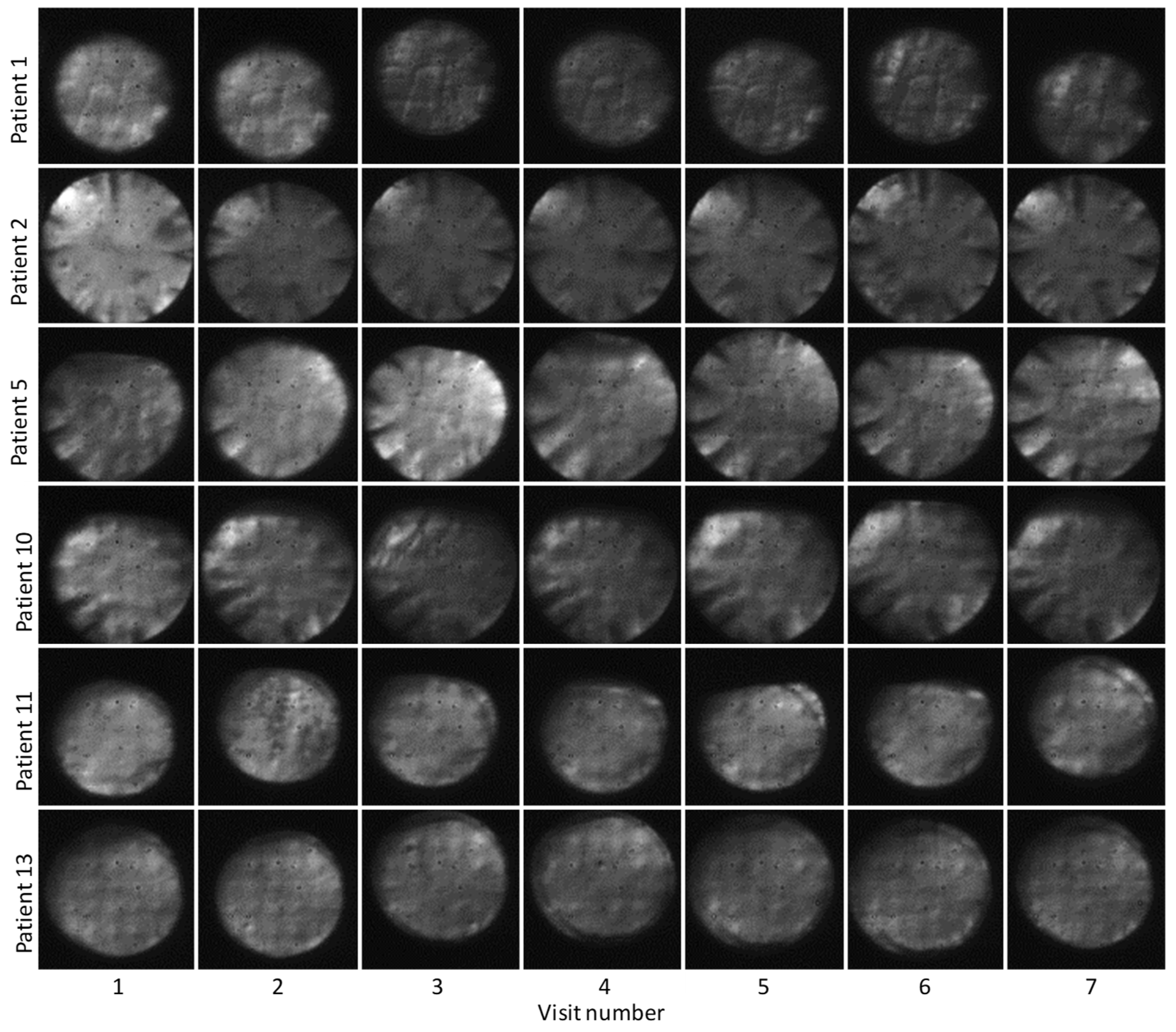
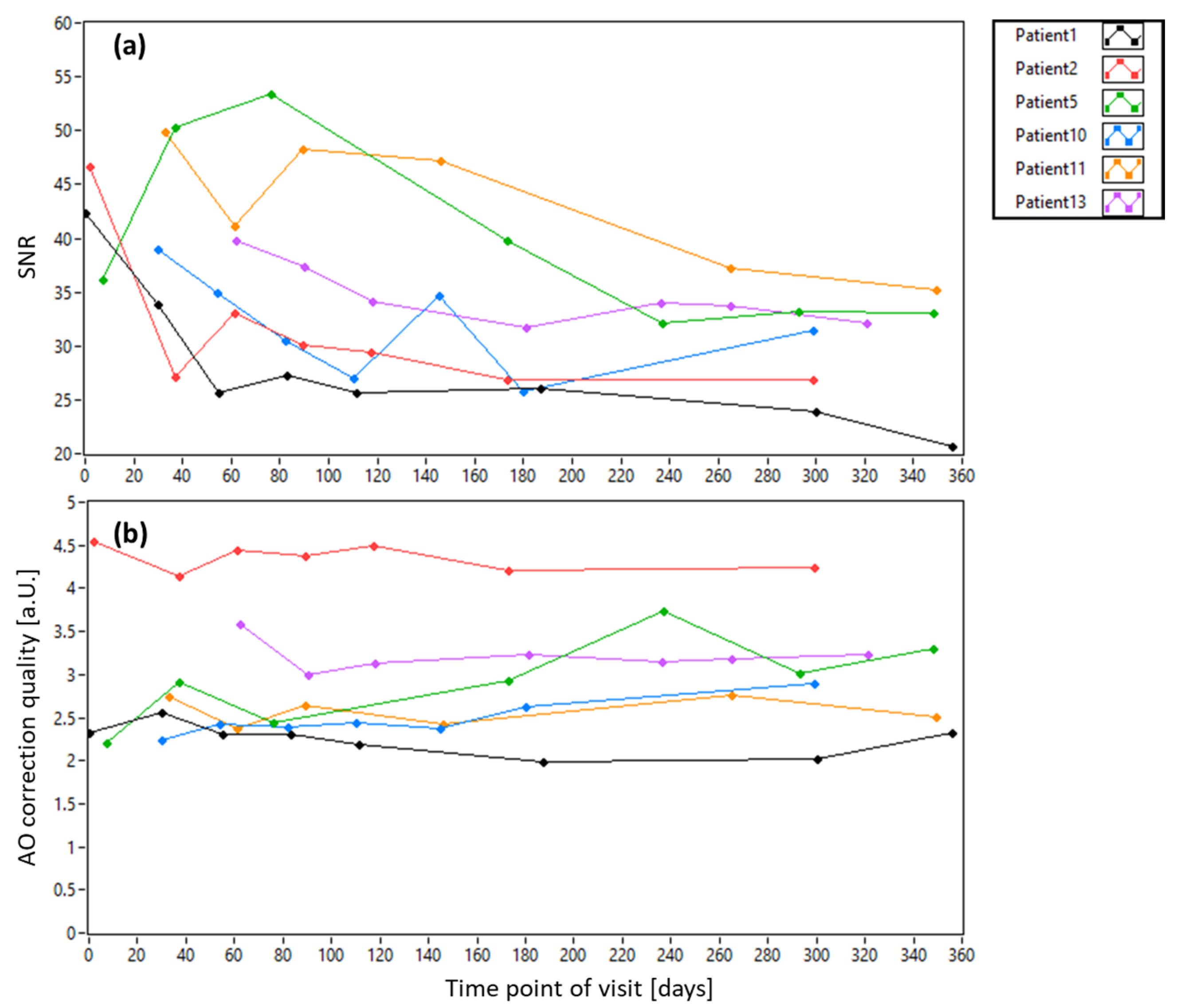
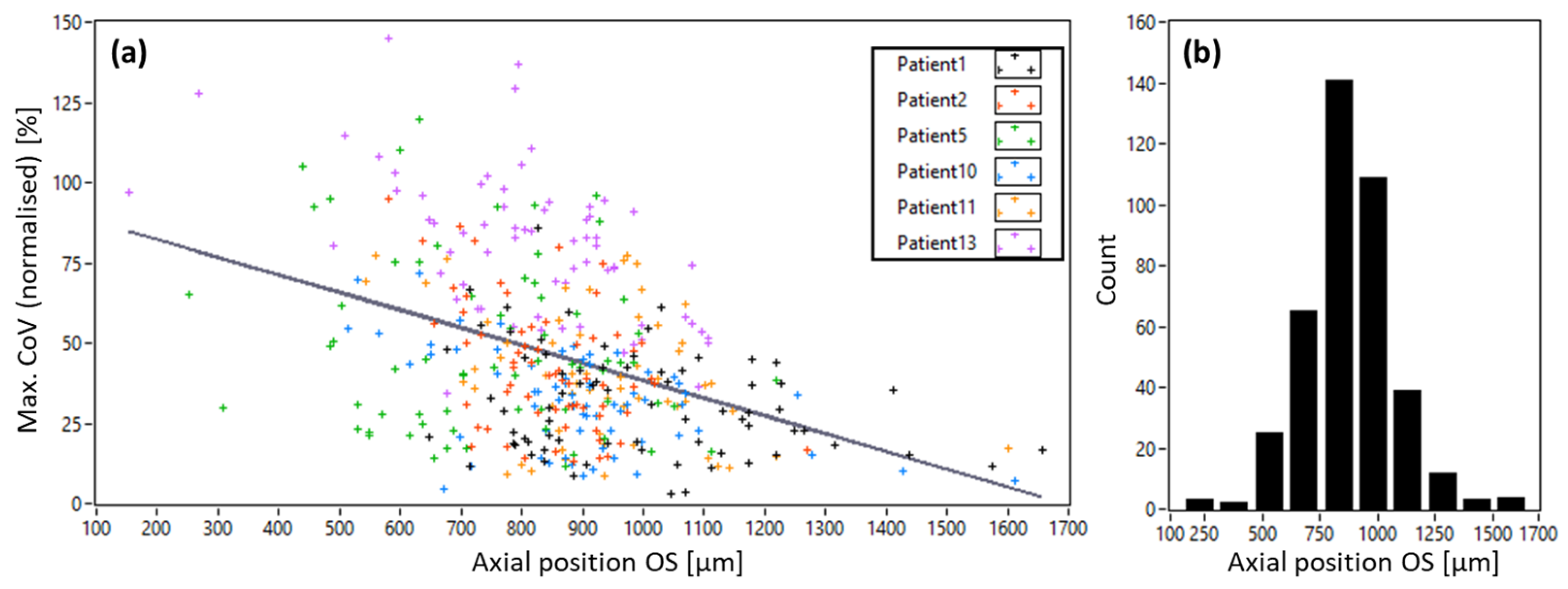
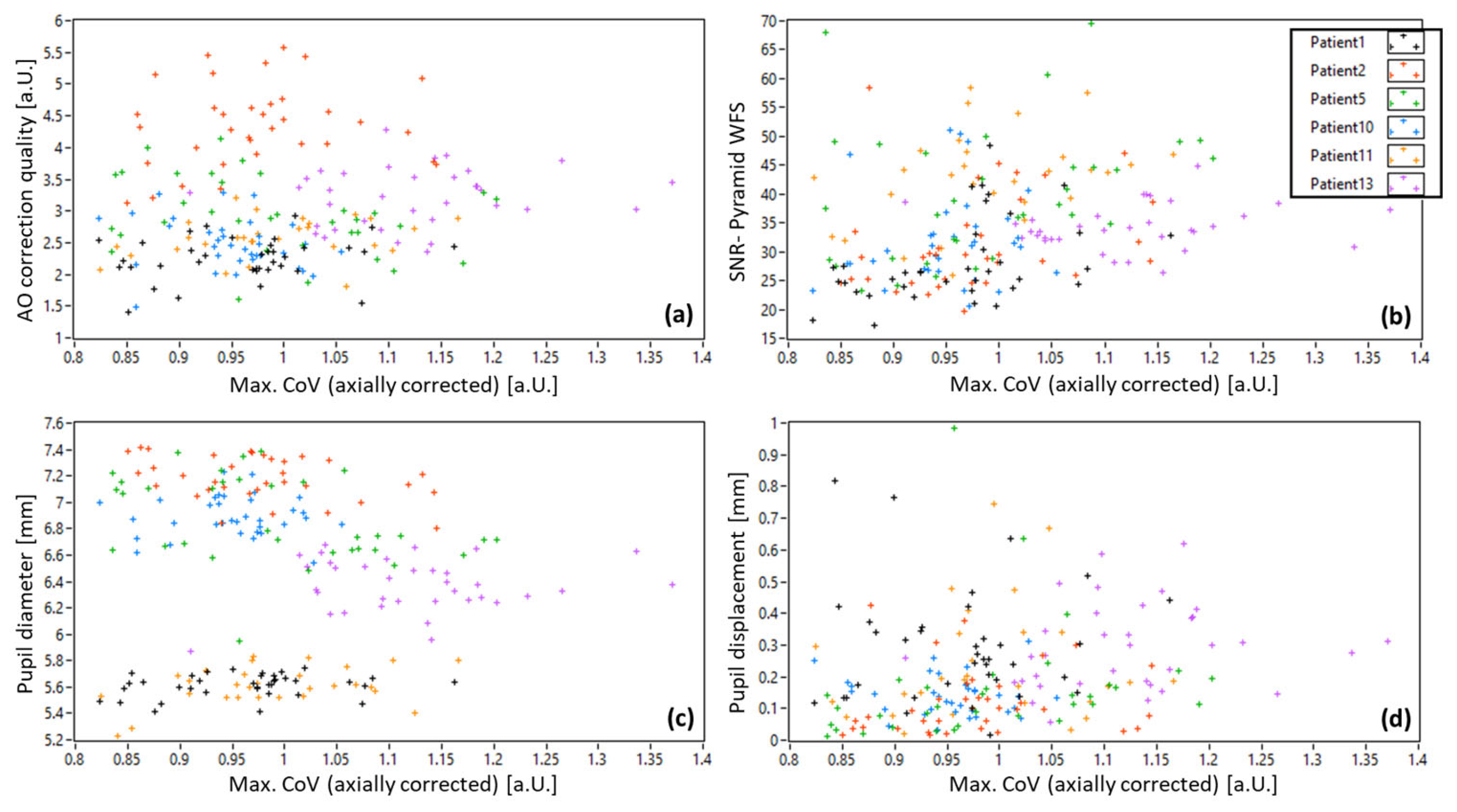
3.1. AO-OCT Image Quality in Patients
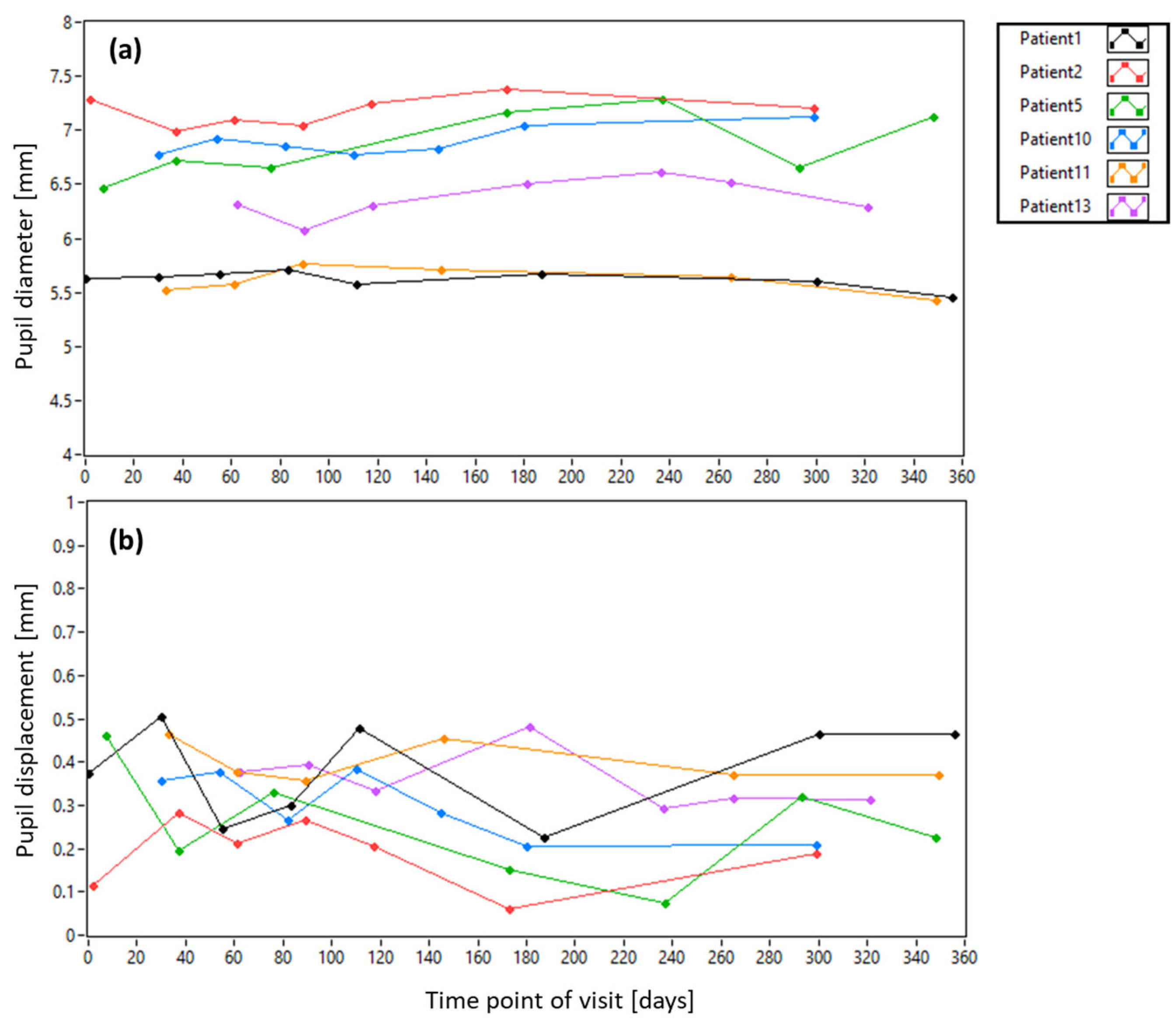
3.2. Pyramid Wavefront Sensor Data in Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonnal, R.S.; Kocaoglu, O.P.; Zawadzki, R.J.; Liu, Z.L.; Miller, D.T.; Werner, J.S. A Review of Adaptive Optics Optical Coherence Tomography: Technical Advances, Scientific Applications, and the Future. Investig. Ophthalmol. Vis. Sci. 2016, 57, Oct51–Oct68. [Google Scholar] [CrossRef] [PubMed]
- Pircher, M.; Zawadzki, R.J. Review of adaptive optics OCT (AO-OCT): Principles and applications for retinal imaging. Biomed. Opt. Express 2017, 8, 2536–2562. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Burns, S.A.; Miller, D.T.; Roorda, A. Evolution of adaptive optics retinal imaging. Biomed. Opt. Express 2023, 14, 1307–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rha, J.T.; Jonnal, R.S.; Miller, D.T. Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina. Opt. Express 2005, 13, 4792–4811. [Google Scholar] [CrossRef]
- Pircher, M.; Zawadzki, R.J.; Evans, J.W.; Werner, J.S.; Hitzenberger, C.K. Simultaneous imaging of human cone mosaic with adaptive optics enhanced scanning laser ophthalmoscopy and high-speed transversal scanning optical coherence tomography. Opt. Lett. 2008, 33, 22–24. [Google Scholar] [CrossRef]
- Felberer, F.; Kroisamer, J.S.; Baumann, B.; Zotter, S.; Schmidt-Erfurth, U.; Hitzenberger, C.K.; Pircher, M. Adaptive optics SLO/OCT for 3D imaging of human photoreceptors in vivo. Biomed. Opt. Express 2014, 5, 439–456. [Google Scholar] [CrossRef]
- Wells-Gray, E.M.; Choi, S.S.; Zawadzki, R.J.; Finn, S.C.; Greiner, C.; Werner, J.S.; Doble, N. Volumetric imaging of rod and cone photoreceptor structure with a combined adaptive optics-optical coherence tomography-scanning laser ophthalmoscope. J. Biomed. Opt. 2018, 23, 1–15. [Google Scholar] [CrossRef]
- Liu, Z.L.; Kocaoglu, O.P.; Miller, D.T. 3D Imaging of Retinal Pigment Epithelial Cells in the Living Human Retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, Oct533–Oct543. [Google Scholar] [CrossRef]
- Shirazi, M.F.; Brunner, E.; Laslandes, M.; Pollreisz, A.; Hitzenberger, C.K.; Pircher, M. Visualizing human photoreceptor and retinal pigment epithelium cell mosaics in a single volume scan over an extended field of view with adaptive optics optical coherence tomography. Biomed. Opt. Express 2020, 11, 4520–4535. [Google Scholar] [CrossRef]
- Liu, Z.; Aghayee, S.; Soltanian-Zadeh, S.; Kovalick, K.; Agrawal, A.; Saeedi, O.; Cukras, C.; Chew, E.Y.; Farsiu, S.; Hammer, D.X. Quantification of Human Photoreceptor–Retinal Pigment Epithelium Macular Topography with Adaptive Optics–Optical Coherence Tomography. Diagnostics 2024, 14, 1518. [Google Scholar] [CrossRef]
- Liu, Z.L.; Kurokawa, K.; Zhang, F.R.; Lee, J.J.; Miller, D.T. Imaging and quantifying ganglion cells and other neurons in the living human retina. Proc. Natl. Acad. Sci. USA 2017, 114, 12803–12808. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Shatokhina, J.; Shirazi, M.F.; Drexler, W.; Leitgeb, R.; Pollreisz, A.; Hitzenberger, C.K.; Ramlau, R.; Pircher, M. Retinal adaptive optics imaging with a pyramid wavefront sensor. Biomed. Opt. Express 2021, 12, 5969–5990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Tam, J.; Saeedi, O.; Hammer, D.X. Trans-retinal cellular imaging with multimodal adaptive optics. Biomed. Opt. Express 2018, 9, 4246–4262. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Kunze, L.; Laidlaw, V.; Jodlbauer, D.; Drexler, W.; Ramlau, R.; Pollreisz, A.; Pircher, M. Improvements on speed, stability and field of view in adaptive optics OCT for anterior retinal imaging using a pyramid wavefront sensor. Biomed. Opt. Express 2024, 15, 6098–6116. [Google Scholar] [CrossRef]
- Reumueller, A.; Schmidt-Erfurth, U.; Salas, M.; Sacu, S.; Drexler, W.; Pircher, M.; Pollreisz, A. Three-Dimensional Adaptive Optics-Assisted Visualization of Photoreceptors in Healthy and Pathologically Aged Eyes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Wollstein, G.; Wang, B.; Schuman, J.S. Adaptive optics optical coherence tomography in glaucoma. Prog. Retin. Eye Res. 2017, 57, 76–88. [Google Scholar] [CrossRef]
- Liu, Z.; Saeedi, O.; Zhang, F.; Villanueva, R.; Asanad, S.; Agrawal, A.; Hammer, D.X. Quantification of Retinal Ganglion Cell Morphology in Human Glaucomatous Eyes. Investig. Ophthalmol. Vis. Sci. 2021, 62, 34. [Google Scholar] [CrossRef]
- Marte, M.E.; Kurokawa, K.; Jung, H.; Liu, Y.; Bernucci, M.T.; King, B.J.; Miller, D.T. Characterizing Presumed Displaced Retinal Ganglion Cells in the Living Human Retina of Healthy and Glaucomatous Eyes. Investig. Ophthalmol. Vis. Sci. 2024, 65, 20. [Google Scholar] [CrossRef]
- Polans, J.; Cunefare, D.; Cole, E.; Keller, B.; Mettu, P.S.; Cousins, S.W.; Allingham, M.J.; Izatt, J.A.; Farsiu, S. Enhanced visualization of peripheral retinal vasculature with wavefront sensorless adaptive optics optical coherence tomography angiography in diabetic patients. Opt. Lett. 2017, 42, 17–20. [Google Scholar] [CrossRef]
- Karst, S.G.; Salas, M.; Hafner, J.; Scholda, C.; Vogl, W.-D.; Drexler, W.; Pircher, M.; Schmidt-Erfurth, U. Three-Dimensional Analysis of Retinal Microaneurysms with Adaptive Optics Optical Coherence Tomography. Retin. J. Retin. Vitr. Dis. 2019, 39, 465–472. [Google Scholar] [CrossRef]
- Brennan, B.D.; Heitkotter, H.; Carroll, J.; Tarima, S.; Cooper, R.F. Quantifying image quality in AOSLO images of photoreceptors. Biomed. Opt. Express 2024, 15, 2849–2862. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.H.; Villamil, M.; Schneider, A.C.; Lawton, P.F.; Young, L.K.; Booth, M.J.; Smithson, H.E. Extended-period AOSLO imaging in the living human retina without pupil dilation: A feasibility study. Biomed. Opt. Express 2024, 15, 4995–5008. [Google Scholar] [CrossRef]
- Tripathi, R.; Nassif, N.; Nelson, J.S.; Park, B.H.; de Boer, J.F. Spectral shaping for non-Gaussian source spectra in optical coherence tomography. Opt. Lett. 2002, 27, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Srinivasan, V.J.; Ko, T.H.; Fujimoto, J.G.; Kowalczyk, A.; Duker, J.S. Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt. Express 2004, 12, 2404–2422. [Google Scholar] [CrossRef]
- Ragazzoni, R. Pupil plane wavefront sensing with an oscillating prism. J. Mod. Opt. 1996, 43, 289–293. [Google Scholar] [CrossRef]
- Ragazzoni, R.; Diolaiti, E.; Vernet, E. A pyramid wavefront sensor with no dynamic modulation. Opt. Commun. 2002, 208, 51–60. [Google Scholar] [CrossRef]
- Leitgeb, R.; Hitzenberger, C.K.; Fercher, A.F. Performance of fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef]
- Wang, Z.; Potsaid, B.; Chen, L.; Doerr, C.; Lee, H.-C.; Nielson, T.; Jayaraman, V.; Cable, A.E.; Swanson, E.; Fujimoto, J.G. Cubic meter volume optical coherence tomography. Optica 2016, 3, 1496–1503. [Google Scholar] [CrossRef]
- Pircher, M.; Baumann, B.; Goetzinger, E.; Sattmann, H.; Hitzenberger, C.K. Simultaneous SLO/OCT imaging of the human retina with axial eye motion correction. Opt. Express 2007, 15, 16922–16932. [Google Scholar] [CrossRef]
- Bedggood, P.; Daaboul, M.; Ashman, R.; Smith, G.; Metha, A. Characteristics of the human isoplanatic patch and implications for adaptive optics retinal imaging. J. Biomed. Opt. 2008, 13, 024008. [Google Scholar] [CrossRef]
- Laslandes, M.; Salas, M.; Hitzenberger, C.K.; Pircher, M. Increasing the field of view of adaptive optics scanning laser ophthalmoscopy. Biomed. Opt. Express 2017, 8, 4811–4826. [Google Scholar] [CrossRef]
- Shirazi, M.F.; Andilla, J.; Lefaudeux, N.; Valdes, C.; Schwarzhans, F.; Durand, M.; Ntatsis, K.; De Jesus, D.A.; Brea, L.S.; Gocho, K.; et al. Multi-modal and multi-scale clinical retinal imaging system with pupil and retinal tracking. Sci. Rep. 2022, 12, 9577. [Google Scholar] [CrossRef]


| Patient | Eye | Age | Sex | DM Type | DR Stage | BCVA | Ocular Condition | Lens Status |
|---|---|---|---|---|---|---|---|---|
| 1 | OS | 79 | F | II | Moderate npDR | 20/20 | DME, PCO inc. | Artificial |
| 2 | OS | 57 | M | II | Moderate npDR | 20/25 | DME, Cat. | Natural |
| 5 | OD | 67 | M | II | Severe npDR | 20/32 | DME, Cat., Dry eye | Natural |
| 10 | OS | 66 | M | II | Moderate npDR | 20/32 | DME, Cat. | Natural |
| 11 | OD | 81 | M | II | Mild npDR | 20/20 | DME | Artificial |
| 13 | OD | 67 | M | II | Severe npDR | 20/32 | DME | Artificial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunner, E.; Kunze, L.; Drexler, W.; Pollreisz, A.; Pircher, M. Image Quality in Adaptive Optics Optical Coherence Tomography of Diabetic Patients. Diagnostics 2025, 15, 429. https://doi.org/10.3390/diagnostics15040429
Brunner E, Kunze L, Drexler W, Pollreisz A, Pircher M. Image Quality in Adaptive Optics Optical Coherence Tomography of Diabetic Patients. Diagnostics. 2025; 15(4):429. https://doi.org/10.3390/diagnostics15040429
Chicago/Turabian StyleBrunner, Elisabeth, Laura Kunze, Wolfgang Drexler, Andreas Pollreisz, and Michael Pircher. 2025. "Image Quality in Adaptive Optics Optical Coherence Tomography of Diabetic Patients" Diagnostics 15, no. 4: 429. https://doi.org/10.3390/diagnostics15040429
APA StyleBrunner, E., Kunze, L., Drexler, W., Pollreisz, A., & Pircher, M. (2025). Image Quality in Adaptive Optics Optical Coherence Tomography of Diabetic Patients. Diagnostics, 15(4), 429. https://doi.org/10.3390/diagnostics15040429







