Deep Learning Models to Detect Anterior Cruciate Ligament Injury on MRI: A Comprehensive Review
Abstract
:1. Introduction
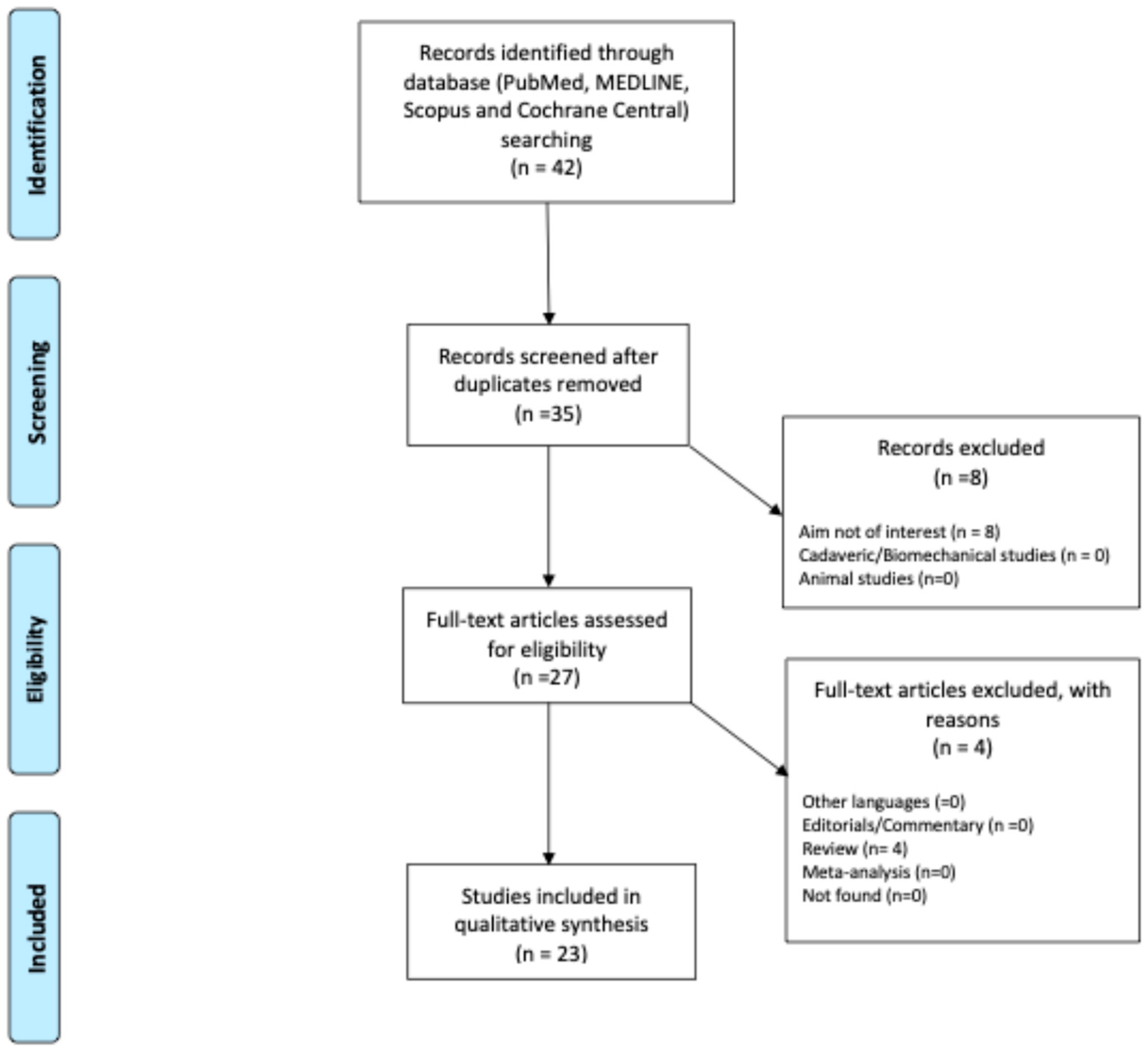
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gianotti, S.M.; Marshall, S.W.; Hume, P.A.; Bunt, L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: A national population-based study. J. Sci. Med. Sport 2009, 12, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Mabrouk, A.; Nielson, J. l: Anterior Cruciate Ligament Knee Injury; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Siouras, A.; Moustakidis, S.; Giannakidis, A.; Chalatsis, G.; Liampas, I.; Vlychou, M.; Hantes, M.; Tasoulis, S.; Tsaopoulos, D. Knee injury detection using deep learning on MRI studies: A systematic review. Diagnostics 2022, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Corona, K.; Cerciello, S.; Vasso, M.; Toro, G.; D’ambrosi, R.; Pola, E.; Ciolli, G.; Mercurio, M.; Panni, A.S. Age over 50 does not predict results in anterior cruciate ligament reconstruction. Orthop. Rev. 2022, 14, 37310. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.J.; Mohd Rahim, M.S.; Salim, N.; Nobanee, H.; Asif, A.A.; Attiq, M.O. MGACA-Net: A novel deep learning based multi-scale guided attention and context aggregation for localization of knee anterior cruciate ligament tears region in MRI images. PeerJ Comput. Sci. 2023, 9, e1483. [Google Scholar] [CrossRef]
- Familiari, F.; Tollefson, L.V.; Izzo, A.; Mercurio, M.; LaPrade, R.F.; Di Vico, G. A High-Grade Lachman’s Exam Predicts a Ramp Tear of the Medial Meniscus in Patients with Anterior Cruciate Ligament Tear: A Prospective Clinical and Radiological Evaluation. J. Clin. Med. 2024, 13, 683. [Google Scholar] [CrossRef]
- Chang, P.D.; Wong, T.T.; Rasiej, M.J. Deep Learning for Detection of Complete Anterior Cruciate Ligament Tear. J. Digit. Imaging 2019, 32, 980–986. [Google Scholar] [CrossRef]
- Mercurio, M.; Corona, K.; Galasso, O.; Cerciello, S.; Morris, B.J.; Guerra, G.; Gasparini, G. Soccer players show the highest seasonal groin pain prevalence and the longest time loss from sport among 500 athletes from major team sports. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2149–2157. [Google Scholar] [CrossRef]
- Liang, C.; Li, X.; Qin, Y.; Li, M.; Ma, Y.; Wang, R.; Xu, X.; Yu, J.; Lv, S.; Luo, H. Effective automatic detection of anterior cruciate ligament injury using convolutional neural network with two attention mechanism modules. BMC Med. Imaging 2023, 23, 120. [Google Scholar] [CrossRef]
- Minici, R.; Mercurio, M.; Iannò, B.; Galasso, O.; Gasparini, G.; Laganà, D. Advantages of the Use of Axial Traction Magnetic Resonance Imaging (MRI) of the Shoulder in Patients with Suspected Rota-Tor Cuff Tears: An Exploratory Pilot Study. Healthc. Switz. 2023, 11, 724. [Google Scholar] [CrossRef]
- Ng, W.H.A.; Griffith, J.F.; Hung, E.H.Y.; Paunipagar, B.; Law, B.K.Y.; Yung, P.S.H. Imaging of the anterior cruciate ligament. World J. Orthop. 2011, 2, 75. [Google Scholar] [CrossRef]
- Holmes, J.; Sacchi, L.; Bellazzi, R. Artificial intelligence in medicine. Ann. R. Coll. Surg. Engl. 2004, 86, 334–338. [Google Scholar]
- Loftus, T.J.; Tighe, P.J.; Filiberto, A.C.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Rashidi, P.; Upchurch, G.R.; Bihorac, A. Artificial intelligence and surgical decision-making. JAMA Surg. 2020, 155, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.C. Machine learning in medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Santomartino, S.M.; Kung, J.; Yi, P.H. Systematic review of artificial intelligence development and evaluation for MRI diagnosis of knee ligament or meniscus tears. Skelet. Radiol. 2024, 53, 445–454. [Google Scholar] [CrossRef]
- Cheng, Q.; Lin, H.; Zhao, J.; Lu, X.; Wang, Q. Application of machine learning-based multi-sequence MRI radiomics in diagnosing anterior cruciate ligament tears. J. Orthop. Surg. 2024, 19, 99. [Google Scholar] [CrossRef]
- Kumar, Y.J.; Wee, S.Y.; Perhakaran, V.K. A Systematic Review on Deep Learning Model in Computer-aided Diagnosis for Anterior Cruciate Ligament Injury. Curr. Med. Imaging 2024, 20, e15734056295157. [Google Scholar] [CrossRef]
- Guermazi, A.; Omoumi, P.; Tordjman, M.; Fritz, J.; Kijowski, R.; Regnard, N.-E.; Carrino, J.; Kahn, C.E.; Knoll, F.; Rueckert, D.; et al. How AI May Transform Musculoskeletal Imaging. Radiology 2024, 310, e230764. [Google Scholar] [CrossRef]
- Althnian, A.; AlSaeed, D.; Al-Baity, H.; Samha, A.; Dris, A.B.; Alzakari, N.; Abou Elwafa, A.; Kurdi, H. Impact of Dataset Size on Classification Performance: An Empirical Evaluation in the Medical Domain. Appl. Sci. 2021, 11, 796. [Google Scholar] [CrossRef]
- Mercurio, M.; Carlisi, G.; Ostojic, M.; Imbrogno, A.; Galasso, O.; Gasparini, G. The Protective Role of the FIFA 11+ Training Program on the Valgus Loading of the Knee in Academy Soccer Players Across a Season. Healthcare 2025, 13, 73. [Google Scholar] [CrossRef]
- D’Ambrosi, R.; Corona, K.; Cerciello, S.; Guerra, G.; Mercurio, M.; Galasso, O.; Valli, F.; Abermann, E.; Fink, C. Combining an Anterolateral Complex Procedure With Anterior Cruciate Ligament Reconstruction Reduces Graft Reinjury Without Increasing the Rate of Complications: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2025, 12, 3635465241285887. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Javed Awan, M.; Mohd Rahim, M.; Salim, N.; Mohammed, M.; Garcia-Zapirain, B.; Abdulkareem, K. Efficient Detection of Knee Anterior Cruciate Ligament from Magnetic Resonance Imaging Using Deep Learning Approach. Diagnostics 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Yang, C.-Y.; Wang, H.-Y.; Ma, H.-L.; Lee, O.K.-S. Artificial Intelligence–Assisted Diagnosis of Anterior Cruciate Ligament Tears From Magnetic Resonance Images: Algorithm Development and Validation Study. JMIR AI 2022, 1, e37508. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.T.; Thuan, N.H.; Van Dung, T.; Van Nho, L.; Tri, N.M.; Vy, V.P.T.; Hoang, L.N.; Phat, N.T.; Chuong, D.A.; Dang, L.H. End-to-end deep learning model for segmentation and severity staging of anterior cruciate ligament injuries from MRI. Diagn. Interv. Imaging 2023, 104, 133–141. [Google Scholar] [CrossRef]
- Germann, C.; Marbach, G.; Civardi, F.; Fucentese, S.F.; Fritz, J.; Sutter, R.; Pfirrmann, C.W.A.; Fritz, B. Deep Convolutional Neural Network–Based Diagnosis of Anterior Cruciate Ligament Tears: Performance Comparison of Homogenous Versus Heterogeneous Knee MRI Cohorts With Different Pulse Sequence Protocols and 1.5-T and 3-T Magnetic Field Strengths. Investig. Radiol. 2020, 55, 499–506. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Yoshino, K.; Hagiwara, S.; Watanabe, A.; Quek, S.T.; Yoshioka, H.; Feng, M. Interpretable and lightweight 3-D deep learning model for automated ACL diagnosis. IEEE J. Biomed. Health Inform. 2021, 25, 2388–2397. [Google Scholar] [CrossRef]
- Joshi, K.; Suganthi, K. Anterior Cruciate Ligament Tear Detection Based on Deep Convolutional Neural Network. Diagnostics 2022, 12, 2314. [Google Scholar] [CrossRef]
- Li, F.; Zhai, P.; Yang, C.; Feng, G.; Yang, J.; Yuan, Y. Automated diagnosis of anterior cruciate ligament via a weighted multi-view network. Front. Bioeng. Biotechnol. 2023, 11, 1268543. [Google Scholar] [CrossRef]
- Li, Z.; Ren, S.; Zhou, R.; Jiang, X.; You, T.; Li, C.; Zhang, W. Deep Learning-Based Magnetic Resonance Imaging Image Features for Diagnosis of Anterior Cruciate Ligament Injury. J. Healthc. Eng. 2021, 2021, 4076175. [Google Scholar] [CrossRef]
- Liu, F.; Guan, B.; Zhou, Z.; Samsonov, A.; Rosas, H.; Lian, K.; Sharma, R.; Kanarek, A.; Kim, J.; Guermazi, A.; et al. Fully Automated Diagnosis of Anterior Cruciate Ligament Tears on Knee MR Images by Using Deep Learning. Radiol. Artif. Intell. 2019, 1, 180091. [Google Scholar] [CrossRef]
- Minamoto, Y.; Akagi, R.; Maki, S.; Shiko, Y.; Tozawa, R.; Kimura, S.; Yamaguchi, S.; Kawasaki, Y.; Ohtori, S.; Sasho, T. Automated detection of anterior cruciate ligament tears using a deep convolutional neural network. BMC Musculoskelet. Disord. 2022, 23, 577. [Google Scholar] [CrossRef] [PubMed]
- Namiri, N.K.; Flament, I.; Astuto, B.; Shah, R.; Tibrewala, R.; Caliva, F.; Link, T.M.; Pedoia, V.; Majumdar, S. Deep Learning for Hierarchical Severity Staging of Anterior Cruciate Ligament Injuries from MRI. Radiol. Artif. Intell. 2020, 2, e190207. [Google Scholar] [CrossRef]
- Richardson, M.L. MR Protocol Optimization With Deep Learning: A Proof of Concept. Curr. Probl. Diagn. Radiol. 2021, 50, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Choi, G.S.; Chang, M.C. Development of convolutional neural network model for diagnosing tear of anterior cruciate ligament using only one knee magnetic resonance image. Medicine 2022, 101, e31510. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Lassalle, L.; Zille, P.; Guillin, R.; Pluot, E.; Adam, C.; Charachon, M.; Brat, H.; Wallaert, M.; d’Assignies, G.; et al. Deep learning to detect anterior cruciate ligament tear on knee MRI: Multi-continental external validation. Eur. Radiol. 2022, 32, 8394–8403. [Google Scholar] [CrossRef]
- Wang, D.; Liu, S.; Ding, J.; Sun, A.; Jiang, D.; Jiang, J.; Zhao, J.; Chen, D.; Ji, G.; Li, N.; et al. A deep learning model enhances clinicians’ diagnostic accuracy to more than 96% for anterior cruciate ligament ruptures on magnetic resonance imaging. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 1197–1205. [Google Scholar] [CrossRef]
- Wang, M.; Yu, C.; Li, M.; Zhang, X.; Jiang, K.; Zhang, Z.; Zhang, X. One-stop detection of anterior cruciate ligament injuries on magnetic resonance imaging using deep learning with multicenter validation. Quant. Imaging Med. Surg. 2024, 14, 3405–3416. [Google Scholar] [CrossRef]
- NIMH Data Archive—NDA. Available online: https://nda.nih.gov/oai%C2%BB (accessed on 24 February 2025).
- Wang, X.; Wu, Y.; Li, J.; Li, Y.; Xu, S. Deep Learning-Assisted Automatic Diagnosis of Anterior Cruciate Ligament Tear in Knee Magnetic Resonance Images. Tomography 2024, 10, 1263–1276. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, S.; Sun, W.; Tan, H.; Lin, K.; Peng, L.; Wang, Z.; Zhang, J. Approaching expert-level accuracy for differentiating ACL tear types on MRI with deep learning. Sci. Rep. 2024, 14, 938. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Zhou, Y.; Lu, G.; Zhou, Q. Deep Learning Approach for Anterior Cruciate Ligament Lesion Detection: Evaluation of Diagnostic Performance Using Arthroscopy as the Reference Standard. J. Magn. Reson. Imaging 2020, 52, 1745–1752. [Google Scholar] [CrossRef]
- Mercurio, M.; Cerciello, S.; Corona, K.; Guerra, G.; Simonetta, R.; Familiari, F.; Galasso, O.; Gasparini, G. Factors Associated With a Successful Return to Performance After Anterior Cruciate Ligament Reconstruction: A Multiparametric Evaluation in Soccer Players. Orthop. J. Sports Med. 2024, 12, 23259671241275663. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.; Cofano, E.; Gasparini, G.; Galasso, O.; Familiari, F.; Sanzo, V.; Ciolli, G.; Corona, K.; Cerciello, S. Isolated ACL Reconstruction Versus Combined ACL and Anterolateral Ligament Reconstruction: Functional Outcomes, Return to Sport, and Survivorship: An Updated Meta-analysis of Comparative Studies. Am. J. Sports Med. 2025, 53, 971–980. [Google Scholar] [CrossRef]
- Bien, N.; Rajpurkar, P.; Ball, R.L.; Irvin, J.; Park, A.; Jones, E.; Bereket, M.; Patel, B.N.; Yeom, K.W.; Shpanskaya, K.; et al. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: Development and retrospective validation of MRNet. PLoS Med. 2018, 15, e1002699. [Google Scholar] [CrossRef] [PubMed]
- Štajduhar, I.; Mamula, M.; Miletić, D.; Ünal, G. Semi-automated detection of anterior cruciate ligament injury from MRI. Comput. Methods Programs Biomed. 2017, 140, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D. Synthetic Data and Data Augmentation. Available online: https://medium.com/@dogankeskin01/synthetic-data-and-data-augmentation-c022029dd660 (accessed on 24 February 2025).
- Wang, G.; He, L.; Yuan, C.; Huang, Y.; Liu, Z.; Liang, C. Pretreatment MR imaging radiomics signatures for response prediction to induction chemotherapy in patients with nasopharyngeal carcinoma. Eur. J. Radiol. 2018, 98, 100–106. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Masui, T.; Terauchi, K.; Yamada, T.; Katyayama, M.; Ichikawa, S.; Noda, Y.; Goshima, S. MRI-based radiomics analysis for differentiating phyllodes tumors of the breast from fibroadenomas. Eur. Radiol. 2022, 32, 4090–4100. [Google Scholar] [CrossRef]
- Wu, M.; Xu, W.; Fei, Y.; Li, Y.; Yuan, J.; Qiu, L.; Zhang, Y.; Chen, G.; Cheng, Y.; Cao, Y.; et al. MRI-based clinical radiomics nomogram may predict the early response after concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma. Front. Oncol. 2023, 13, 1192953. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Kiryati, N.; Konen, E.; Eshed, I.; Mayer, A. Knee injury detection using MRI with efficiently-layered network (ELNet). In Proceedings of the Third Conference on Medical Imaging with Deep Learning, Virtual, 6–8 July 2020; pp. 784–794. [Google Scholar]
- Oquab, M.; Darcet, T.; Moutakanni, T.; Vo, H.; Szafraniec, M.; Khalidov, V.; Fernandez, P.; Haziza, D.; Massa, F.; El-Nouby, A.; et al. Dinov2: Learning robust visual features without supervision. arXiv 2023, arXiv:2304.07193. [Google Scholar]
- Chen, S.; Ma, K.; Zheng, Y. Med3d: Transfer learning for 3d medical image analysis. arXiv 2019, arXiv:1904.00625. [Google Scholar]
- Familiari, F.; Galasso, O.; Massazza, F.; Mercurio, M.; Fox, H.; Srikumaran, U.; Gasparini, G. Artificial Intelligence in the Management of Rotator Cuff Tears. Int. J. Environ. Res. Public. Health 2022, 19, 16779. [Google Scholar] [CrossRef]
- Cerciello, S.; Mercurio, M.; Corona, K.; Proietti, L.; Di Vico, G.; Giordano, M.C.; Morris, B.J. Posterior Cruciate Buckling Angle Variations Are Associated with Different Patterns of Medial Meniscus Tears in Anterior-Cruciate-Deficient Knees: Results of a Prospective Comparative Magnetic Imaging Resonance Study. Healthcare 2024, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, Y.; Xu, B. Three-dimensional reconstruction with dual-source computed tomography for evaluating graft deformation and bone tunnel position following reconstruction of the anterior cruciate ligament. Med. Eng. Phys. 2022, 110, 103858. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Singh, M.; Karimi, D.; Kim, J.-Y.; Flannery, S.W.; Ecklund, K.; Murray, M.M.; Fleming, B.C.; Gholipour, A.; Kiapour, A.M.; et al. LigaNET: A multi-modal deep learning approach to predict the risk of subsequent anterior cruciate ligament injury after surgery. medRxiv 2023. [Google Scholar] [CrossRef]
- Liuzzi, P.; Nesi, E.; Campagnini, S.; Mari, F.; Dimauro, I.; Carta, N.; Rocchi, J.; Bergamini, E.; Mannini, A.; Mariani, P. Predicting 60-day Recovery Outcomes After ACL Surgery Using Machine Learning. Gait Posture 2024, 114, S26. [Google Scholar] [CrossRef]
- Park, S.G.; Park, J.; Choi, H.R.; Lee, J.H.; Cho, S.T.; Lee, Y.G.; Ahn, H.; Pak, S. Deep Learning Model for Real-time Semantic Segmentation During Intraoperative Robotic Prostatectomy. Eur. Urol. Open Sci. 2024, 62, 47–53. [Google Scholar] [CrossRef]
- Baker, L.A.; Momen, M.; Chan, K.; Bollig, N.; Lopes, F.B.; Rosa, G.J.; Todhunter, R.J.; Binversie, E.E.; Sample, S.J.; Muir, P. Bayesian and machine learning models for genomic prediction of anterior cruciate ligament rupture in the canine model. G3 Genes Genomes Genet. 2020, 10, 2619–2628. [Google Scholar] [CrossRef]


| Authors and Year | Where | Journal | Method | Software | % Accuracy and % Specificity | Dataset |
|---|---|---|---|---|---|---|
| Awan et al. 2023 [4] | Pakistan | PeerJ Computer Science | MGACA | Python (version 9.3.12); Python Software Foundation, Wilmington, DE, USA | 98%–NA | In-house dataset (15,265 images) |
| Awan et al. 2021 [23] | Pakistan | Diagnostics | ResNet-14 CNN | Python (version 3.6); Python Software Foundation, Wilmington, DE, USA | 92–94% | In-house dataset (917 knees sagittal plane) |
| Chang et al. 2019 [7] | USA | Journal of Imaging Informatics in Medicine | CNN | Python (version 3.5; Python Software Foundation, Wilmington, DE, USA | 96–100% | In-house dataset |
| Chen et al. 2022 [24] | Taiwan | JMIR AI. | CNN | Python (version 3.x); Python Software Foundation, Wilmington, Delaware, USA and PyTorch (version 1.1.x) Meta AI, Menlo Park, CA, USA | 96–96% | In-house dataset (1000 cases) |
| Cheng et al. 2024 [16] | China | Journal of Orthopaedic Surgery and Research | SVM | Python (version 3.6); Python Software Foundation, Wilmington, DE, USA | 93–93% | In-house dataset (526 patients) |
| Dung et al. 2023 [25] | Vietnam | Diagnostic and Interventional Imaging | DCLU-Net CNN | TensorFlow (Available online: https://www.tensorflow.org, Accessed on: 12 December 2024) Google, Mountain View, CA, USA PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | 90–89% | In-house dataset (expanded—247 patients) |
| Germann et al. 2020 [26] | Switzerland | Invest Radiol. | DCNN | TensorFlow (version 1.11) Google, Mountain View, CA, USA | AUC 93–93% | In-house dataset (5802 MRI) |
| Jeon et al. 2021 [27] | Japan | IEEE Journal of Biomedical and Health Informatics | CNN | PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | AUC 98–95% | Chiba and Stanford datasets+ data augmentation |
| Joshi and Suganthi 2022 [28] | India | Diagnostics | CPDCNN | Python (version 3.x), Python Software Foundation Beaverton, OR, USA | 96%–NA | Stanford dataset |
| Li et al. 2023 [29] | China | Frontiers in Bioengineering and Biotechnology | 3D CNN | PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | AUC 97% and 93% for each dataset–NA | In-house dataset + Stanford dataset |
| Li et al. 2021 [30] | China | Journal of Healthcare Engineering | pretrained VGG16 | TensorFlow (Available online: https://www.tensorflow.org, Accessed on: 12 December 2024) Google, Mountain View, California, USA PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | 92–91% | In-house dataset (30 patients) |
| Liang et al. 2023 [9] | China | BMC Medical Imaging | Res-Net CNN | TensorFlow (Available online: https://www.tensorflow.org, Accessed on: 12 December 2024) Google, Mountain View, CA, USA PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | 81–65% | In-house dataset (468 images) + data augmentation |
| Liu et al. 2019 [31] | USA | Radiology: Artificial Intelligence | CNN | Python (version 2.7); Python Software Foundation, Wilmington, DE, USA | AUC 98–96% | In-house dataset (300 subjects) |
| Minamoto et al. 2022 [32] | Japan | BMC Musculoskeletal Disorders | CNN | Python (version 3.6.7); Python Software Foundation, Wilmington, DE, USA | 88–86% | In-house dataset (100 epochs) + data augmentation |
| Namiri et al. 2020 [33] | USA | Radiology: Artificial Intelligence | 2D e 3D CNN | TensorFlow (Available online: https://www.tensorflow.org, Accessed on: 12 December 2024) Google, Mountain View, CA, USA PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | 92% and 89–90% and 88% | In-house dataset (1243 knee MRI) |
| Richardson. 2021 [34] | USA | Current Problem in Diagnostic Radiology | CNN | Python (version 1.2.2); Python Software Foundation, Wilmington, DE, USA | 99–99% | In-house dataset (2007 images) |
| Shin et al. 2022 [35] | Korea | Medicine | VGGNet model CNN | TensorFlow (Available online: https://www.tensorflow.org, Accessed on: 12 December 2024) Google, Mountain View, CA, USA PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | 94%–NA | In-house dataset (130 images) |
| Tran et al. 2022 [36] | France | European Radiology | CNN | TensorFlow (Available online: https://www.tensorflow.org, Accessed on: 12 December 2024) Google, Mountain View, CA, USA | AUC 94–91% | In-house dataset vs. two esternal dataset (Stanford dataset and KneeMRI) |
| Wang et al. 2024 [37] | China | Arthroscopy | CNN | TensorFlow (Available online: https://www.tensorflow.org, Accessed on: 12 December 2024) Google, Mountain View, CA, USA PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024): Meta AI, Menlo Park, CA, USA | 96–95% | Internal dataset (22,767 MRIs) vs. external validation dataset (4086 MRIs) |
| Wang et al. 2024 [38] | China | QIMS | CNN (YOLOv5m and ResNet-18) | PyTorch (version 1.11.0) Meta AI, Menlo Park, CA, USA | 95–95% | OAI dataset [39] (1589 knees) vs. external (Stanford and kneeMRI dataset) |
| Wang et al. 2024 [40] | China | Tomography | SGNET model CNN | PyTorch (Available online: https://pytorch.org, Accessed on: 12 December 2024) Meta AI, Menlo Park, CA, USA | 92–92% | Stanford dataset |
| Xue et al. 2024 [41] | China | Nature | U-Net CNN | Python (version 2.7); Python Software Foundation, Wilmington, DE, USA | 99–97% | In-house dataset (862 participants) |
| Zhang et al. 2020 [42] | China | Journal of Magnetic Resonance Imaging | 3D DenseNet CNN | ITK-SNAP software (v. 3.6; Available online: http://www.itksnap.org, Accessed on: 12 December 2024) | 96–94% | In-house dataset (408 subjects) + data augmentation |
| Criteria | Total | Quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| Awan et al. (2021) [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Awan et al. (2023) [5] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | High |
| Chang et al. (2019) [7] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 | High |
| Chen et al. (2022) [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Cheng et al. (2024) [16] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Dung et al. (2023) [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Germann et al. (2020) [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Jeon et al. (2021) [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Joshi, Suganthi (2022) [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Li et al. (2023) [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Li et al. (2021) [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Liang et al. (2023) [9] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Liu et al. (2019) [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Minamoto et al. (2022) [32] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 | High |
| Namiri et al. (2020) [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Richardson (2021) [34] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Shin et al. (2022) [35] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | High |
| Tran et al. (2022) [36] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Wang et al. (2024) [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Wang et al. (2024) [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Wang et al. (2024) [37] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Xue et al. (2024) [41] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
| Zhang et al. (2020) [42] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercurio, M.; Denami, F.; Melissaridou, D.; Corona, K.; Cerciello, S.; Laganà, D.; Gasparini, G., on behalf of the IORS; Minici, R. Deep Learning Models to Detect Anterior Cruciate Ligament Injury on MRI: A Comprehensive Review. Diagnostics 2025, 15, 776. https://doi.org/10.3390/diagnostics15060776
Mercurio M, Denami F, Melissaridou D, Corona K, Cerciello S, Laganà D, Gasparini G on behalf of the IORS, Minici R. Deep Learning Models to Detect Anterior Cruciate Ligament Injury on MRI: A Comprehensive Review. Diagnostics. 2025; 15(6):776. https://doi.org/10.3390/diagnostics15060776
Chicago/Turabian StyleMercurio, Michele, Federica Denami, Dimitra Melissaridou, Katia Corona, Simone Cerciello, Domenico Laganà, Giorgio Gasparini on behalf of the IORS, and Roberto Minici. 2025. "Deep Learning Models to Detect Anterior Cruciate Ligament Injury on MRI: A Comprehensive Review" Diagnostics 15, no. 6: 776. https://doi.org/10.3390/diagnostics15060776
APA StyleMercurio, M., Denami, F., Melissaridou, D., Corona, K., Cerciello, S., Laganà, D., Gasparini, G., on behalf of the IORS, & Minici, R. (2025). Deep Learning Models to Detect Anterior Cruciate Ligament Injury on MRI: A Comprehensive Review. Diagnostics, 15(6), 776. https://doi.org/10.3390/diagnostics15060776










