Exploring Fat Fraction and Vertebral Bone Quality Score in Lumbar Spine Magnetic Resonance Imaging: A Cross-Sectional Study on Associations and Clinical Implication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Magnetic Resonance Imaging
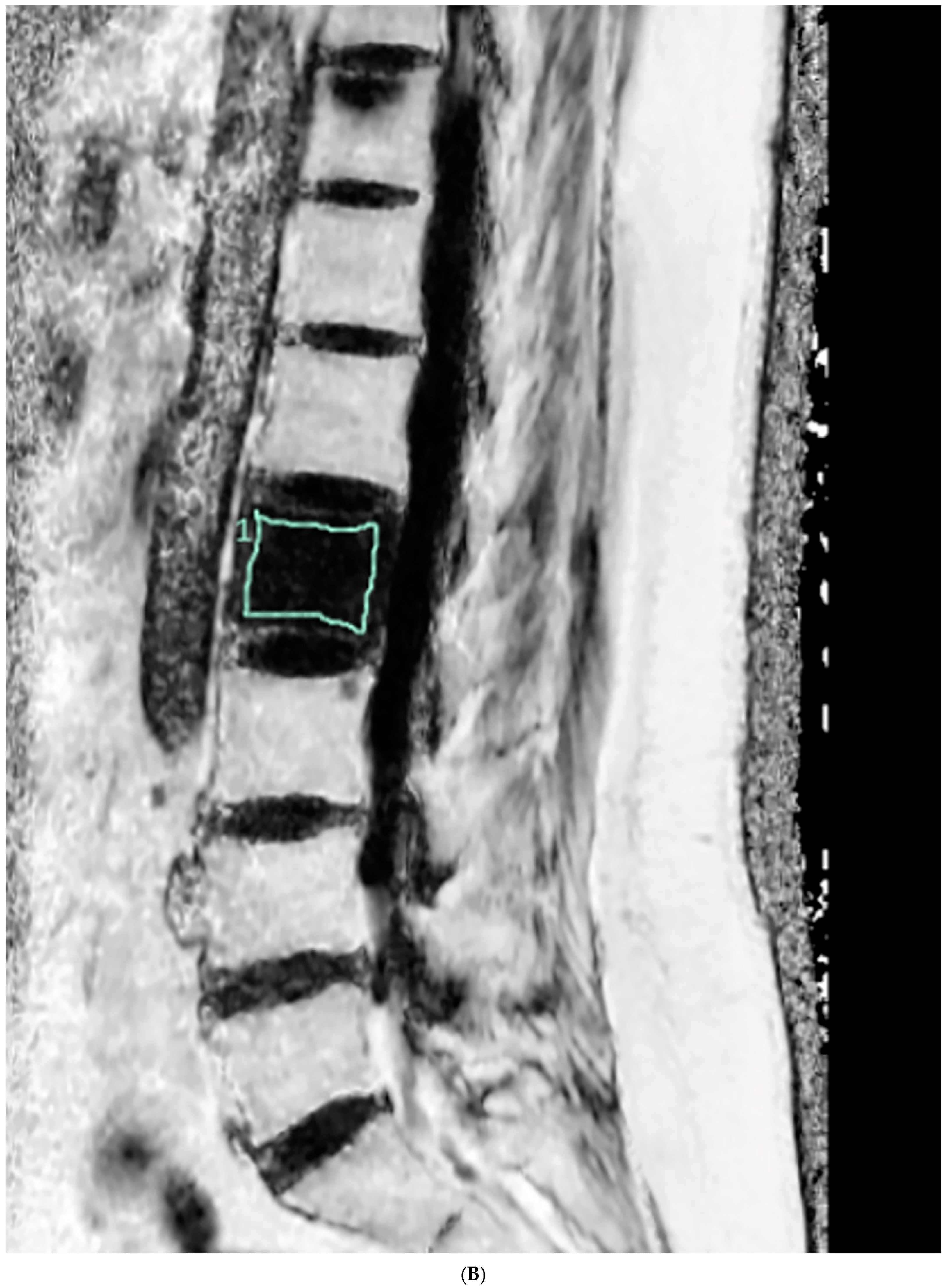
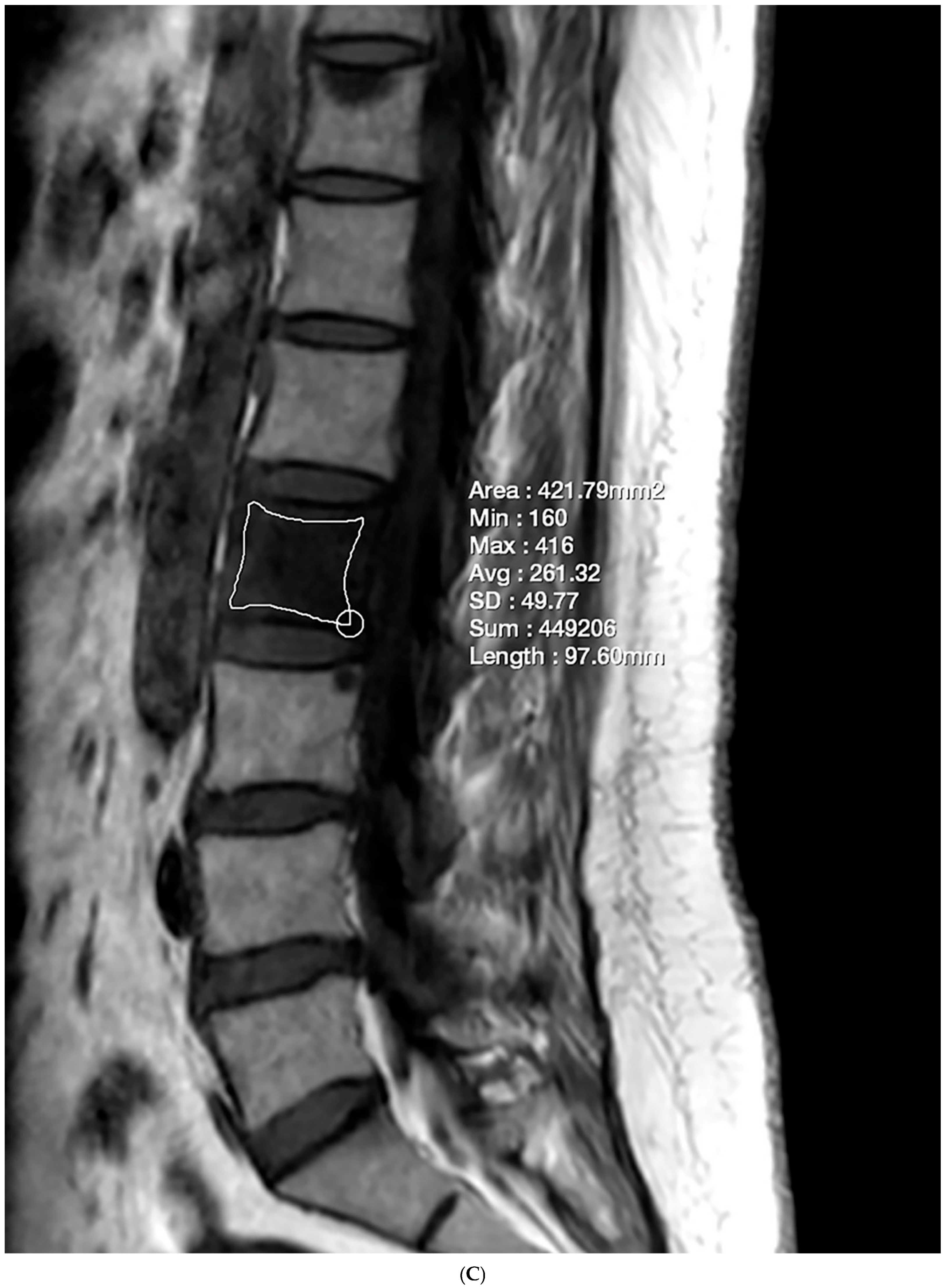
2.3. Fat Fraction Measurement
2.4. Vertebral Bone Quality Score Measurement
2.5. Evaluation for Vertebral Lesions
- The typical imaging finding in all conventional MRI sequences.
- In cases of an acute vertebral fracture, findings diagnosed with CT or plain radiography, with accompanying trauma history.
- In cases other than fractures, the typical imaging appearance in MRI was taken at least 6 months before or after the evaluation.
2.6. Statistical Analysis
3. Results
3.1. Reproducibility
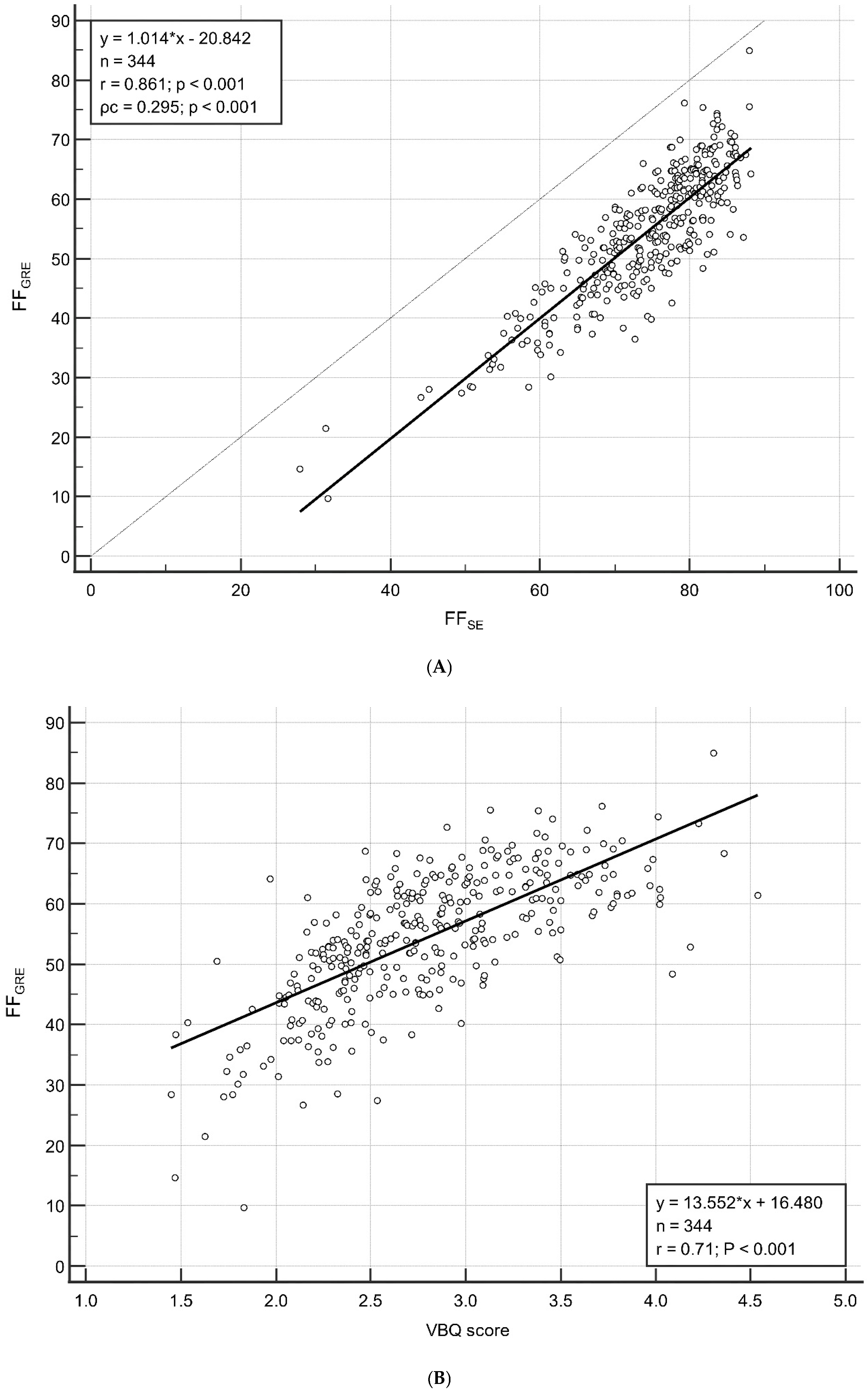
3.2. Correlation Between FFGRE and FFSE/VBQ Score
3.3. Correlation Between Age and FF/VBQ Score
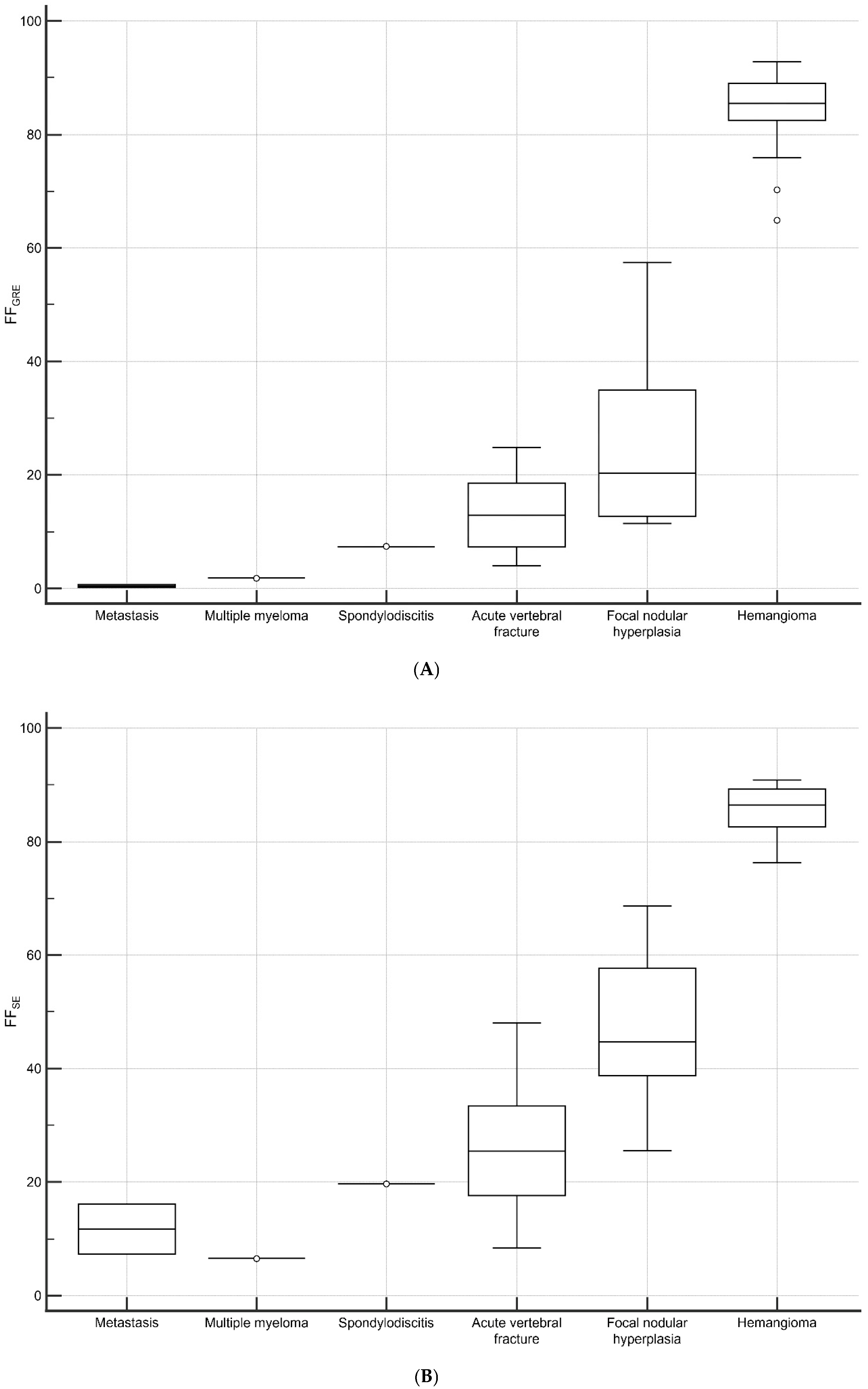
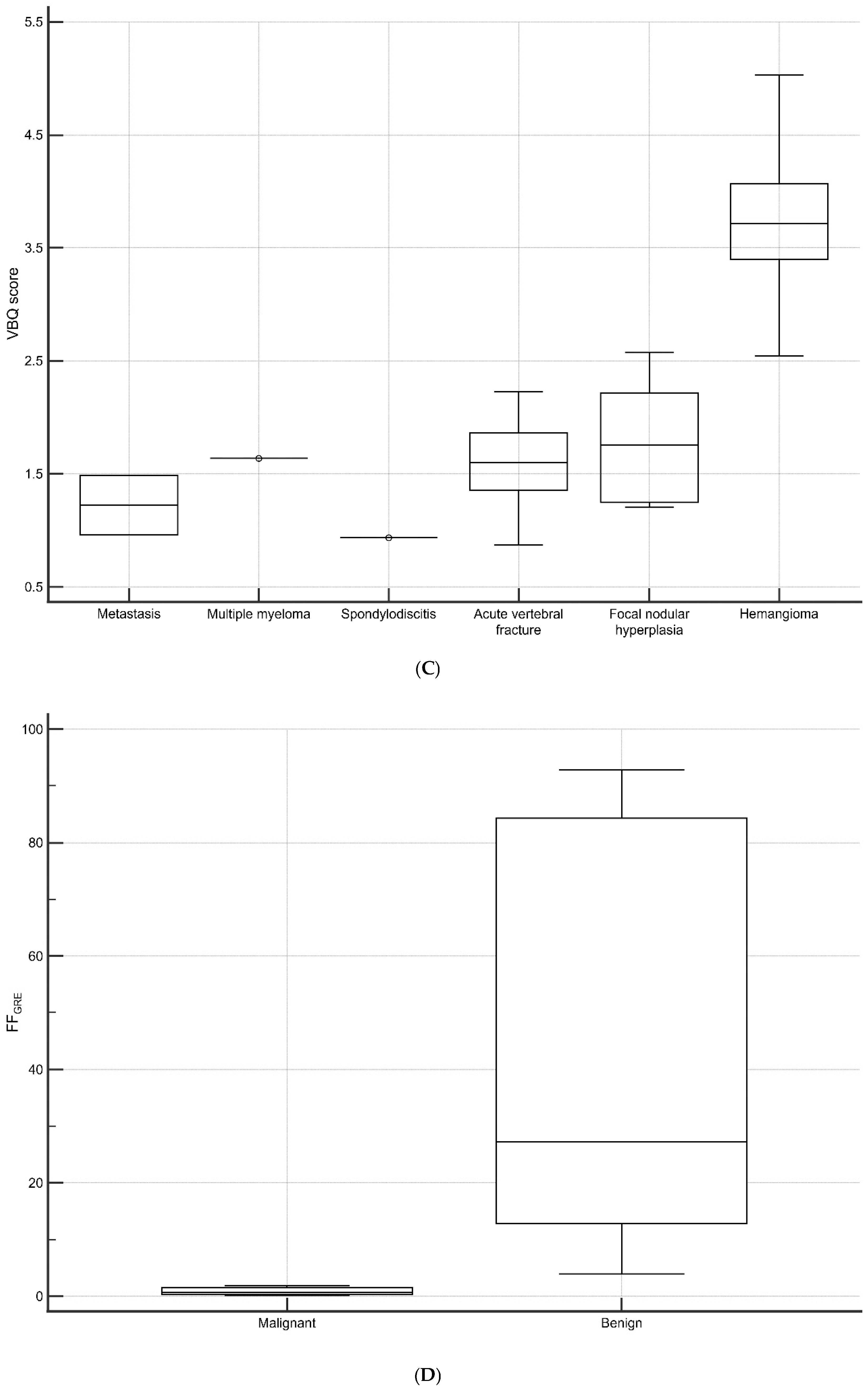
3.4. Vertebral Lesions Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMAT | Bone marrow adipose tissue |
| CSE-MRI | Chemical shift-encoded magnetic resonance imaging |
| GRE | Gradient-echo |
| FF | Fat fraction |
| SE | Spin-echo |
| VBQ | Vertebral bone quality |
| TR | Repetition time |
| TE | Echo time |
| FOV | Field of view |
| ROIs | regions of interest |
| SI | Signal intensity |
| CSF | Cerebrospinal fluid |
| CI | confidence interval |
References
- Veldhuis-Vlug, A.G.; Rosen, C.J. Clinical implications of bone marrow adiposity. J. Intern. Med. 2018, 283, 121–139. [Google Scholar] [CrossRef]
- Piotrowska, K.; Tarnowski, M. Bone Marrow Adipocytes-Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients 2021, 13, 1412. [Google Scholar] [CrossRef]
- Hanrahan, C.J.; Shah, L.M. MRI of spinal bone marrow: Part 2, T1-weighted imaging-based differential diagnosis. Am. J. Roentgenol. 2011, 197, 1309–1321. [Google Scholar] [CrossRef]
- Martel, D.; Leporq, B.; Bruno, M.; Regatte, R.R.; Honig, S.; Chang, G. Chemical shift-encoded MRI for assessment of bone marrow adipose tissue fat composition: Pilot study in premenopausal versus postmenopausal women. Magn. Reson. Imaging 2018, 53, 148–155. [Google Scholar] [CrossRef]
- Santopaolo, M.; Gu, Y.; Spinetti, G.; Madeddu, P. Bone marrow fat: Friend or foe in people with diabetes mellitus? Clin. Sci. 2020, 134, 1031–1048. [Google Scholar] [CrossRef]
- Karampinos, D.C.; Melkus, G.; Baum, T.; Bauer, J.S.; Rummeny, E.J.; Krug, R. Bone marrow fat quantification in the presence of trabecular bone: Initial comparison between water-fat imaging and single-voxel MRS. Magn. Reson. Med. 2014, 71, 1158–1165. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hong, S.H.; Kim, D.H.; Choi, J.Y.; Chae, H.D.; Jeong, B.M.; Ahn, J.M.; Kang, H.S. Measurement of fat content in vertebral marrow using a modified dixon sequence to differentiate benign from malignant processes. J. Magn. Reson. Imaging 2017, 45, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A. MR spectroscopy of the liver: Principles and clinical applications. Radiographics 2009, 29, 1653–1664. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.K.; Lee, S.J.; Choo, H.J.; Park, J.W.; Kim, K.Y. Simultaneous Estimation of the Fat Fraction and R(2)(*) via T(2)(*)-Corrected 6-Echo Dixon Volumetric Interpolated Breath-hold Examination Imaging for Osteopenia and Osteoporosis Detection: Correlations with Sex, Age, and Menopause. Korean J. Radiol. 2019, 20, 916–930. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, H.J.; Yu, S.M.; Hong, S.H.; Choi, J.Y.; Chae, H.D. Fat Quantification in the Vertebral Body: Comparison of Modified Dixon Technique with Single-Voxel Magnetic Resonance Spectroscopy. Korean J. Radiol. 2019, 20, 126–133. [Google Scholar] [CrossRef]
- Li, G.; Xu, Z.; Li, X.; Zuo, X.; Chang, S.; Wu, D.; Dai, Y. Adding marrow R2 * to proton density fat fraction improves the discrimination of osteopenia and osteoporosis in postmenopausal women assessed with 3D FACT sequence. Menopause 2021, 28, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhao, Y.J.; Ni, J.M.; Jiang, Y.; Chen, F.M.; Wang, Z.J.; Zhang, Z.Y. Adding liver R2* quantification to proton density fat fraction MRI of vertebral bone marrow improves the prediction of osteoporosis. Eur. Radiol. 2022, 32, 7108–7116. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, F.C.; Luetkens, J.A.; Wagenhauser, P.J.; Meier-Schroers, M.; Kuetting, D.L.; Feisst, A.; Gieseke, J.; Schmeel, L.C.; Traber, F.; Schild, H.H.; et al. Proton density fat fraction (PDFF) MRI for differentiation of benign and malignant vertebral lesions. Eur. Radiol. 2018, 28, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Do Huh, J. Differentiation of bone metastases from benign red marrow depositions of the spine: The role of fat-suppressed T2-weighted imaging compared to fat fraction map. Eur. Radiol. 2022, 32, 6730–6738. [Google Scholar] [CrossRef]
- Zanchi, F.; Richard, R.; Hussami, M.; Monier, A.; Knebel, J.F.; Omoumi, P. MRI of non-specific low back pain and/or lumbar radiculopathy: Do we need T1 when using a sagittal T2-weighted Dixon sequence? Eur. Radiol. 2020, 30, 2583–2593. [Google Scholar] [CrossRef]
- Ehresman, J.; Pennington, Z.; Schilling, A.; Lubelski, D.; Ahmed, A.K.; Cottrill, E.; Khan, M.; Sciubba, D.M. Novel MRI-based score for assessment of bone density in operative spine patients. Spine J. 2020, 20, 556–562. [Google Scholar] [CrossRef]
- Salzmann, S.N.; Okano, I.; Jones, C.; Zhu, J.; Lu, S.; Onyekwere, I.; Balaji, V.; Reisener, M.J.; Chiapparelli, E.; Shue, J.; et al. Preoperative MRI-based vertebral bone quality (VBQ) score assessment in patients undergoing lumbar spinal fusion. Spine J. 2022, 22, 1301–1308. [Google Scholar] [CrossRef]
- Pu, M.; Zhong, W.; Heng, H.; Yu, J.; Wu, H.; Jin, Y.; Zhang, P.; Shen, Y. Vertebral bone quality score provides preoperative bone density assessment for patients undergoing lumbar spine surgery: A retrospective study. J. Neurosurg. Spine 2023, 38, 705–714. [Google Scholar] [CrossRef]
- Li, R.; Yin, Y.; Ji, W.; Wu, X.; Jiang, H.; Chen, J.; Zhu, Q. MRI-based vertebral bone quality score effectively reflects bone quality in patients with osteoporotic vertebral compressive fractures. Eur. Spine J. 2022, 31, 1131–1137. [Google Scholar] [CrossRef]
- Kadri, A.; Binkley, N.; Hernando, D.; Anderson, P.A. Opportunistic Use of Lumbar Magnetic Resonance Imaging for Osteoporosis Screening. Osteoporos. Int. 2022, 33, 861–869. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Chen, J.; Zhu, H.; Tian, H.; Wang, L. MRI-based vertebral bone quality score is a comprehensive index reflecting the quality of bone and paravertebral muscle. Spine J. 2024, 24, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Liu, H.; Zhang, X.; Liao, H.; He, P.; Yang, H.; Yang, H.; Qu, B. Comprehensive Diagnostic Value of Vertebral Bone Quality Scores and Paravertebral Muscle Quality Parameters in Osteoporotic Vertebral Fractures. World Neurosurg. 2025, 194, 123503. [Google Scholar] [CrossRef] [PubMed]
- Eggers, H.; Bornert, P. Chemical shift encoding-based water-fat separation methods. J. Magn. Reson. Imaging 2014, 40, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, Z.; Gu, H.; Li, X.; Yuan, W.; Chang, S.; Fan, J.; Calimente, H.; Hu, J. Comparison of chemical shift-encoded water-fat MRI and MR spectroscopy in quantification of marrow fat in postmenopausal females. J. Magn. Reson. Imaging 2017, 45, 66–73. [Google Scholar] [CrossRef]
- Haueise, T.; Schick, F.; Stefan, N.; Machann, J. Comparison of the accuracy of commercial two-point and multi-echo Dixon MRI for quantification of fat in liver, paravertebral muscles, and vertebral bone marrow. Eur. J. Radiol. 2024, 172, 111359. [Google Scholar] [CrossRef]
- Shah, L.M.; Hanrahan, C.J. MRI of spinal bone marrow: Part I, techniques and normal age-related appearances. Am. J. Roentgenol. 2011, 197, 1298–1308. [Google Scholar] [CrossRef]
- Ehresman, J.; Schilling, A.; Pennington, Z.; Gui, C.; Chen, X.; Lubelski, D.; Ahmed, A.K.; Cottrill, E.; Khan, M.; Redmond, K.J.; et al. A novel MRI-based score assessing trabecular bone quality to predict vertebral compression fractures in patients with spinal metastasis. J. Neurosurg. Spine 2019, 32, 499–506. [Google Scholar] [CrossRef]
- Malkiewicz, A.; Dziedzic, M. Bone marrow reconversion—imaging of physiological changes in bone marrow. Pol. J. Radiol. 2012, 77, 45–50. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, D.; Jiang, Y.; Ma, X.; Zhang, Y.; Chang, R. Correlation of R2* with fat fraction and bone mineral density and its role in quantitative assessment of osteoporosis. Eur. Radiol. 2023, 33, 6001–6008. [Google Scholar] [CrossRef]
- Gassert, F.G.; Kranz, J.; Gassert, F.T.; Schwaiger, B.J.; Bogner, C.; Makowski, M.R.; Glanz, L.; Stelter, J.; Baum, T.; Braren, R.; et al. Longitudinal MR-based proton-density fat fraction (PDFF) and T2* for the assessment of associations between bone marrow changes and myelotoxic chemotherapy. Eur. Radiol. 2023, 34, 2437–2444. [Google Scholar] [CrossRef]
- Al Saedi, A.; Chen, L.; Phu, S.; Vogrin, S.; Miao, D.; Ferland, G.; Gaudreau, P.; Duque, G. Age-Related Increases in Marrow Fat Volumes have Regional Impacts on Bone Cell Numbers and Structure. Calcif. Tissue Int. 2020, 107, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Tyagi, S.; Myers, D.; Duque, G. Good, Bad, or Ugly: The Biological Roles of Bone Marrow Fat. Curr. Osteoporos. Rep. 2018, 16, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F.; Yeung, D.K.; Ma, H.T.; Leung, J.C.; Kwok, T.C.; Leung, P.C. Bone marrow fat content in the elderly: A reversal of sex difference seen in younger subjects. J. Magn. Reson. Imaging 2012, 36, 225–230. [Google Scholar] [CrossRef]
- Zajick, D.C., Jr.; Morrison, W.B.; Schweitzer, M.E.; Parellada, J.A.; Carrino, J.A. Benign and malignant processes: Normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology 2005, 237, 590–596. [Google Scholar] [CrossRef]
- Douis, H.; Davies, A.M.; Jeys, L.; Sian, P. Chemical shift MRI can aid in the diagnosis of indeterminate skeletal lesions of the spine. Eur. Radiol. 2016, 26, 932–940. [Google Scholar] [CrossRef]
- Schmeel, F.C.; Lakghomi, A.; Lehnen, N.C.; Haase, R.; Banat, M.; Wach, J.; Handke, N.; Vatter, H.; Radbruch, A.; Attenberger, U.; et al. Proton Density Fat Fraction Spine MRI for Differentiation of Erosive Vertebral Endplate Degeneration and Infectious Spondylitis. Diagnostics 2021, 12, 78. [Google Scholar] [CrossRef]
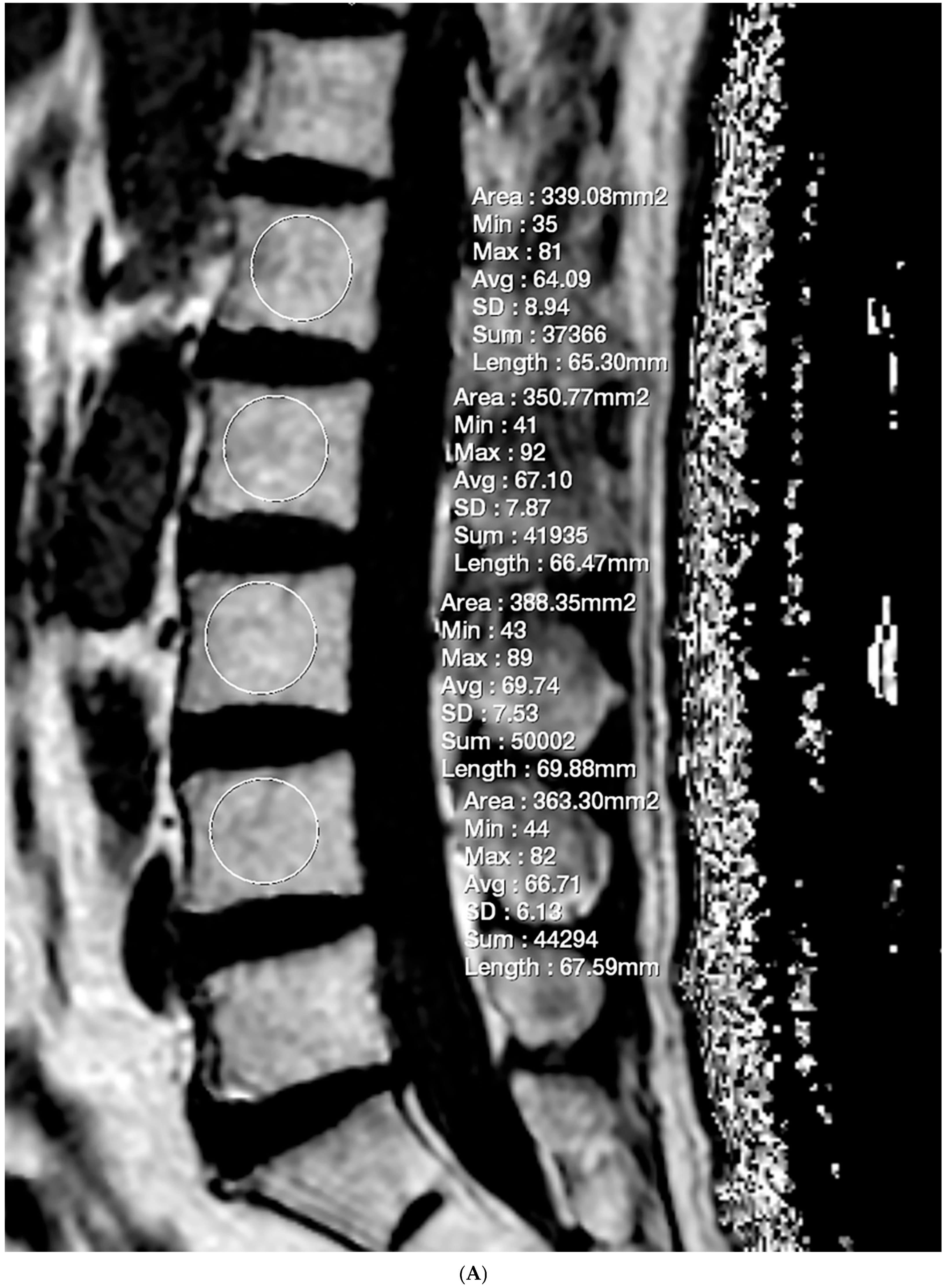
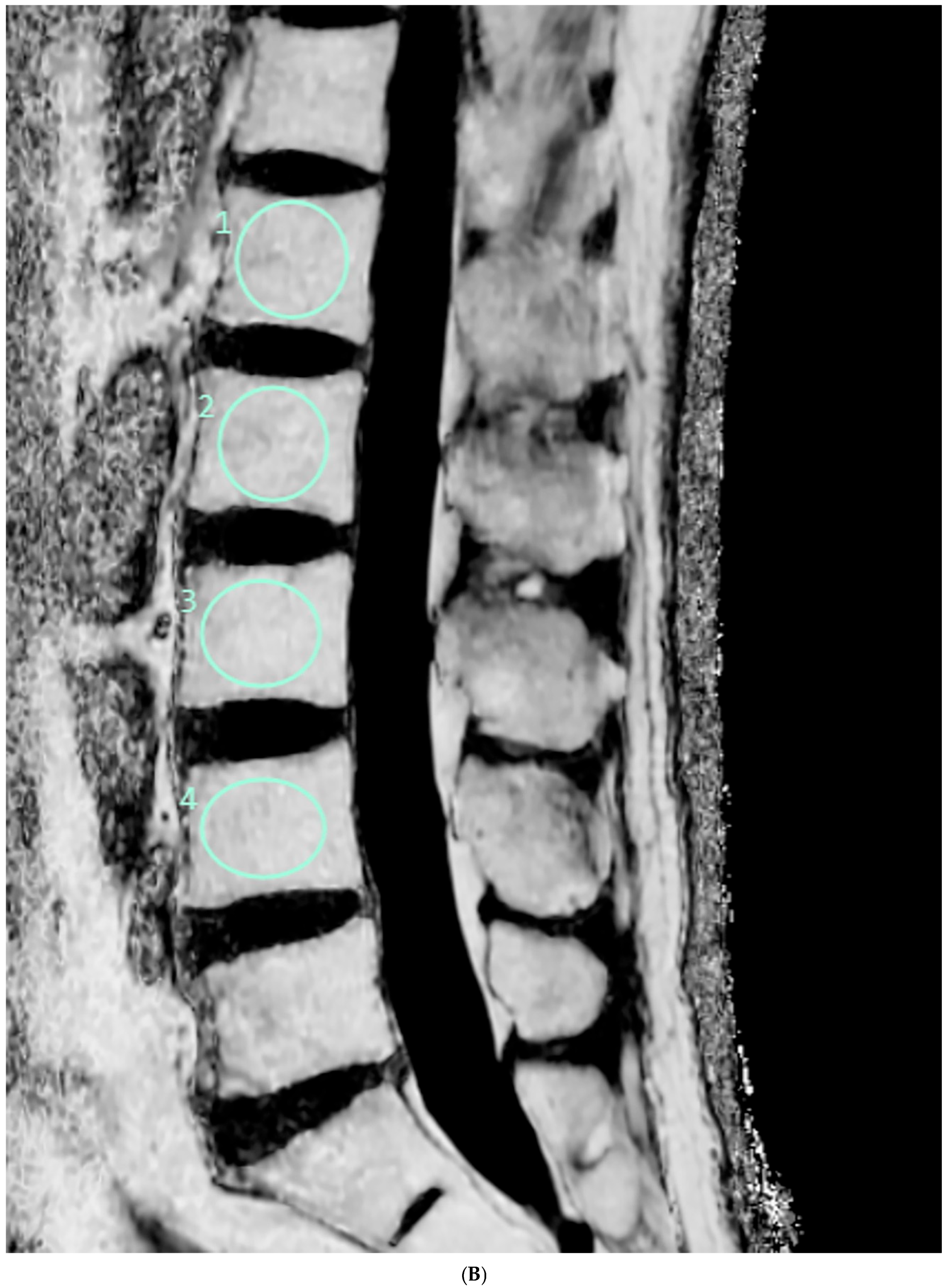

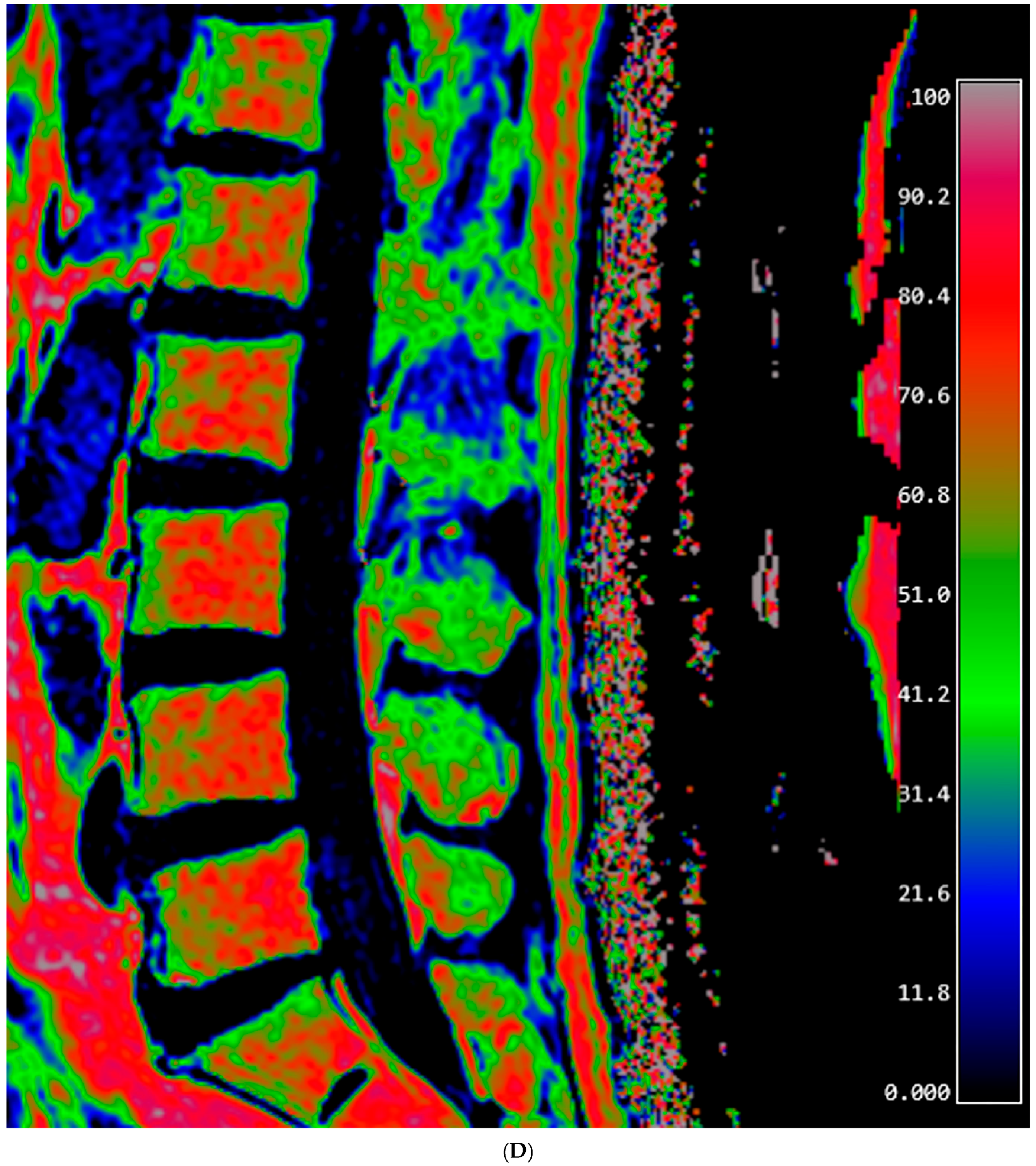

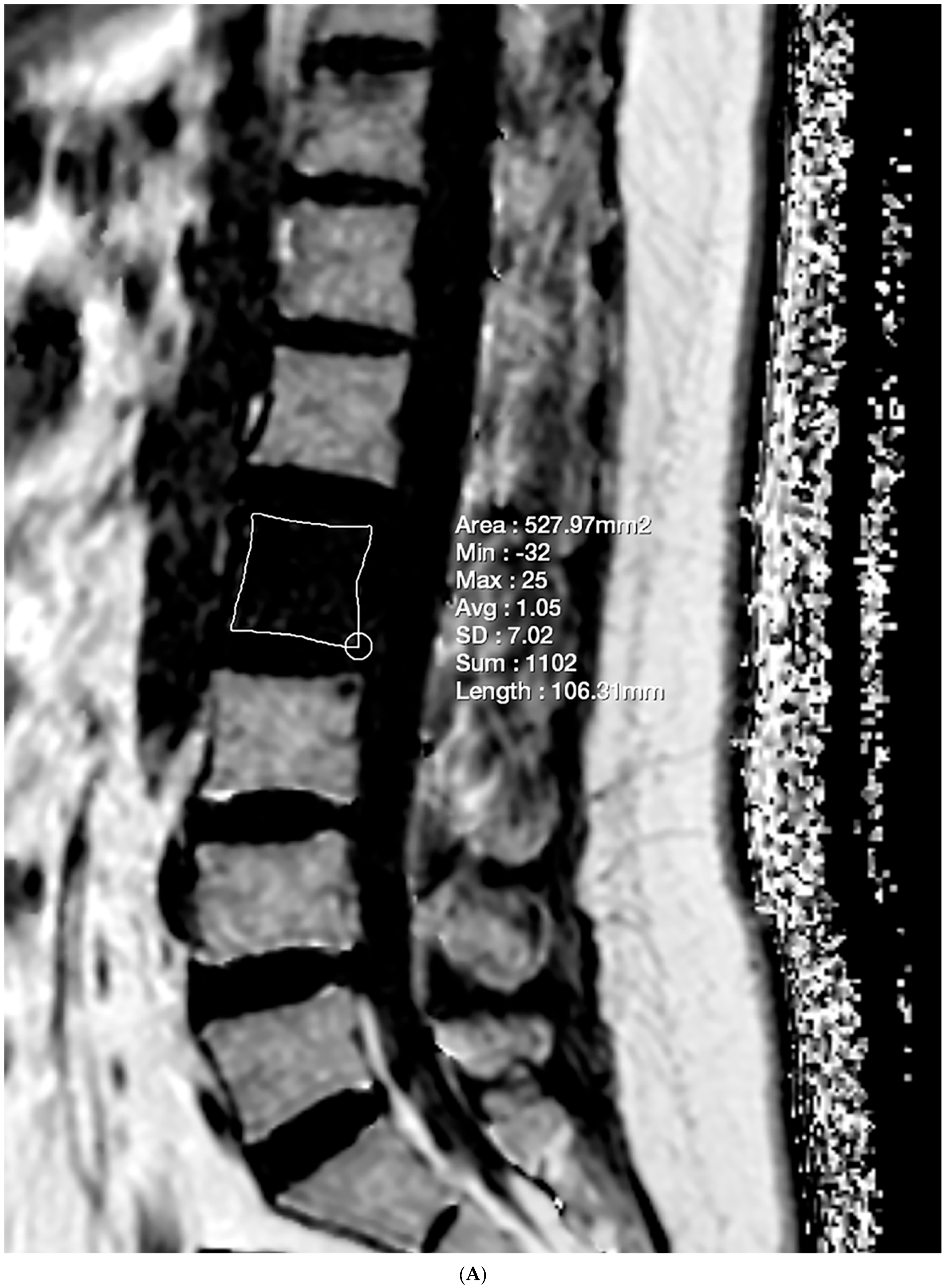

| Mean ± Standard Deviation | p | |
|---|---|---|
| FFGRE | 54.31 ± 11.04 | <0.001 |
| FFSE | 74.13 ± 9.37 | |
| VBQ score | 2.79 ± 0.58 |
| FFGRE | FFSE | VBQ Score | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| All (n = 344) | 0.583 | <0.001 | 0.477 | <0.001 | 0.468 | <0.001 |
| Age < 50 (n = 79) | 0.538 | <0.001 | 0.488 | <0.001 | 0.372 | 0.001 |
| Age ≥ 50 (n = 265) | 0.199 | 0.001 | 0.111 | 0.070 | 0.183 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Hwang, J.; Kwack, K.-S.; Lee, K.H.; Yun, J.S. Exploring Fat Fraction and Vertebral Bone Quality Score in Lumbar Spine Magnetic Resonance Imaging: A Cross-Sectional Study on Associations and Clinical Implication. Diagnostics 2025, 15, 503. https://doi.org/10.3390/diagnostics15040503
Park S, Hwang J, Kwack K-S, Lee KH, Yun JS. Exploring Fat Fraction and Vertebral Bone Quality Score in Lumbar Spine Magnetic Resonance Imaging: A Cross-Sectional Study on Associations and Clinical Implication. Diagnostics. 2025; 15(4):503. https://doi.org/10.3390/diagnostics15040503
Chicago/Turabian StylePark, Sunghoon, Jinwoo Hwang, Kyu-Sung Kwack, Kyu Hong Lee, and Jae Sung Yun. 2025. "Exploring Fat Fraction and Vertebral Bone Quality Score in Lumbar Spine Magnetic Resonance Imaging: A Cross-Sectional Study on Associations and Clinical Implication" Diagnostics 15, no. 4: 503. https://doi.org/10.3390/diagnostics15040503
APA StylePark, S., Hwang, J., Kwack, K.-S., Lee, K. H., & Yun, J. S. (2025). Exploring Fat Fraction and Vertebral Bone Quality Score in Lumbar Spine Magnetic Resonance Imaging: A Cross-Sectional Study on Associations and Clinical Implication. Diagnostics, 15(4), 503. https://doi.org/10.3390/diagnostics15040503






