Distinct Phenotypic and Molecular Characteristics of CD34− and CD34+ Hematopoietic Stem/Progenitor Cell Subsets in Cord Blood and Bone Marrow Samples: Implications for Clinical Applications
Abstract
1. Introduction
2. Materials and Methods
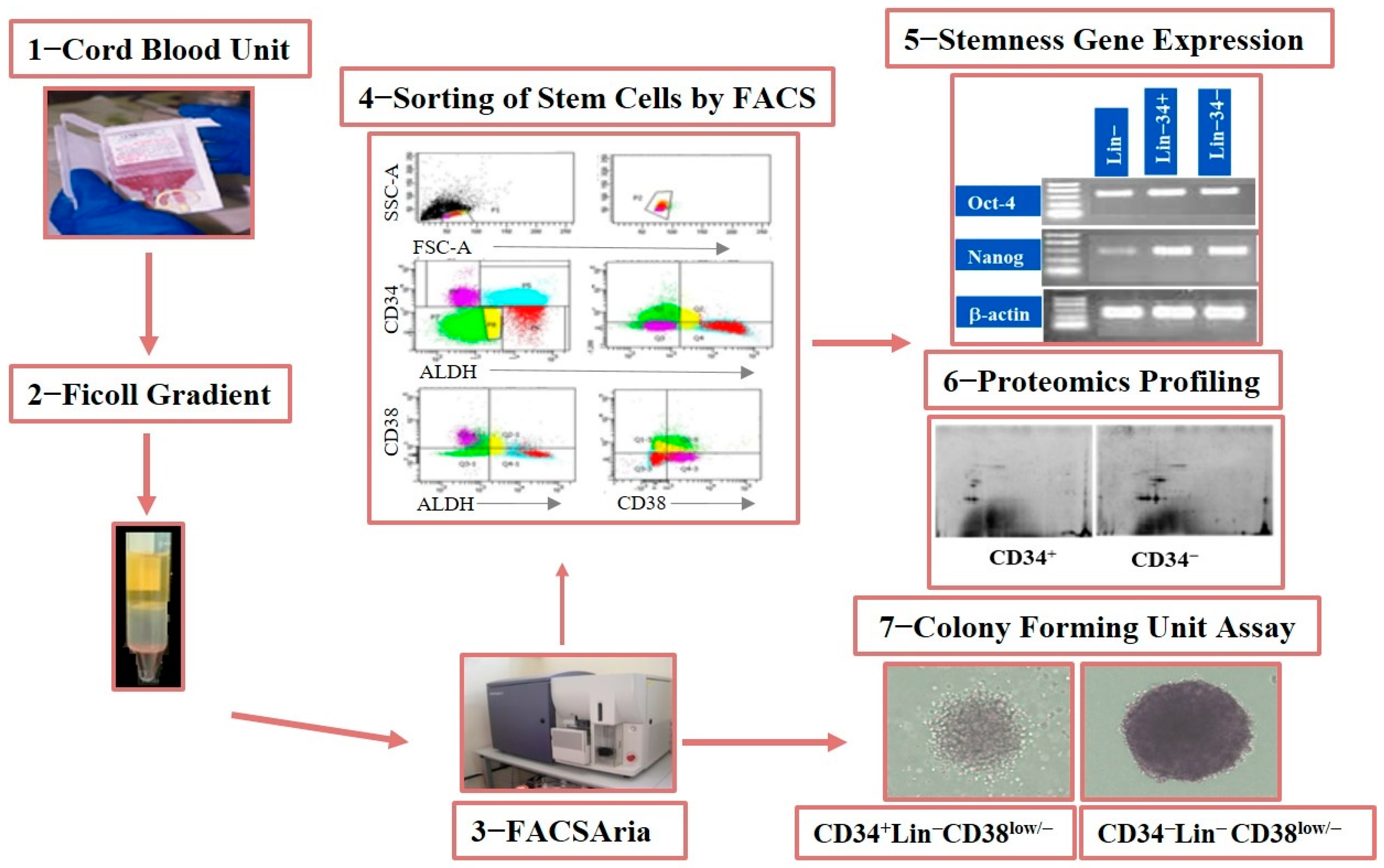
2.1. Donors, Human CB/BM Collection, and Cell Isolation
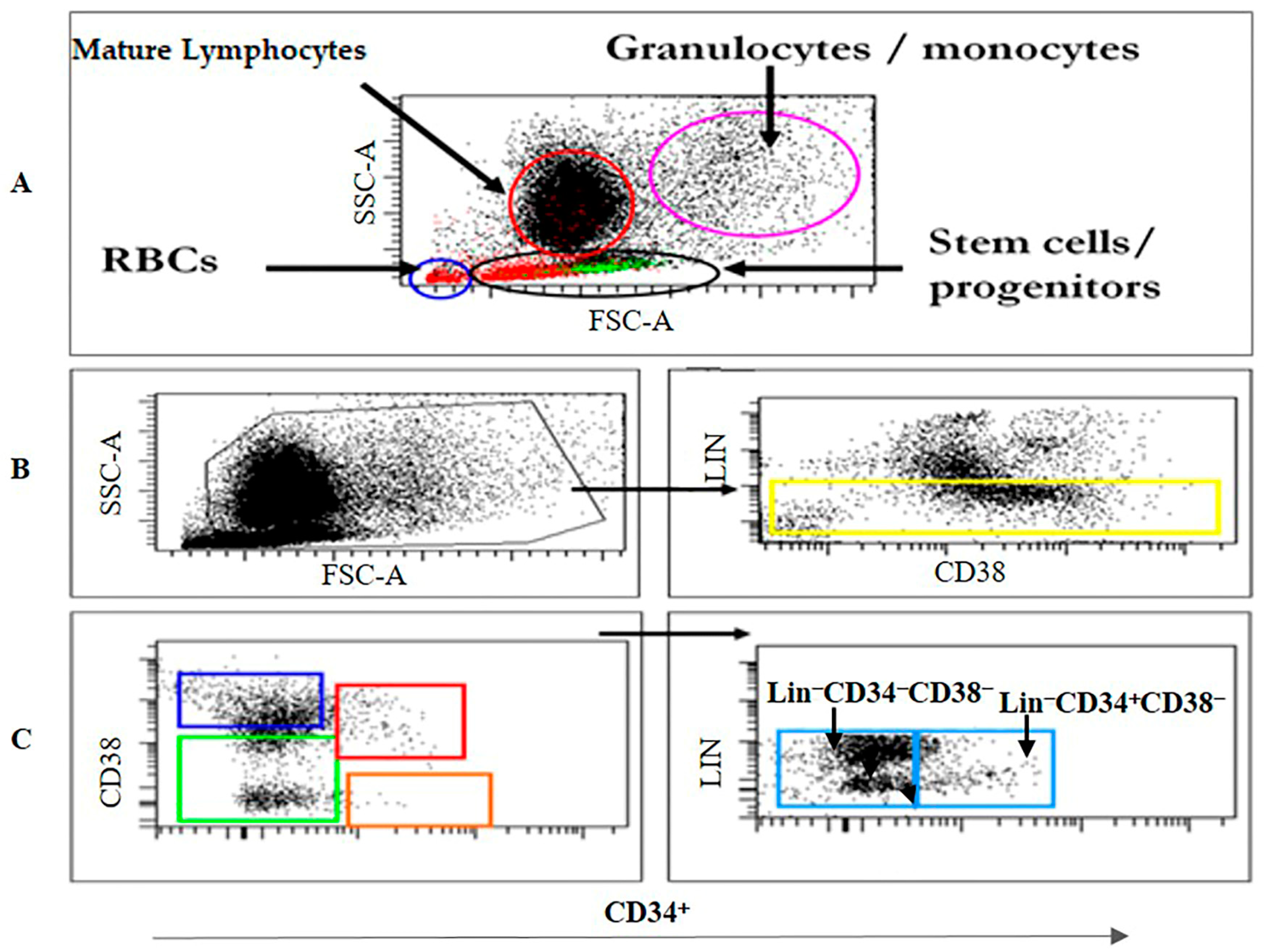
2.2. Immunophenotyping by Flow Cytometry
2.3. Cell Sorting and Isolation of Lin− Cell Subsets
2.4. RNA Isolation, cDNA Synthesis, and RT-PCR
2.5. Analysis of the Gene Expression Profiles of Sorted (+/−) and (−/−) HSC/HPCs by Real-Time PCR
2.6. Proteomics
2.6.1. Sample Preparation and Protein Extraction
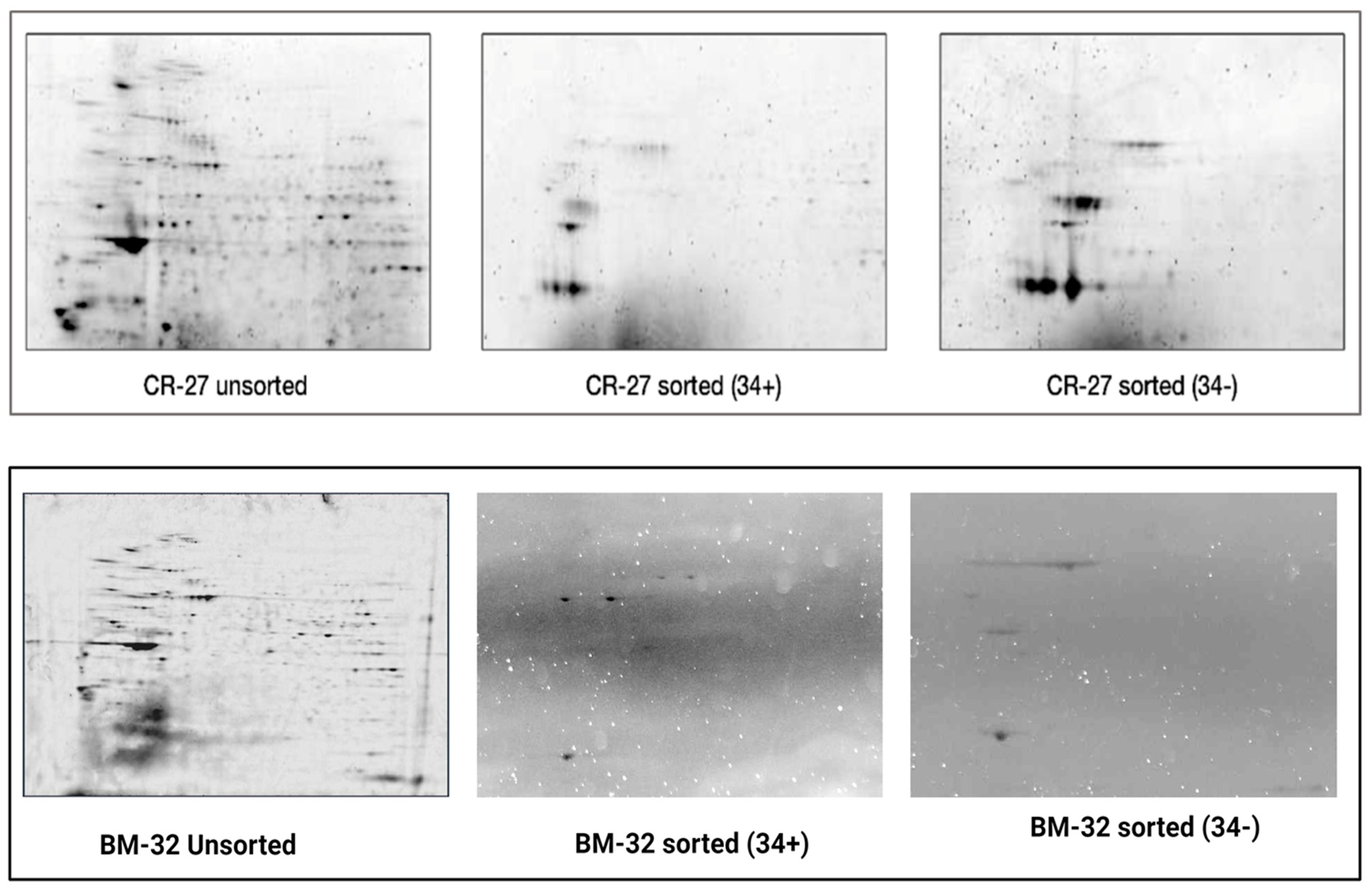
2.6.2. Sample Quality Control by 2-DE Electrophoresis
2.6.3. Protein in Solution–Digestion Prior to LC–MS Analysis
2.6.4. Protein Identification Using LC/MSGS
2.7. Colony-Forming Assay
2.8. Statistical Analysis
3. Results
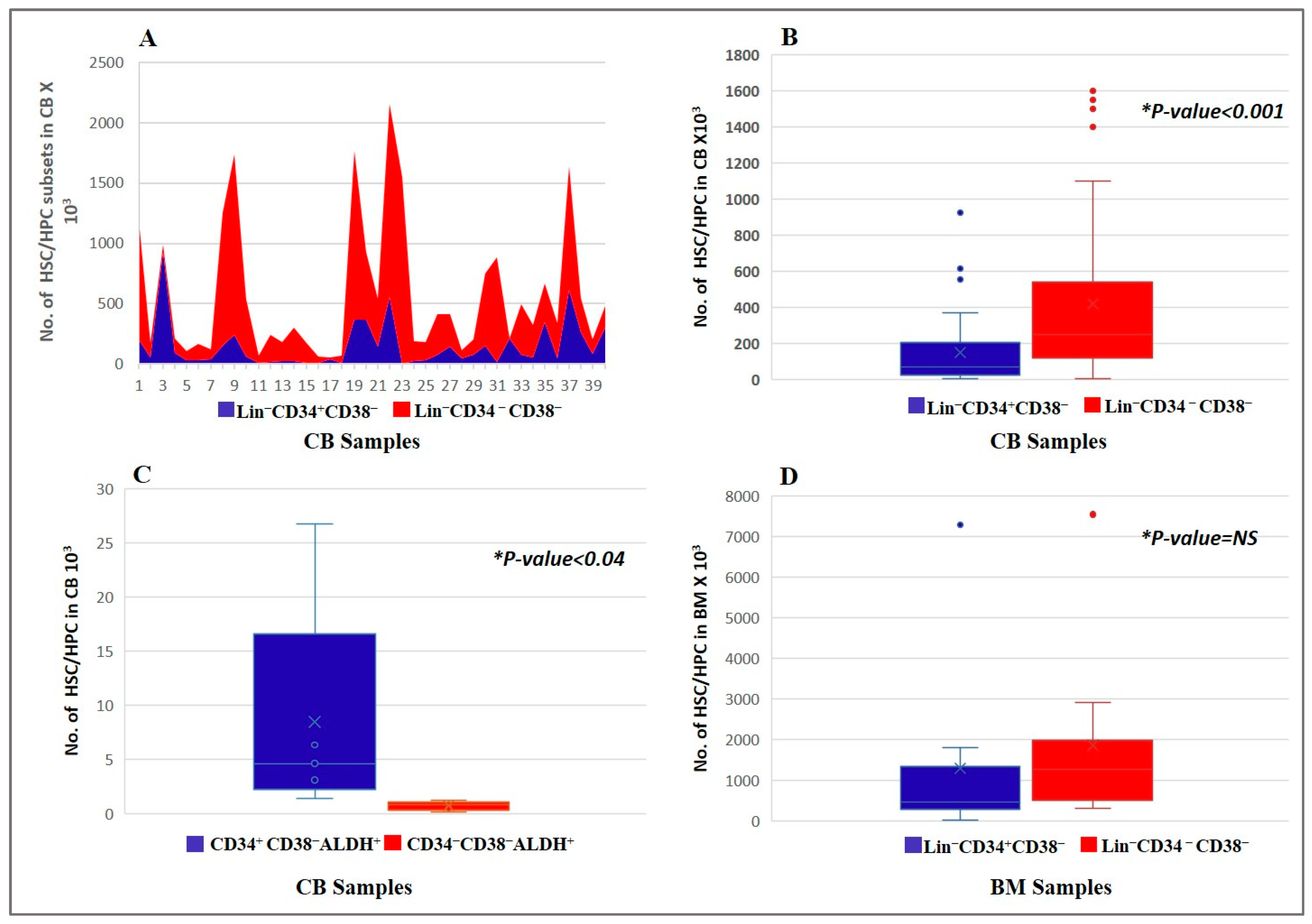
3.1. Kinetics of the HSC/HPC Subsets in CB and BM Samples
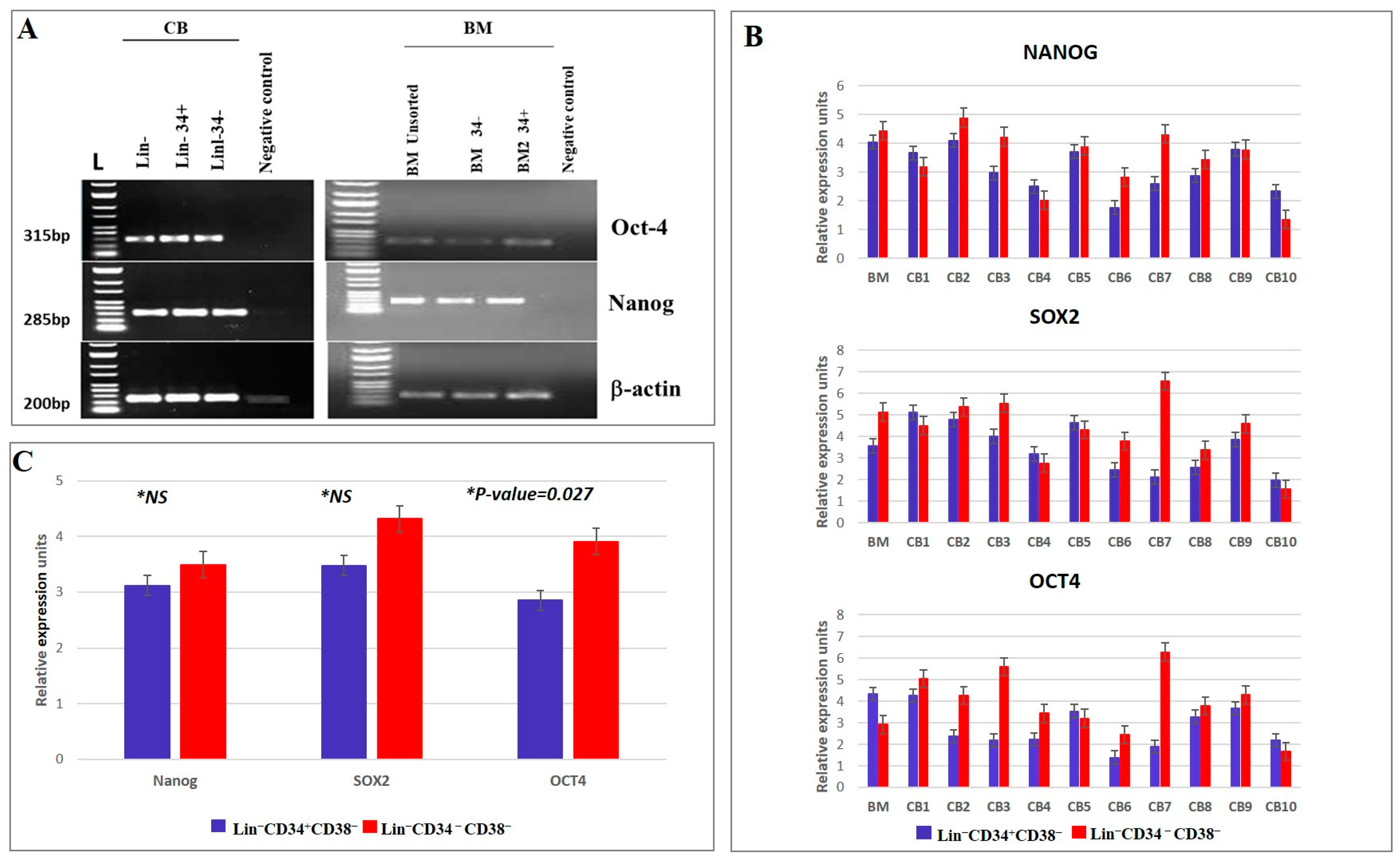
3.2. Gene Expression Profiles of CD34+ and CD34− Cells in CB and BM Samples
3.3. Global Protein Expression Profiles of CD34+ and CD34− Cells in CB and BM Samples
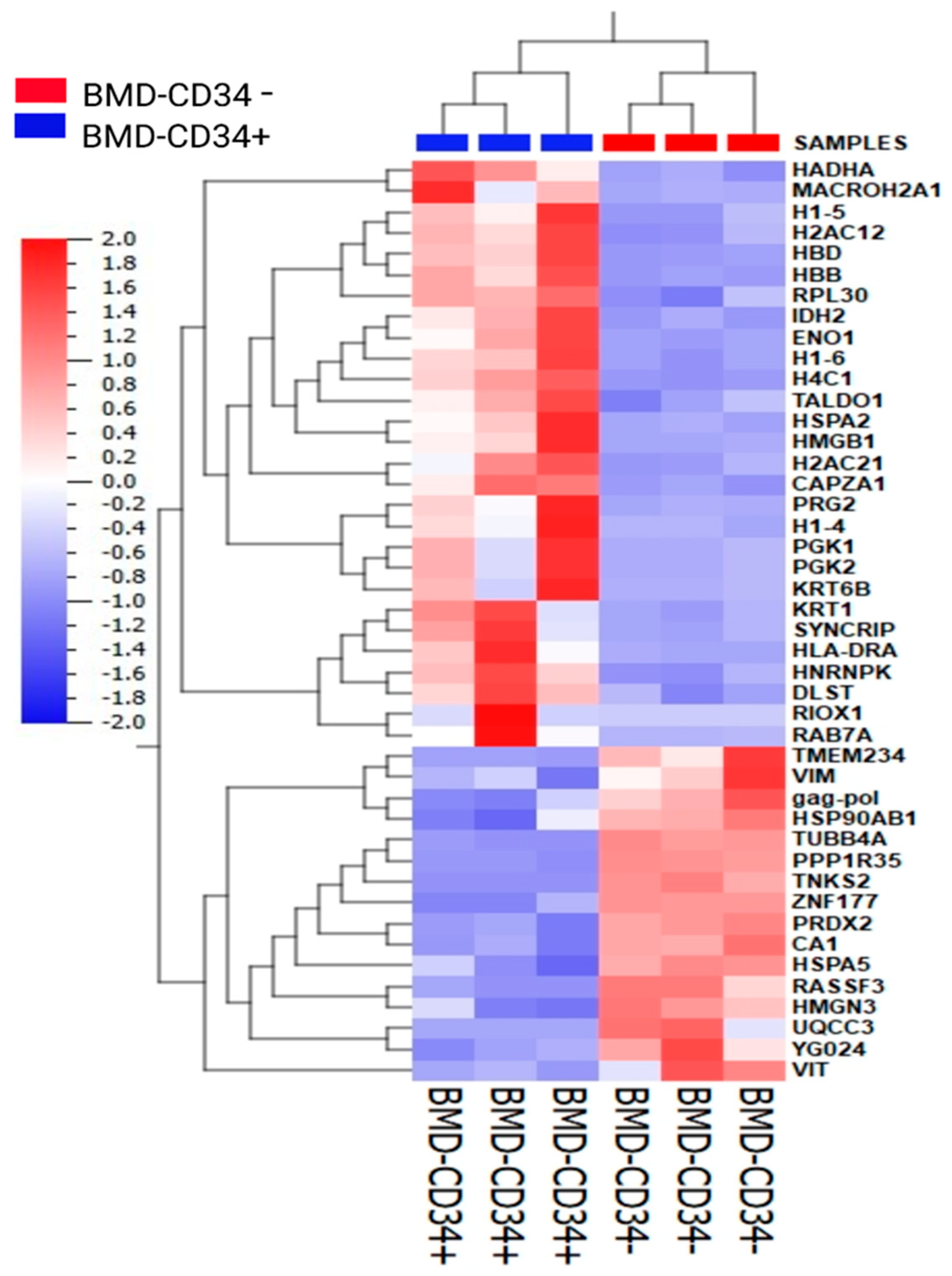
3.4. Protein Expression Changes and Annotation of the Identified Proteins Using Knowledge-Based Ingenuity Pathway Analysis
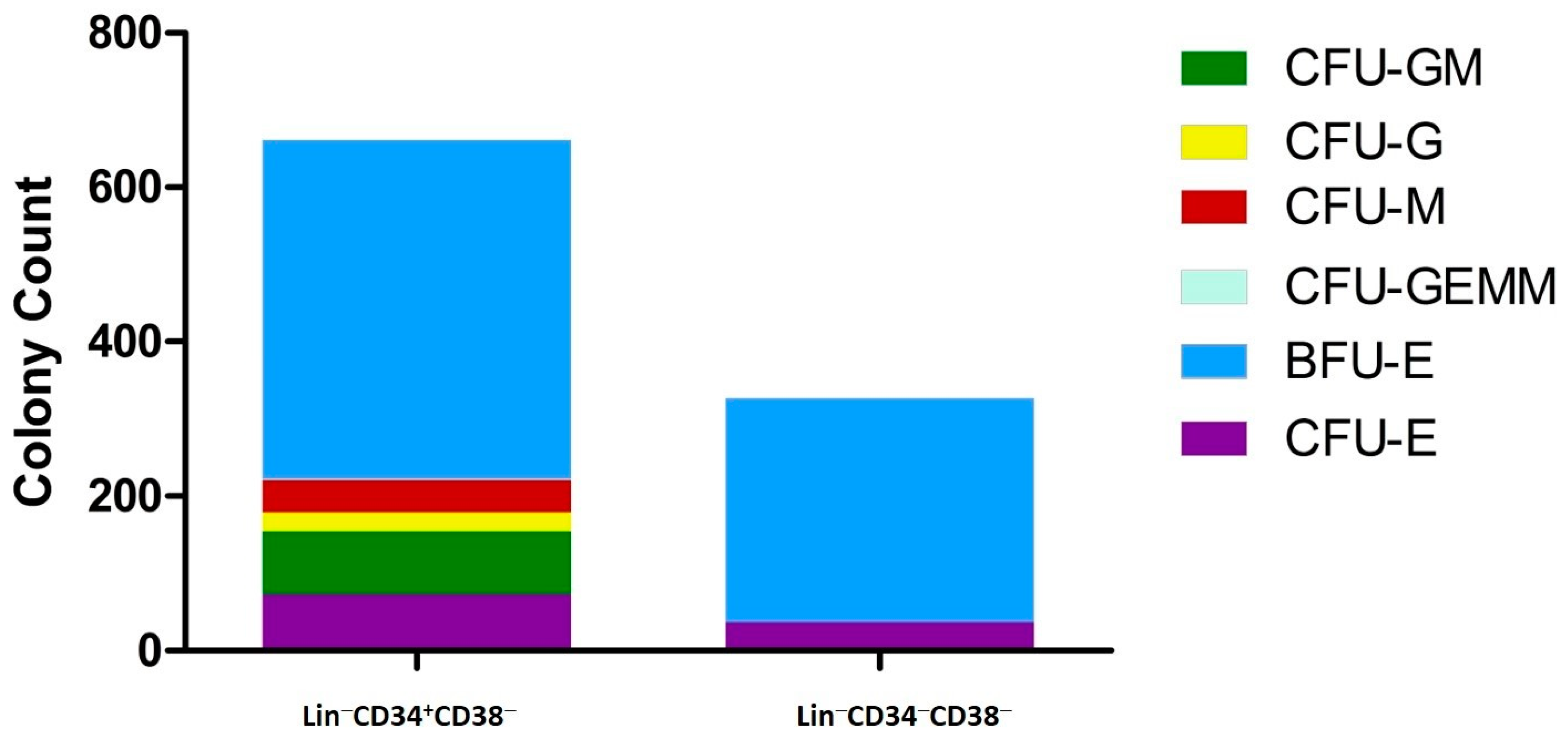
3.5. Functional Analysis—Colony Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaheen, M.; Almohareb, F.; Aljohani, N.; Ayas, M.; Chaudhri, N.; Abosoudah, I.; Alotaibi, S.; Alshahrani, M.; Alsharif, F.; Akhtar, S.; et al. Hematopoietic stem cell transplantation in Saudi Arabia between 1984 and 2016: Experience from four leading tertiary care hematopoietic stem cell transplantation centers. Hematol. Oncol. Stem Cell Ther. 2021, 14, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, H.J.; Eaves, C.J.; Eaves, A.C.; Dragowska, W.; Lansdorp, P.M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood 1989, 74, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Hanks, S.; Thompson, J.M.; Dugan, M.J.; Akard, L.P. Transplantation of hematopoietic stem cells from the peripheral blood. J. Cell. Mol. Med. 2005, 9, 37–50. [Google Scholar] [CrossRef][Green Version]
- Larochelle, A.; Vormoor, J.; Hanenberg, H.; Wang, J.C.; Bhatia, M.; Lapidot, T.; Moritz, T.; Murdoch, B.; Xiao, X.L.; Kato, I.; et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: Implications for gene therapy. Nat. Med. 1996, 2, 1329–1337. [Google Scholar] [CrossRef]
- Bhatia, M.; Bonnet, D.; Kapp, U.; Wang, J.C.; Murdoch, B.; Dick, J.E. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J. Exp. Med. 1997, 186, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Wang, J.C.; Kapp, U.; Bonnet, D.; Dick, J.E. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 5320–5325. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.E.; Bhatia, M.; Gan, O.; Kapp, U.; Wang, J.C. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells 1997, 15, 199–203; discussion 204–207. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Bonnet, D.; Murdoch, B.; Gan, O.I.; Dick, J.E. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat. Med. 1998, 4, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H.; Manz, M.G. Impact of inflammation on early hematopoiesis and the microenvironment. Int. J. Hematol. 2017, 106, 27–33. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sezaki, M.; Takizawa, H. Development of the hematopoietic system: Role of inflammatory factors. Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e341. [Google Scholar] [CrossRef] [PubMed]
- DiGiusto, D.; Chen, S.; Combs, J.; Webb, S.; Namikawa, R.; Tsukamoto, A.; Chen, B.P.; Galy, A.H. Human fetal bone marrow early progenitors for T, B, and myeloid cells are found exclusively in the population expressing high levels of CD34. Blood 1994, 84, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Fackler, M.J.; Civin, C.I.; May, W.S. CD34: Structure, biology, and clinical utility. Blood 1996, 87, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Simmons, P.J.; Levesque, J.P.; Haylock, D.N. Mucin-like molecules as modulators of the survival and proliferation of primitive hematopoietic cells. Ann. N. Y. Acad. Sci. 2001, 938, 196–206; discussion 206–207. [Google Scholar] [CrossRef]
- Krause, D.S.; Kapadia, S.U.; Raj, N.B.; May, W.S. Regulation of CD34 expression in differentiating M1 cells. Exp. Hematol. 1997, 25, 1051–1061. [Google Scholar]
- Dooley, D.C.; Oppenlander, B.K. Phenotypic and functional analyses of CD34NEG hematopoietic precursors from mobilized peripheral blood. Methods Mol. Biol. 2004, 263, 201–218. [Google Scholar]
- Dooley, D.C.; Oppenlander, B.K.; Xiao, M. Analysis of primitive CD34- and CD34+ hematopoietic cells from adults: Gain and loss of CD34 antigen by undifferentiated cells are closely linked to proliferative status in culture. Stem Cells 2004, 22, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Lataillade, J.J.; Clay, D.; David, C.; Boutin, L.; Guerton, B.; Drouet, M.; Hérodin, F.; Le Bousse-Kerdilès, M.C. Phenotypic and functional characteristics of CD34+cells are related to their anatomical environment: Is their versatility a prerequisite for their bio-availability? J. Leuko Biol. 2005, 77, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef]
- Anjos-Afonso, F.; Currie, E.; Palmer, H.G.; Foster, K.E.; Taussig, D.C.; Bonnet, D. CD34(-) cells at the apex of the human hematopoietic stem cell hierarchy have distinctive cellular and molecular signatures. Cell Stem Cell 2013, 13, 161–174. [Google Scholar] [CrossRef]
- Sonoda, Y. Immunophenotype and functional characteristics of human primitive CD34-negative hematopoietic stem cells: The significance of the intra-bone marrow injection. J. Autoimmun. 2008, 30, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, A.; Al-Omar, H.M.; Manogaran, P.S.; Almohareb, F.; Alhussein, K. Prevalence of the BCR/ABL fusion gene and T cell stimulation capacity of dendritic cells in chronic myelogenous leukemia. Am. J. Transl. Res. 2023, 15, 967–981. [Google Scholar] [PubMed]
- Osawa, M.; Hanada, K.; Hamada, H.; Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 1996, 273, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, H. Hematopoietic stem cells: Are they CD34-positive or CD34-negative? Nat. Med. 1998, 4, 1009–1010. [Google Scholar] [CrossRef]
- Wang, J.; Kimura, T.; Asada, R.; Harada, S.; Yokota, S.; Kawamoto, Y.; Fujimura, Y.; Tsuji, T.; Ikehara, S.; Sonoda, Y. SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood 2003, 101, 2924–2931. [Google Scholar] [CrossRef][Green Version]
- Goodell, M.A.; Rosenzweig, M.; Kim, H.; Marks, D.F.; DeMaria, M.; Paradis, G.; Grupp, S.A.; Sieff, C.A.; Mulligan, R.C.; Johnson, R.P. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat. Med. 1997, 3, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y. Human CD34-negative hematopoietic stem cells: The current understanding of their biological nature. Exp. Hematol. 2021, 96, 13–26. [Google Scholar] [CrossRef]
- Hess, D.A.; Meyerrose, T.E.; Wirthlin, L.; Craft, T.P.; Herrbrich, P.E.; Creer, M.H.; Nolta, J.A. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood 2004, 104, 1648–1655. [Google Scholar] [CrossRef]
- Croker, A.K.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.D.; Hess, D.A.; Allan, A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2009, 13, 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.; Hess, D.A.; Nolta, J.A. Recent advances in hematopoietic stem cell biology. Curr. Opin. Hematol. 2004, 11, 392–398. [Google Scholar] [CrossRef]
- Storms, R.W.; Green, P.D.; Safford, K.M.; Niedzwiecki, D.; Cogle, C.R.; Colvin, O.M.; Chao, N.J.; Rice, H.E.; Smith, C.A. Distinct hematopoietic progenitor compartments are delineated by the expression of aldehyde dehydrogenase and CD34. Blood 2005, 106, 95–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pierre-Louis, O.; Clay, D.; Brunet de la Grange, P.; Blazsek, I.; Desterke, C.; Guerto, B.; Blondeau, C.; Malfuson, J.V.; Prat, M.; Bennaceur-Griscelli, A.; et al. Dual SP/ALDH functionalities refine the human hematopoietic Lin-CD34+CD38- stem/progenitor cell compartment. Stem Cells 2009, 27, 2552–2562. [Google Scholar] [CrossRef]
- Gaafar, A.; Veress, B.; Permin, H.; Kharazmi, A.; Theander, T.G.; el Hassan, A.M. Characterization of the local and systemic immune responses in patients with cutaneous leishmaniasis due to Leishmania major. Clin. Immunol. 1999, 91, 314–320. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Colak, D.; Alaiya, A.A.; Kaya, N.; Muiya, N.P.; AlHarazi, O.; Shinwari, Z.; Andres, E.; Dzimiri, N. Integrated left ventricular global transcriptome and proteome profiling in human end-stage dilated cardiomyopathy. PLoS ONE 2016, 11, e0162669. [Google Scholar] [CrossRef]
- Alkhayal, Z.; Shinwari, Z.; Gaafar, A.; Alaiya, A. Proteomic profiling of the first human dental pulp mesenchymal stem/stromal cells from carbonic anhydrase II deficiency osteopetrosis patients. Int. J. Mol. Sci. 2020, 22, 380. [Google Scholar] [CrossRef]
- Alkhayal, Z.; Shinwari, Z.; Gaafar, A.; Alaiya, A. Fluconazole-induced protein changes in osteogenic and immune metabolic pathways of dental Pulp mesenchymal stem cells of osteopetrosis patients. Int. J. Mol. Sci. 2023, 24, 13841. [Google Scholar] [CrossRef]
- Alaiya, A.A.; Aljurf, M.; Shinwari, Z.; Almohareb, F.; Malhan, H.; Alzahrani, H.; Owaidah, T.; Fox, J.; Alsharif, F.; Mohamed, S.Y.; et al. Protein signatures as potential surrogate biomarkers for stratification and prediction of treatment response in chronic myeloid leukemia patients. Int. J. Oncol. 2016, 49, 913–933. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef]
- Civin, C.I.; Strauss, L.C.; Brovall, C.; Fackler, M.J.; Schwartz, J.F.; Shaper, J.H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J. Immunol. 1984, 133, 157–165. [Google Scholar] [CrossRef]
- Sumide, K.; Matsuoka, Y.; Kawamura, H.; Nakatsuka, R.; Fujioka, T.; Asano, H.; Takihara, Y.; Sonoda, Y. A revised road map for the commitment of human cord blood CD34-negative hematopoietic stem cells. Nat. Commun. 2018, 9, 2202. [Google Scholar] [CrossRef] [PubMed]
- Chute, J.P.; Muramoto, G.G.; Whitesides, J.; Colvin, M.; Safi, R.; Chao, N.J.; McDonnell, D.P. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11707–11712. [Google Scholar] [CrossRef]
- Hess, D.A.; Craft, T.P.; Wirthlin, L.; Hohm, S.; Zhou, P.; Eades, W.C.; Creer, M.H.; Sands, M.S.; Nolta, J.A. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells 2008, 26, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.J.; Bonnet, D. The combined use of Hoechst efflux ability and aldehyde dehydrogenase activity to identify murine and human hematopoietic stem cells. Exp. Hematol. 2007, 35, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ando, K.; Chargui, J.; Kawada, H.; Sato, T.; Tsuji, T.; Hotta, T.; Kato, S. Ex vivo generation of CD34(+) cells from CD34(-) hematopoietic cells. Blood 1999, 94, 4053–4059. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, E.D.; Almeida-Porada, G.; Livingston, A.G.; Flake, A.W.; Ogawa, M. Human bone marrow CD34- cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp. Hematol. 1998, 26, 353–360. [Google Scholar] [PubMed]
- Kimura, T.; Asada, R.; Wang, J.; Kimura, T.; Morioka, M.; Matsui, K.; Kobayashi, K.; Henmi, K.; Imai, S.; Kita, M.; et al. Identification of long-term repopulating potential of human cord blood-derived CD34-flt3- severe combined immunodeficiency-repopulating cells by intra-bone marrow injection. Stem Cells 2007, 25, 1348–1355. [Google Scholar] [CrossRef]
- Ishii, M.; Matsuoka, Y.; Sasaki, Y.; Nakatsuka, R.; Takahashi, M.; Nakamoto, T.; Yasuda, K.; Matsui, K.; Asano, H.; Uemura, Y.; et al. Development of a high-resolution purification method for precise functional characterization of primitive human cord blood-derived CD34-negative SCID-repopulating cells. Exp. Hematol. 2011, 39, 203–213.e1. [Google Scholar] [CrossRef] [PubMed]
- Notta, F.; Doulatov, S.; Laurenti, E.; Poeppl, A.; Jurisica, I.; Dick, J.E. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 2011, 333, 218–221. [Google Scholar] [CrossRef]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Galan-Caridad, J.M.; Harel, S.; Arenzana, T.L.; Hou, Z.E.; Doetsch, F.K.; Mirny, L.A.; Reizis, B. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell 2007, 129, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.; Subedi, R. Defining molecular phenotypes of mesenchymal and hematopoietic stem cells derived from peripheral blood of acute lymphocytic leukemia patients for regenerative stem cell therapy. J. Stem Cells Regen. Med. 2011, 7, 29–40. [Google Scholar] [PubMed]
- Potdar, P.D.; D’Souza, S.B. Isolation of Oct4+, Nanog+ and SOX2- mesenchymal cells from peripheral blood of a diabetes mellitus patient. Hum. Cell 2011, 24, 51–55. [Google Scholar] [CrossRef]
- Lee, Y.J.; Wang, J.K.; Pai, Y.M.; Frost, A.; Viprakasit, V.; Ekwattanakit, S.; Chin, H.C.; Liu, J.Y. Culture of leukocyte-derived cells from human peripheral blood: Increased expression of pluripotent genes OCT4, NANOG, SOX2, self-renewal gene TERT and plasticity. Medicine 2023, 102, e32746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, W.; Li, Q.; Wang, Q.; Ru, Y.; Xiong, X.; Yan, F.; Pan, T.; Lin, W.; Li, X. IDH2 compensates for IDH1 mutation to maintain cell survival under hypoxic conditions in IDH1mutant tumor cells. Mol. Med. Rep. 2019, 20, 1893–1900. [Google Scholar] [PubMed]
- Lin, C.Y.; Wu, C.L.; Lee, K.Z.; Chen, Y.J.; Zhang, P.H.; Chang, C.Y.; Harn, H.J.; Lin, S.Z.; Tsai, H.J. Extracellular Pgk1 enhances neurite outgrowth of motoneurons through Nogo66/NgR-independent targeting of NogoA. eLife 2019, 8, e49175. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Bak, Y.; Park, Y.H.; Jang, G.B.; Nam, J.S.; Yoo, J.E.; Park, Y.N.; Bak, I.S.; Kim, J.M.; Yoon, D.Y.; et al. Peroxiredoxin II is essential for maintaining stemness by redox regulation in liver cancer cells. Stem Cells 2016, 34, 1188–1197. [Google Scholar] [CrossRef]
- Jansen, M.; Yang, F.C.; Cancelas, J.A.; Bailey, J.R.; Williams, D.A. Rac2-deficient hematopoietic stem cells show defective interaction with the hematopoietic microenvironment and long-term engraftment failure. Stem Cells 2005, 23, 335–346. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer | Product Size (bp) |
|---|---|---|
| Oct4 | F GAAGGTATTCAGCCAAACGAC R GTTACAGAACCACACTCGGA | 315 |
| Nanog | F TGCAAATGTCTTCTGCTGAGAT R GTTCAGGATGTTGGAGAGTTC | 285 |
| Sox−2 | F ATGCACCGCTACGACGTGA R CTTTTGCACCCCTCCCATTT | 437 |
| Human GAPDH | F GTCAGTGCTGGACCTGACCT R CACCACCATGTTGCTGTAGC | 255 |
| Accession | Symbol | Entrez Gene Name-Description | IPA Expr p-Value Ranking | * Proteomics Expr Fold-Change: CD34+/− | Location | Type(s) |
|---|---|---|---|---|---|---|
| P00915 | CA1 | carbonic anhydrase 1 | 5.29 × 10−3 | −5115 | Cytoplasm | Enzyme |
| P52907 | CAPZA1 | capping actin protein of muscle Z-line subunit alpha 1 | 2.92 × 10−3 | 2442 | Cytoplasm | Other |
| P36957 | DLST | dihydrolipoamide S-succinyltransferase | 6.64 × 10−3 | 2461 | Cytoplasm | Enzyme |
| P16401 | H1-5 | H1.5 linker histone, cluster member | 1.16 × 10−2 | 2595 | Nucleus | Other |
| P22492 | H1-6 | H1.6 linker histone, cluster member | 2.71 × 10−3 | 2787 | Nucleus | Other |
| Q8IUE6 | H2AC21 | H2A clustered histone 21 | 1.04 × 10−2 | 3055 | Nucleus | Other |
| P40939 | HADHA | hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha | 4.45 × 10−3 | 6879 | Cytoplasm | Enzyme |
| P02042 | HBD | hemoglobin subunit delta | 1.33 × 10−3 | 3325 | Other | Transporter |
| Q15651 | HMGN3 | high mobility group nucleosomal binding domain 3 | 4.94 × 10−2 | −5861 | Nucleus | Other |
| P61978 | HNRNPK | heterogeneous nuclear ribonucleoprotein K | 3.36 × 10−3 | 2732 | Nucleus | Other |
| P54652 | HSPA2 | heat shock protein family A (Hsp70) member 2 | 8.41 × 10−3 | 3396 | Cytoplasm | Other |
| P48735 | IDH2 | isocitrate dehydrogenase (NADP(+)) 2 | 2.04 × 10−3 | 9253 | Cytoplasm | Enzyme |
| P04264 | KRT1 | keratin 1 | 1.57 × 10−2 | 4856 | Cytoplasm | Other |
| P00558 | PGK1 | phosphoglycerate kinase 1 | 3.06 × 10−2 | 3595 | Cytoplasm | Kinase |
| P07205 | PGK2 | phosphoglycerate kinase 2 | 3.06 × 10−2 | 3595 | Cytoplasm | Kinase |
| Q8TAP8 | PPP1R35 | protein phosphatase 1 regulatory subunit 35 | 1.90 × 10−2 | −34,676 | Cytoplasm | Other |
| P32119 | PRDX2 | peroxiredoxin 2 | 2.85 × 10−2 | −8288 | Cytoplasm | Enzyme |
| P13727 | PRG2 | proteoglycan 2, pro-eosinophil major basic protein | 9.77 × 10−3 | 4217 | Extracellular Space | Other |
| P51149 | RAB7A | RAB7A, member RAS oncogene family | 3.48 × 10−2 | 3120 | Cytoplasm | Enzyme |
| Q86WH2 | RASSF3 | Ras association domain family member 3 | 4.71 × 10−3 | −17,993 | Other | Other |
| Q9H6W3 | RIOX1 | ribosomal oxygenase 1 | 5.18 × 10−3 | 1000,000 | Nucleus | Enzyme |
| P62888 | RPL30 | ribosomal protein L30 | 6.50 × 10−3 | 3379 | Cytoplasm | Other |
| O60506 | SYNCRIP | synaptotagmin binding cytoplasmic RNA interacting protein | 2.00 × 10−2 | 3041 | Nucleus | Other |
| P37837 | TALDO1 | transaldolase 1 | 1.49 × 10−2 | 3146 | Cytoplasm | Enzyme |
| Q8WY98 | TMEM234 | transmembrane protein 234 | 4.12 × 10−2 | −45,290 | Other | Other |
| Q9H2K2 | TNKS2 | tankyrase 2 | 2.18 × 10−3 | −273,322 | Nucleus | Enzyme |
| P04350 | TUBB4A | tubulin beta 4A class IVa | 2.14 × 10−2 | −68,781 | Cytoplasm | Other |
| Q6UW78 | UQCC3 | ubiquinol–cytochrome c reductase complex assembly factor 3 | 1.76 × 10−2 | −168,107 | Extracellular Space | Other |
| Q6UXI7 | VIT | vitrin | 1.79 × 10−2 | −8856 | Extracellular Space | Other |
| Factors | Median | Min | Q1 | Q3 | Max | Median | Min | Q1 | Q3 | Max | Median | Min | Q1 | Q3 | Max | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Group | CD34- | CD34+ | ||||||||||||||
| Colonies | 41 | 1 | 1 | 104.25 | 200 | 1 | 1 | 1 | 65.75 | 200 | 58.50 | 10 | 34 | 140.20 | 199 | NS * |
| CFU-E | 0 | 0 | 0 | 1 | 64 | 0 | 0 | 0 | 1 | 35 | 0.50 | 0 | 0 | 5.50 | 64 | NA+ |
| BFU-E | 5 | 0 | 0 | 65.75 | 200 | 1 | 0 | 0 | 57 | 200 | 20.5 | 0 | 0 | 124.20 | 199 | NA |
| CFU-GM | 0 | 0 | 0 | 0 | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.25 | 74 | NA |
| CFU-G | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | NA |
| CFU-M | 0 | 0 | 0 | 0 | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 | NA |
| CFU-GEMM | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaafar, A.; Hamza, F.N.; Yousif, R.; Shinwari, Z.; Alotaibi, A.G.; Iqniebi, A.; Al-Hussein, K.; Al-Mazrou, A.; Manogaran, P.S.; Elhassan, T.; et al. Distinct Phenotypic and Molecular Characteristics of CD34− and CD34+ Hematopoietic Stem/Progenitor Cell Subsets in Cord Blood and Bone Marrow Samples: Implications for Clinical Applications. Diagnostics 2025, 15, 447. https://doi.org/10.3390/diagnostics15040447
Gaafar A, Hamza FN, Yousif R, Shinwari Z, Alotaibi AG, Iqniebi A, Al-Hussein K, Al-Mazrou A, Manogaran PS, Elhassan T, et al. Distinct Phenotypic and Molecular Characteristics of CD34− and CD34+ Hematopoietic Stem/Progenitor Cell Subsets in Cord Blood and Bone Marrow Samples: Implications for Clinical Applications. Diagnostics. 2025; 15(4):447. https://doi.org/10.3390/diagnostics15040447
Chicago/Turabian StyleGaafar, Ameera, Fatheia Nabeil Hamza, Rama Yousif, Zakia Shinwari, Aminah Ghazi Alotaibi, Alia Iqniebi, Khalid Al-Hussein, Amer Al-Mazrou, Pulicat Subramanian Manogaran, Tusneem Elhassan, and et al. 2025. "Distinct Phenotypic and Molecular Characteristics of CD34− and CD34+ Hematopoietic Stem/Progenitor Cell Subsets in Cord Blood and Bone Marrow Samples: Implications for Clinical Applications" Diagnostics 15, no. 4: 447. https://doi.org/10.3390/diagnostics15040447
APA StyleGaafar, A., Hamza, F. N., Yousif, R., Shinwari, Z., Alotaibi, A. G., Iqniebi, A., Al-Hussein, K., Al-Mazrou, A., Manogaran, P. S., Elhassan, T., Marquez-Méndez, M., Aljurf, M., Al-Humaidan, H., & Alaiya, A. (2025). Distinct Phenotypic and Molecular Characteristics of CD34− and CD34+ Hematopoietic Stem/Progenitor Cell Subsets in Cord Blood and Bone Marrow Samples: Implications for Clinical Applications. Diagnostics, 15(4), 447. https://doi.org/10.3390/diagnostics15040447






