Central Venous Pressure as a Predictor of Acute Kidney Injury in Cardiac Surgery: A Systematic Review of Observational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Research Strategy
2.4. Data Extraction and Analysis
2.5. Bias Assessment
3. Results
3.1. Predicting AKI in Relation to CVP
3.2. The Role of Postoperative CVP in Predicting AKI
3.3. CVP and the Need for Renal Replacement Therapy
3.4. CVP as a Limited Indicator of AKI
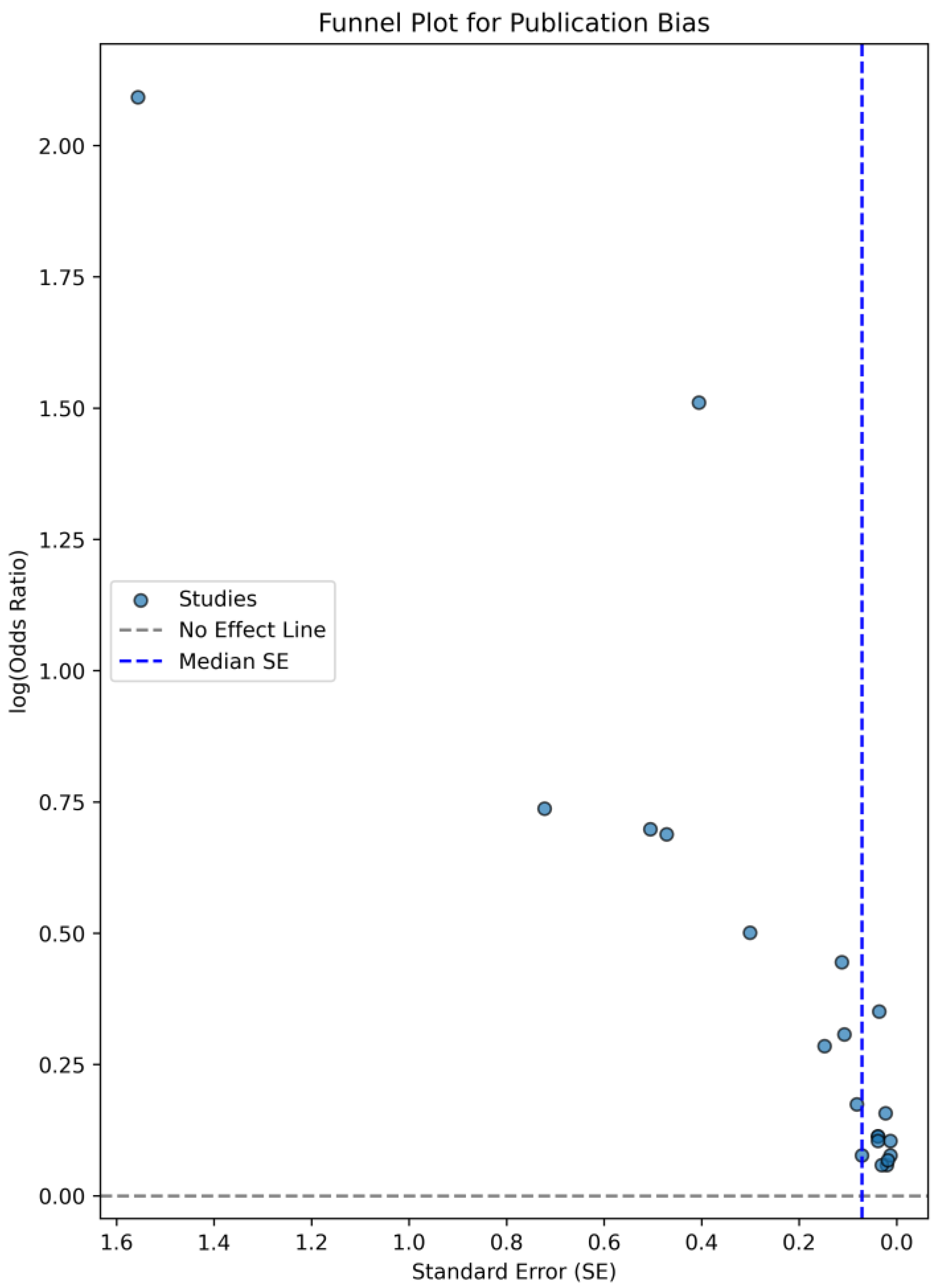
3.5. Association Between CVP Levels and AKI Risk—A Forest Plot Analysis
4. Discussion
4.1. Mechanisms Linking Elevated CVP to AKI
4.2. Surgical and CPB-Related Factors Influencing CVP and AKI
4.3. CVP Thresholds to Predict AKI
4.4. Clinical Implications and Strategies for CVP Management in Cardiac Surgery
4.5. The Impact of Comorbidities on AKI Risk and CVP Levels
4.6. Limitations
4.7. Gaps in the Research and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef]
- Rajesh, K.; Spring, K.J.; Smokovski, I.; Upmanyue, V.; Mehndiratta, M.M.; Strippoli, G.F.M.; Beran, R.G.; Bhaskar, S.M.M. The impact of chronic kidney disease on prognosis in acute stroke: Unraveling the pathophysiology and clinical complexity for optimal management. Clin. Exp. Nephrol. 2025, 29, 149–172. [Google Scholar] [CrossRef]
- Pollock, D.M. Rethinking Ischemic Acute Kidney Injury as Nephrotoxicity. Function 2024, 5, zqae020. [Google Scholar] [CrossRef]
- Lannemyr, L.; Bragadottir, G.; Krumbholz, V.; Redfors, B.; Sellgren, J.; Ricksten, S.E. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology 2017, 126, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e493. [Google Scholar] [CrossRef]
- Meng, L. Heterogeneous impact of hypotension on organ perfusion and outcomes: A narrative review. Br. J. Anaesth. 2021, 127, 845–861. [Google Scholar] [CrossRef]
- Heinrich, T.; Bähring, R.; Larena-Avellaneda, A.; Querengässer, J.; Solbrig, O.; Ehmke, H.; Schwoerer, A.P. Bridging vascular physiology to vascular medicine: An integrative laboratory class. Adv. Physiol. Educ. 2023, 47, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Luthe, S.K.; Nasu, M. Blood pressure and acute kidney injury. Crit Care 2017, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Chen, H.; Shang, X.; Yu, R. Optimization of central venous pressure during the perioperative period is associated with improved prognosis of high-risk operation patients. J. Intensive Med. 2022, 3, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Guinot, P.G.; Abou-Arab, O.; Longrois, D.; Dupont, H. Right ventricular systolic dysfunction and vena cava dilatation precede alteration of renal function in adult patients undergoing cardiac surgery: An observational study. Eur. J. Anaesthesiol. 2015, 32, 535–542. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J.; Zhao, L. High central venous pressure is associated with acute kidney injury and mortality in patients underwent cardiopulmonary bypass surgery. J. Crit. Care 2018, 48, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Wang, Y.; Zhang, Y.; Jiang, W.; Shen, B.; Ding, X. A novel machine learning algorithm, Bayesian networks model, to predict the high-risk patients with cardiac surgery-associated acute kidney injury. Clin. Cardiol. 2020, 43, 752–761. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Benkreira, A.; Robillard, P.; Bouabdallaoui, N.; Chassé, M.; Desjardins, G.; Lamarche, Y.; White, M.; Bouchard, J.; Denault, A. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated with Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J. Am. Heart Assoc. 2018, 7, e009961. [Google Scholar] [CrossRef]
- Chen, L.; Hong, L.; Ma, A.; Chen, Y.; Xiao, Y.; Jiang, F.; Huang, R.; Zhang, C.; Bu, X.; Ge, Y.; et al. Intraoperative venous congestion rather than hypotension is associated with acute adverse kidney events after cardiac surgery: A retrospective cohort study. Br. J. Anaesth. 2022, 128, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.G.; Shotwell, M.S.; Morse, J.; Liang, Y.; Wanderer, J.P.; Absi, T.S.; Balsara, K.R.; Levack, M.M.; Shah, A.S.; Hernandez, A.; et al. Intraoperative venous congestion and acute kidney injury in cardiac surgery: An observational cohort study. Br. J. Anaesth. 2021, 126, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; He, W.; Xie, Y.; Lv, W.; Li, Y.; Li, H.; Huang, J.; Huang, J.; Chen, Y.; Guo, Q.; et al. A LASSO-derived clinical score to predict severe acute kidney injury in the cardiac surgery recovery unit: A large retrospective cohort study using the MIMIC database. BMJ Open 2022, 12, e060258. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, S.; Bakaeen, F.; Tang, W.W.; Donaldson, C.; Taliercio, J.D.; Huml, A.; Gadegbeku, C.A.; Gillinov, A.M.; Insler, S.D. Hemodynamic Determinants of Cardiac Surgery-Associated Acute Kidney Injury. Crit. Care Explor. 2024, 6, e1063. [Google Scholar] [CrossRef]
- Gül, I.; Cerit, L.; Senturk, B.; Zungur, M.; Alkan, M.B.; Kemal, H.; Cerit, Z.; Yaman, B.; Usalp, S.; Duygu, H. The Negative Effect of Mean Perfusion Pressure on the Development of Acute Kidney Injury After Transcatheter Aortic Valve Implantation. Braz. J. Cardiovasc. Surg. 2018, 33, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Schiefenhövel, F.; Trauzeddel, R.F.; Sander, M.; Heringlake, M.; Groesdonk, H.V.; Grubitzsch, H.; Kruppa, J.; Berger, C.; Treskatsch, S.; Balzer, F. High Central Venous Pressure After Cardiac Surgery Might Depict Hemodynamic Deterioration Associated with Increased Morbidity and Mortality. J. Clin. Med. 2021, 10, 3945. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.J.; Liu, J.; Hu, J.F.; Xiao, L.Q.; Chen, X. Insight into Kidney Protection by Vacuum-Assisted Venous Drainage in Adult Cardiac Operation–A Multicenter Study. Circ. J. 2023, 87, 551–559. [Google Scholar] [CrossRef] [PubMed]
- McCoy, I.E.; Montez-Rath, M.E.; Chertow, G.M.; Chang, T.I. Central venous pressure and the risk of diuretic-associated acute kidney injury in patients after cardiac surgery. Am. Heart J. 2020, 221, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yu, J.; Chang, S.C.; Xu, J.; Xu, S.; Jiang, W.; Shen, B.; Zhuang, Y.; Wang, C.; Ding, X.; et al. Postoperative diastolic perfusion pressure is associated with the development of acute kidney injury in patients after cardiac surgery: A retrospective analysis. BMC Nephrol. 2019, 20, 458. [Google Scholar] [CrossRef] [PubMed]
- Kotani, Y.; Yoshida, T.; Kumasawa, J.; Kamei, J.; Taguchi, A.; Kido, K.; Yamaguchi, N.; Kariya, T.; Nakasone, M.; Mikami, N.; et al. The impact of relative hypotension on acute kidney injury progression after cardiac surgery: A multicenter retrospective cohort study. Ann. Intensive Care 2021, 11, 178. [Google Scholar] [CrossRef]
- Kang, W.; Wu, X. Pre-, Intra-, and Post-Operative Factors for Kidney Injury of Patients Underwent Cardiac Surgery: A Retrospective Cohort Study. Med. Sci. Monit. 2019, 25, 5841–5849. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Shen, B.; Wang, Y.; Xu, J.; Luo, Z.; Teng, J. Potentially Modifiable Predictors for Renal Replacement Therapy in Patients with Cardiac Surgery Associated-Acute Kidney Injury: A Propensity Score-Matched Case-Control Study. Braz. J. Cardiovasc. Surg. 2019, 34, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Houchin, A.; Nazir, N.; Leonardo, V.; Flynn, B.C. Comparing the associations of central venous pressure and pulmonary artery pulsatility index with postoperative renal injury. Front. Cardiovasc. Med. 2022, 9, 967596. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Yan Dai, Q.; Qi, H.; Wang, Z.; Chen, X. Intraoperative central venous pressure during cardiopulmonary bypass is an alternative indicator for early prediction of acute kidney injury in adult cardiac surgery. J. Cardiothorac. Surg. 2024, 19, 262. [Google Scholar] [CrossRef]
- Vandenberghe, W.; Bové, T.; De Somer, F.; Herck, I.; François, K.; Peperstraete, H.; Dhondt, A.; Martens, T.; Schaubroeck, H.; Philipsen, T.; et al. Impact of mean perfusion pressure and vasoactive drugs on occurrence and reversal of cardiac surgery-associate acute kidney injury: A cohort study. J. Crit. Care 2022, 71, 154101. [Google Scholar] [CrossRef] [PubMed]
- Barbu, M.; Hjärpe, A.; Martinsson, A.; Dellgren, G.; Ricksten, S.; Lannemyr, L.; Pivodic, A.; Taha, A.; Jeppsson, A. Cardiopulmonary bypass management and acute kidney injury in cardiac surgery patients. Acta Anaesthesiol. Scand. 2024, 68, 328–336. [Google Scholar] [CrossRef]
- Li, Z.T.; Huang, D.B.; Zhao, J.F.; Li, H.; Fu, S.Q.; Wang, W. Comparison of various surrogate markers for venous congestion in predicting acute kidney injury following cardiac surgery: A cohort study. J. Crit. Care 2024, 79, 154441. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Kalam, Y.; Broad, J.; Ho, T.; Parker, F.; Lee, M.; Bellomo, R. Decreased mean perfusion pressure as an independent predictor of acute kidney injury after cardiac surgery. Heart Vessel. 2020, 35, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Xin, D.; Xiaoting, W.; Dawei, L. High Central Venous Pressure and Right Ventricle Size Are Related to Non-Decreased Left Ventricle Stroke Volume After Negative Fluid Balance in Critically Ill Patients: A Single Prospective Observational Study. Front. Med. 2021, 8, 715099. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Wang, X.T.; Liu, D.W. Association between elevated central venous pressure and outcomes in critically ill patients. Ann. Intensive Care 2017, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.; Wang, X.; Liu, D. Impact of central venous pressure during the first 24 h and its time-course on the lactate levels and clinical outcomes of patients who underwent coronary artery bypass grafting. Front. Cardiovasc. Med. 2023, 10, 1036285. [Google Scholar] [CrossRef]
- Persichini, R.; Lai, C.; Teboul, J.L.; Adda, I.; Guérin, L.; Monnet, X. Venous return and mean systemic filling pressure: Physiology and clinical applications. Crit. Care 2022, 26, 150. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.R.; Griesdale, D.E.; Walley, K.R.; Sheel, A.W. Clinical review: Guyton—The role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit. Care 2010, 14, 243. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Honore, P.M.; Spapen, H.D.; Liu, D. Renal failure in critically ill patients, beware of applying (central venous) pressure on the kidney. Ann. Intensive Care 2018, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Gutierrez, A.; Ibrahim, H.N. The Kidney in Heart Failure: The Role of Venous Congestion. Methodist DeBakey Cardiovasc. J. 2022, 18, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Liao, L.; Pan, L.; Pinhu, L. Association Between the Central Venous Pressure and All-Cause Mortality in Critically Ill Patients with Acute Kidney Injury. Int. J. Gen. Med. 2021, 14, 8019–8027. [Google Scholar] [CrossRef] [PubMed]
- Vellinga, N.A.; Ince, C.; Boerma, E.C. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: A hypothesis generating post hoc analysis. BMC Anesthesiol. 2013, 13, 17. [Google Scholar] [CrossRef]
- Ross, E.A. Congestive renal failure: The pathophysiology and treatment of renal venous hypertension. J. Card. Fail. 2012, 18, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Feng, J.; Sellke, F.W. Strategies to attenuate maladaptive inflammatory response associated with cardiopulmonary bypass. Front. Surg. 2024, 11, 1224068. [Google Scholar] [CrossRef]
- Dutta, A.; Saha, S.; Bahl, A.; Mittal, A.; Basak, T. A comprehensive review of acute cardio-renal syndrome: Need for novel biomarkers. Front. Pharmacol. 2023, 14, 1152055. [Google Scholar] [CrossRef]
- Thurman, J.M. Triggers of inflammation after renal ischemia/reperfusion. Clin. Immunol. 2007, 123, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Tannvik, T.D.; Rimehaug, A.E.; Skjaervold, N.K.; Kirkeby-Garstad, I. Post cardiac surgery stunning reduces stroke work, but leaves cardiac power output unchanged in patients with normal ejection fraction. Physiol. Rep. 2018, 6, e13781. [Google Scholar] [CrossRef] [PubMed]
- Roshanali, F.; Yousefnia, M.A.; Mandegar, M.H.; Rayatzadeh, H.; Alinejad, S. Decreased right ventricular function after coronary artery bypass grafting. Tex. Heart Inst. J. 2008, 35, 250–255. [Google Scholar] [PubMed]
- Lemay, S.E.; Grobs, Y.; Boucherat, O. Right Ventricular Dysfunction in Pulmonary Hypertension: Is Resistin a Promising Target? J. Am. Heart Assoc. 2023, 12, e8285. [Google Scholar] [CrossRef]
- Raut, M.S.; Maheshwari, A.; Desurkar, V.; Bhavsar, R. Rising Central venous pressure: Impending right-sided failure? Ann. Card. Anaesth. 2017, 20, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lian, H.; Zhang, Q.; Zhao, H.; Wang, X. Can central venous pressure help identify acute right ventricular dysfunction in mechanically ventilated critically ill patients? Ann. Intensive Care 2024, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Dupuis, C.; Simon, C.; Gayat, E.; Mateo, J.; Lukaszewicz, A.C.; Payen, D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: A retrospective observational study. Crit. Care 2013, 17, R278. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Guo, Q.; Wang, J.; Zou, Y.; Chen, Z.; Wang, J.; Zhang, Y. Central venous pressure and acute kidney injury in critically ill patients with multiple comorbidities: A large retrospective cohort study. BMC Nephrol. 2022, 23, 83. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, W.; Wen, Y.; Zheng, J.; Chen, J.; Yu, S.; Chen, X.; Chen, W.; Xiong, X.; Wen, D.; et al. Association Between Wait Time of Central Venous Pressure Measurement and Outcomes in Critical Patients with Acute Kidney Injury: A Retrospective Cohort Study. Front. Public Health 2022, 10, 893683. [Google Scholar] [CrossRef]
- Mishra, R.C.; Sodhi, K.; Prakash, K.C.; Tyagi, N.; Chanchalani, G.; Annigeri, R.A.; Govil, D.; Savio, R.D.; Subbarayan, B.; Arora, N.; et al. ISCCM Guidelines on Acute Kidney Injury and Renal Replacement Therapy. Indian J. Crit. Care Med. 2022, 26 (Suppl. S2), S13–S42. [Google Scholar] [PubMed]
- Desebbe, O.; Mondor, W.; Gergele, L.; Raphael, D.; Vallier, S. Variations of pulse pressure and central venous pressure may predict fluid responsiveness in mechanically ventilated patients during lung recruitment manoeuvre: An ancillary study. BMC Anesthesiol. 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Gatto, P.; Giusti, G.; Pizzamiglio, F.; Previtali, I.; Vignati, C.; Arena, R. Pathophysiology of cardiorenal syndrome in decompensated heart failure: Role of lung-right heart-kidney interaction. Int. J. Cardiol. 2013, 169, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Milne, B.; Gilbey, T.; Kunst, G. Perioperative Management of the Patient at High-Risk for Cardiac Surgery-Associated Acute Kidney Injury. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4460–4482. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Bihorac, A.; Hobson, C.; Koyner, J.L.; Shaw, A.; Arnaoutakis, G.J.; Ding, X.; Engelman, D.T.; Gasparovic, H.; et al. Cardiac and Vascular Surgery-Associated Acute Kidney Injury: The 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group. J. Am. Heart Assoc. 2018, 7, e008834. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-J.; Xi, X.-M.; Jia, H.-M.; Zheng, X.; Wang, M.-P.; Li, W. Risk factors, clinical features and outcome of new-onset acute kidney injury among critically ill patients: A database analysis based on prospective cohort study. BMC Nephrol. 2021, 22, 289. [Google Scholar] [CrossRef]
- Vonk Noordegraaf, A.; Chin, K.M.; Haddad, F. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, R.; Hossain, N.; Butt, S.; Bhellar, Z.; Fatima, E.; Imtiaz, S.; Moosa, P.G.; Abbas, K.; Jafri, S.B.; Khan, S. Efficacy of multiple Biomarkers: NGAL, KIM1, Cystatin C and IL18 in predicting pregnancy related acute kidney injury. Pak. J. Med. Sci. 2023, 39, 34–40. [Google Scholar] [CrossRef]


| Stage | Serum Creatinine | Urine |
|---|---|---|
| 1 | 1.5–1.9 times baseline OR 26.5 μmol/L) rise | <0.5 mL/kg/h for 6–12 h |
| 2 | 2.0–2.9 times baseline | <0.5 mL/kg/h for ≥12 h |
| 3 | 3.0 times baseline OR 4.0 mg/dL OR inflation of RRT OR Decrease in eGFR to <35 mL/min per 1.73 m2 (patients < 18 years) | <0.3 mL/kg/h for ≥24 h OR Anuria for ≥12 h |
| Stage | Serum Creatinine | GFR Criteria | Urine Output |
|---|---|---|---|
| Risk (R) | 1.5 times baseline | >25% decrease | <0.5 mL/kg/h for 6 h |
| Injury (I) | 2.0 times baseline | >50% decrease | <0.5 mL/kg/h for 12 h |
| Failure (F) | 3.0 times baseline OR creatinine ≥ 4 mg/dL with acute rise ≥ 0.5 mg/dL | >75% decrease | <0.3 mL/kg/h for 24 h OR anuria for 12 h |
| Loss (L) | Persistent AKI with complete loss of kidney function for >4 weeks | ||
| ERSD (E) | Complete loss of kidney function for >3 months | ||
| Stage | Serum Creatinine | Urine |
|---|---|---|
| 1 | 0.3 mg/dL (26.4 μmol/L) rise or 150–200% increase from baseline | <0.5 mL/kg/h in 6 h |
| 2 | 1.5 times baseline OR 50% increase | <0.5 mL/kg/h in 12 h |
| 3 | 3.0 times baseline OR Increase in serum creatinine 354 μmol/L, with an acute increase by at least 44 μmol/L OR inflation of RRT OR | <0.3 mL/kg/h in 24 h OR Anuria for 12 h |
| Risk Factor | Points Assigned |
|---|---|
| Female sex | 1 |
| COPD requiring treatment | 1 |
| Congestive heart failure | 2 |
| LVEF < 35% | 2 |
| Diabetes mellitus and insulin treatment | 1 |
| Preoperative creatinine > 1.2 mg/dL | 2 |
| Use of intra-aortic balloon pump | 2 |
| CPB time > 120 min | 3 |
| Reoperation | 2 |
| Risk Factor | Points Assigned |
|---|---|
| Age ≥ 65 years | 2 |
| Emergency surgery | 1 |
| Congestive heart failure | 2 |
| LVEF < 35% | 2 |
| Preoperative creatinine > 1.2 mg/dL | 2 |
| Use of intra-aortic balloon pump | 2 |
| CPB time > 120 min | 3 |
| Reoperation | 2 |
| Study, Year | Country | Design | Population Size | AKI Classification | NOS Score | CVP Threshold |
|---|---|---|---|---|---|---|
| Yang et al., 2018, [11] | China | Prospective Study | 1941 patients underwent cardiac surgery | KDIGO | 7 stars | CVP > 10 cmH2O at the end of surgery |
| Li et al., 2020, [12] | China | Prospective Study | 5533 participants underwent cardiac surgery | KDIGO | 8 stars | Postoperative CVP of ≥10 mmHg |
| Beaubien-Souligny et al., 2018, [13] | Canada | Prospective Observational Cohort Study | 145 patients undergoing cardiac surgery | KDIGO | 6 stars | Higher CVP measurements at the end of surgery were associated with AKI |
| Chen et al., 2022, [14] | China | Retrospective Cohort Study | 5127 patients undergoing cardiac surgery | KDIGO | 6 stars | Intraoperative venous congestion above 16 or 20 mmHg resulted in a 7% or 11% increase in AKI risk |
| Lopez et. al., 2020, [15] | USA | Prospective Study | 435 patients undergoing cardiac surgery | KDIGO | 6 stars | Increased intraoperative venous congestion was independently associated with the development of postoperative AKI |
| Huang et al., 2022 [16] | China | Retrospective Cohort Study | 6271 patients admitted to the CSRU | KDIGO | 9 stars | CVP of 11.2 mmHg in the severe AKI group (p < 0.001) |
| Demirjian et al., 2024, [17] | Cleveland, OH | Retrospective Observational Study | 40,426 patients undergoing cardiac surgery | KDIGO | 7 stars | Elevated CVP and low cardiac index had the strongest association with AKI |
| Gül et al., 2018, [18] | Cyprus | Prospective Study | 147 patients undergone TAVI procedure | AKIN | 8 stars | The mean value of CVP was 13.2 ± 2.9 mmHg in HR-G vs. LR-G (10.9 ± 2.5 mmHg) |
| Schiefenhövel et al., 2021, [19] | Germany | Retrospective Observational Cohort Study | 9802 patients | KDIGO | 7 stars | High CVP group (>11 mmHg) had a higher incidence of AKI |
| Wang et al., 2023, [20] | China | Retrospective Study | 15,387 patients undergoing cardiac surgery | KDIGO | 9 stars | The average CVP values were significantly higher in the AKI group (CVP of 4 mmHg) compared to the non-AKI group (CVP of 2 mmHg) |
| McCoy et al., 2020, [21] | California | Retrospective Cohort Study | 4164 post-cardiac surgical patients | KDIGO | 6 stars | CVP > 12 mmHg associated with 54% probability of AKI |
| Jin et al., 2019, [22] | China | Retrospective Study | 300 patients underwent cardiac surgery | KDIGO | 7 stars | The peak value of postoperative CVP independently contributed to the development of AKI |
| Kotani et al., 2021, [23] | Japan | Retrospective Study | 746 patients undergoing cardiac procedure | KDIGO | 8 stars | CVP of 9.4 cmH2O at the AKI group |
| Kang et al., 2019, [24] | China | Retrospective Cohort Study | 1468 patients undergoing cardiac surgery | KDIGO | 7 stars | CVP < 6 cmH2O was important clinical factor that increased the risk of post-operative AKI |
| Jiang et al., 2019, [25] | China | Retrospective Case–control Study | 1773 cardiac surgery patients | KDIGO | 7 stars | CVP > 10 mmHg is a predictor of AKI-RRT |
| Wei et al., 2022. [26] | United States | Retrospective Cohort Study | 1288 patients undergoing cardiac surgery | KDIGO | 7 stars | Patients who developed CSAKI had mean CVP of 11.5 mmHg |
| Wang et al., 2024, [27] | China | Retrospective Study | 2048 patients undergoing cardiac procedure | KDIGO | 8 stars | Elevated CVP (≥6.5 cmH2O) in CPB during cardiac operation is associated with an increased risk of AKI |
| Vandenberghe et al., 2022, [28] | Belgium | Retrospective Cohort Study | 3415 patients undergoing cardiac surgery | KDIGO | 8 stars | CVP above 14 mmHg associated with AKI |
| Barbu et al., 2023, [29] | Sweden | Observational Study | 2661 coronary artery bypass grafting and/or valve patients operated on | KDIGO | 7 stars | There were no significant differences between AKI and non-AKI patients in terms of CVP |
| Li et al., 2024, [30] | China | Prospective Observational Cohort Study | 230 patients undergoing cardiac surgery | KDIGO | 7 stars | CVP exhibited strong predictive ability for the escalation of CSA-AKI rates |
| Hu et al., 2020. [31] | Australia | Retrospective Observational Study | 513 patients undergoing CPB | RIFLE | 5 stars | Confirmation of the association of baseline CVP as an independent predictor of the development of AKICS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griva, P.; Griva, V.; Samara, D.; Talliou, C.; Panagouli, K.; Roungeris, L. Central Venous Pressure as a Predictor of Acute Kidney Injury in Cardiac Surgery: A Systematic Review of Observational Studies. Diagnostics 2025, 15, 530. https://doi.org/10.3390/diagnostics15050530
Griva P, Griva V, Samara D, Talliou C, Panagouli K, Roungeris L. Central Venous Pressure as a Predictor of Acute Kidney Injury in Cardiac Surgery: A Systematic Review of Observational Studies. Diagnostics. 2025; 15(5):530. https://doi.org/10.3390/diagnostics15050530
Chicago/Turabian StyleGriva, Panagiota, Vasiliki Griva, Dimitra Samara, Christina Talliou, Konstantina Panagouli, and Loizos Roungeris. 2025. "Central Venous Pressure as a Predictor of Acute Kidney Injury in Cardiac Surgery: A Systematic Review of Observational Studies" Diagnostics 15, no. 5: 530. https://doi.org/10.3390/diagnostics15050530
APA StyleGriva, P., Griva, V., Samara, D., Talliou, C., Panagouli, K., & Roungeris, L. (2025). Central Venous Pressure as a Predictor of Acute Kidney Injury in Cardiac Surgery: A Systematic Review of Observational Studies. Diagnostics, 15(5), 530. https://doi.org/10.3390/diagnostics15050530






