Perspectives on Reducing Barriers to the Adoption of Digital and Computational Pathology Technology by Clinical Labs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
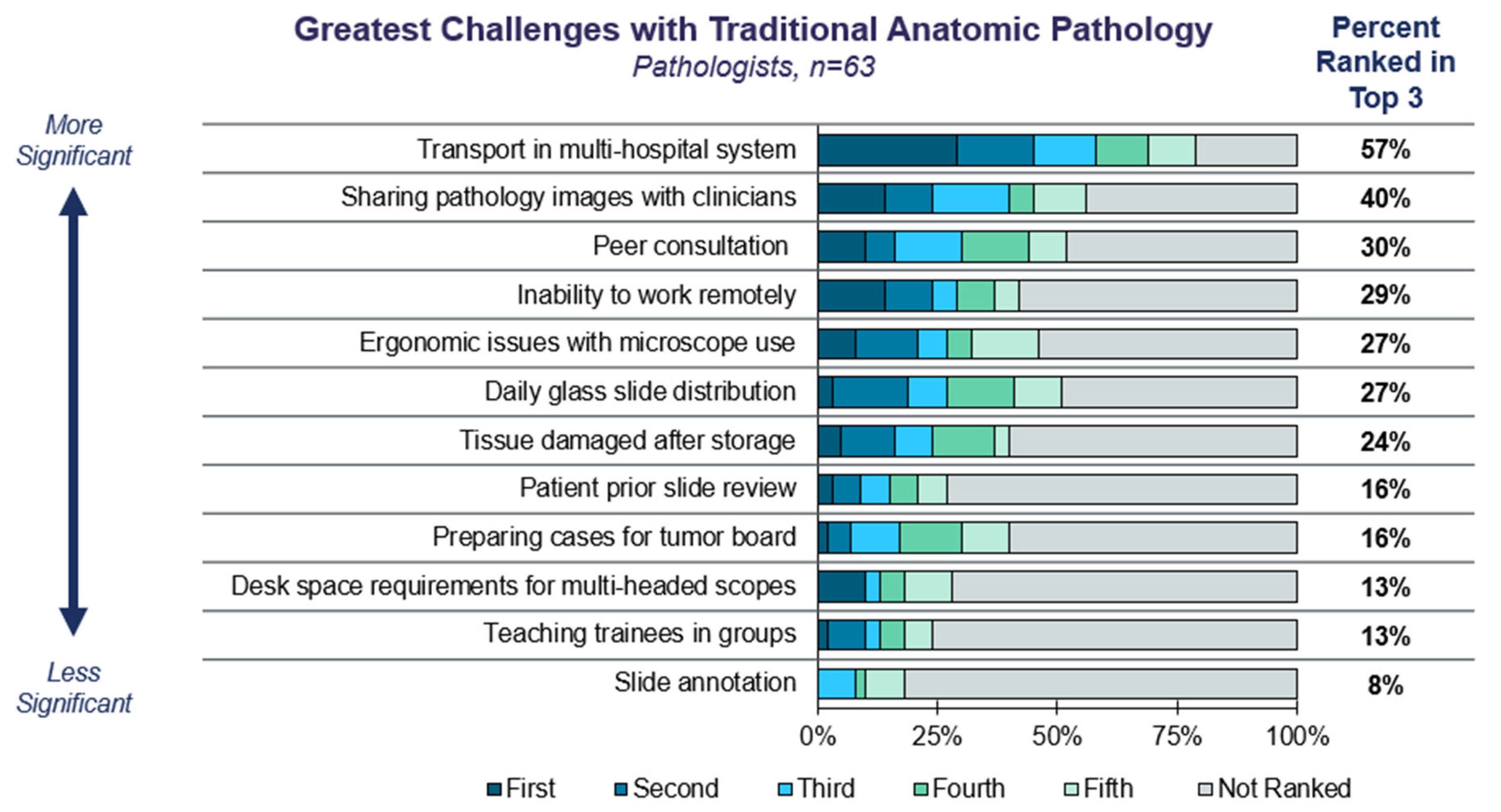
3.1. Current Trends in Anatomic Pathology
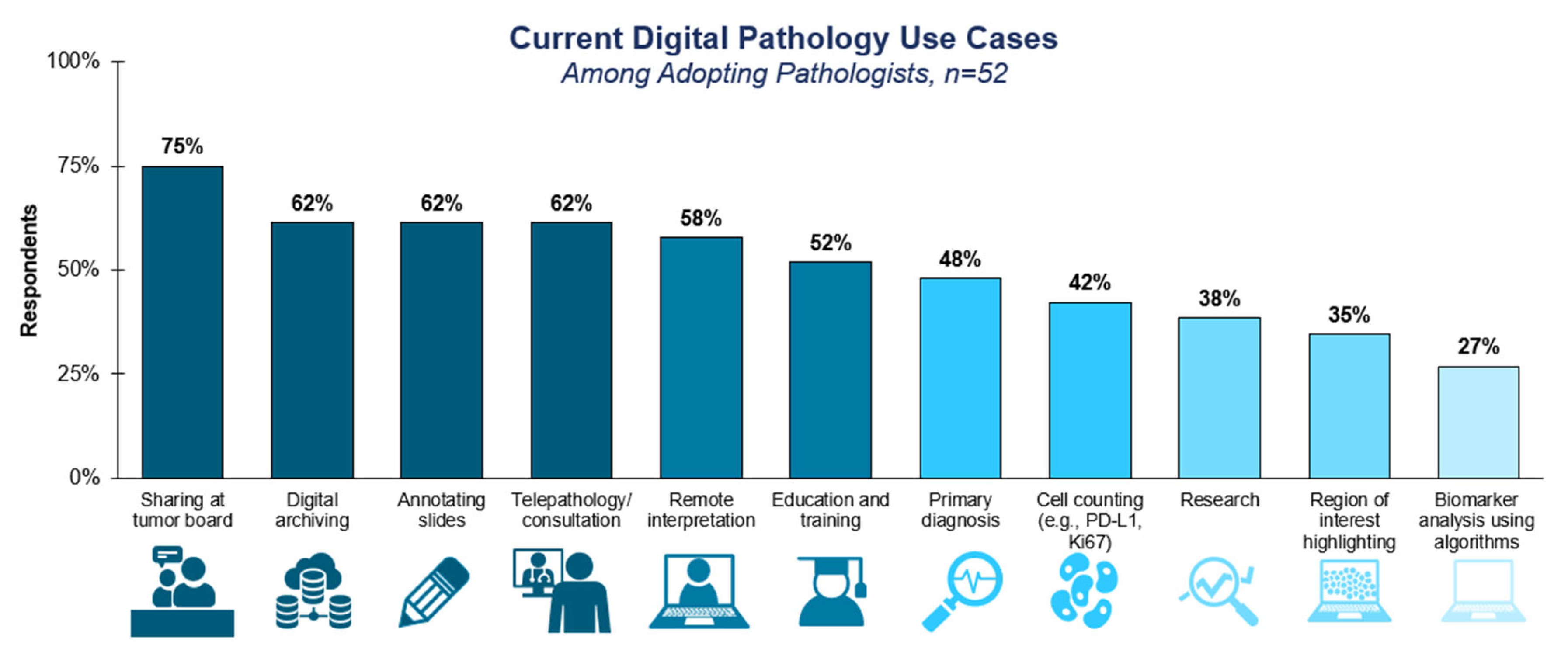
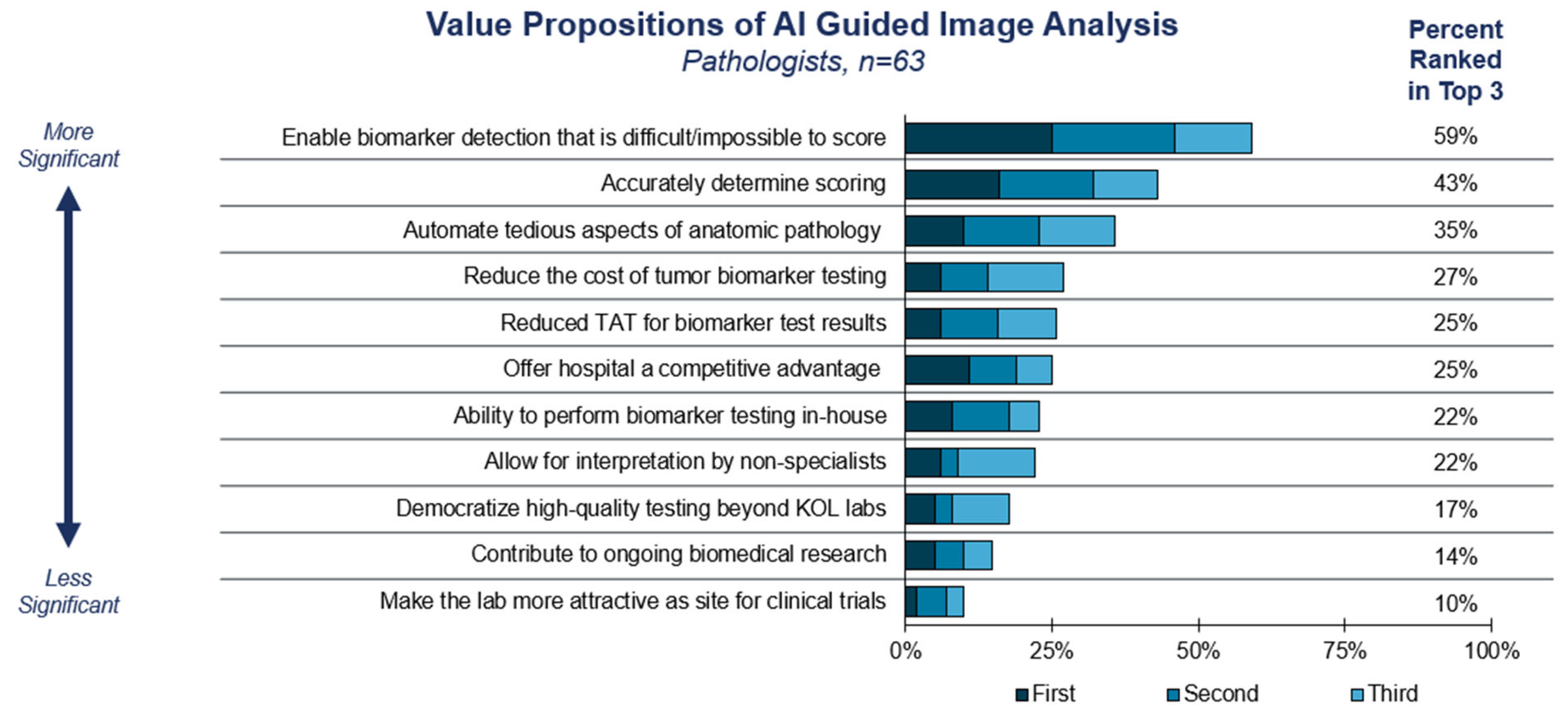
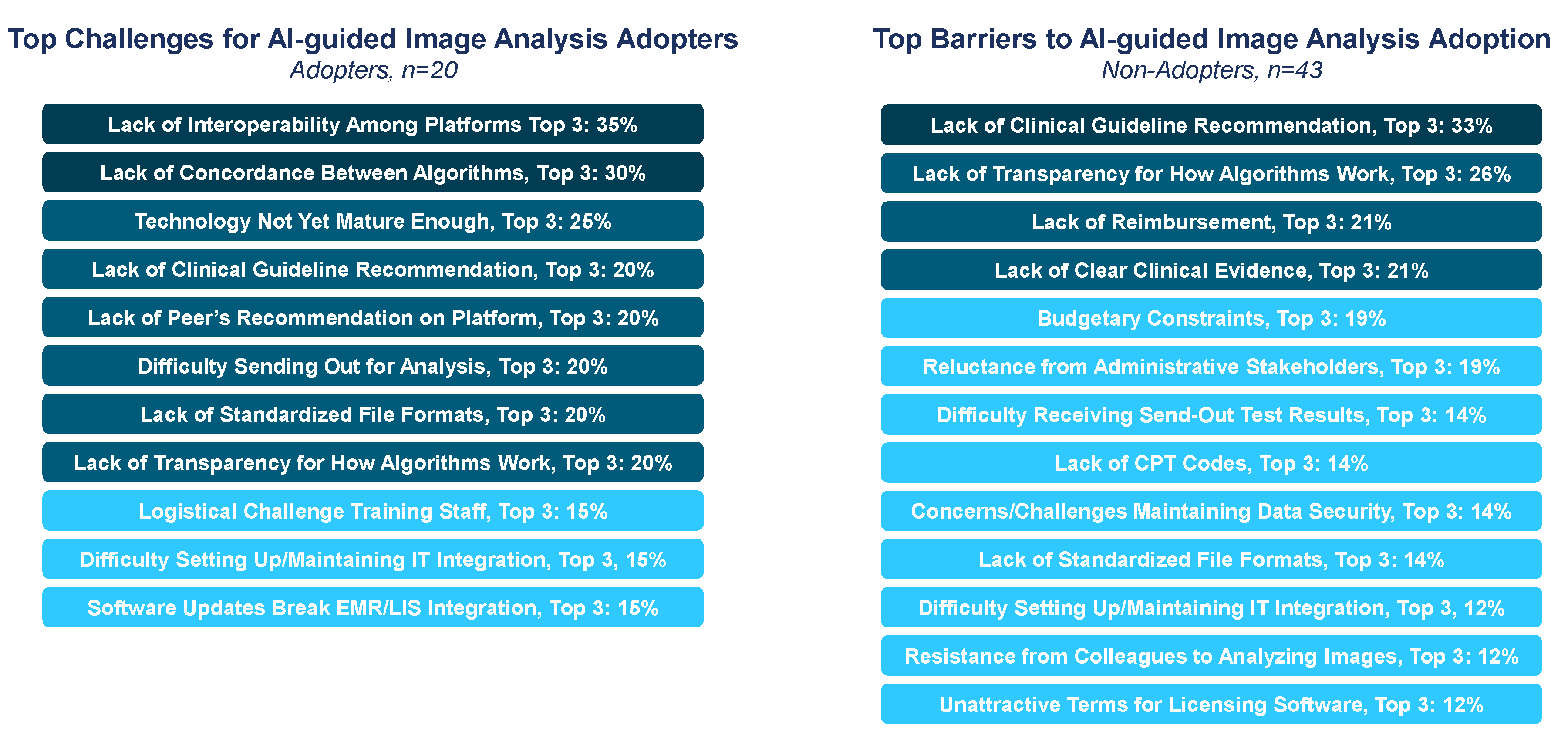
3.2. Adoption Considerations for Computational Pathology
3.3. Role for Guidelines in Promoting DP/CP Adoption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahn, S.W.; Plass, M.; Moinfar, F. Digital Pathology: Advantages, Limitations and Emerging Perspectives. J. Clin. Med. 2020, 9, 3697. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; L’Imperio, V.; Merolla, F.; Girolami, I.; Leoni, E.; Della Mea, V.; Pagni, F.; Fraggetta, F. The slow-paced digital evolution of pathology: Lights and shadows from a multifaceted board. Pathologica 2023, 115, 127–136. [Google Scholar] [CrossRef]
- Robboy, S.J.; Weintraub, S.; Horvath, A.E.; Jensen, B.W.; Alexander, C.B.; Fody, E.P.; Crawford, J.M.; Clark, J.R.; Cantor-Weinberg, J.; Joshi, M.G. Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Arch. Pathol. Lab. Med. 2013, 137, 1723–1732. [Google Scholar] [CrossRef]
- Bonert, M.; Zafar, U.; Maung, R.; El-Shinnawy, I.; Kak, I.; Cutz, J.-C.; Naqvi, A.; Juergens, R.A.; Finley, C.; Salama, S.; et al. Evolution of anatomic pathology workload from 2011 to 2019 assessed in a regional hospital laboratory via 574,093 pathology reports. PLoS ONE 2021, 16, e0253876. [Google Scholar] [CrossRef]
- Grizzle, W.E.; Bell, W.C.; Sexton, K.C. Issues in collecting, processing and storing human tissues and associated information to support biomedical research. Cancer Biomark. Sect. A Dis. Markers 2010, 9, 531–549. [Google Scholar] [CrossRef]
- Robert, M.E.; Rüschoff, J.; Jasani, B.; Graham, R.P.; Badve, S.S.; Rodriguez-Justo, M.; Kodach, L.L.; Srivastava, A.; Wang, H.L.; Tang, L.H.; et al. High Interobserver Variability Among Pathologists Using Combined Positive Score to Evaluate PD-L1 Expression in Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma. Mod. Pathol. 2023, 36, 100154. [Google Scholar] [CrossRef]
- Brunnström, H.; Johansson, A.; Westbom-Fremer, S.; Backman, M.; Djureinovic, D.; Patthey, A.; Isaksson-Mettävainio, M.; Gulyas, M.; Micke, P. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: Inter-pathologist variability is higher than assay variability. Mod. Pathol. 2017, 30, 1411–1421. [Google Scholar] [CrossRef]
- Verghese, G.; Lennerz, J.K.; Ruta, D.; Ng, W.; Thavaraj, S.; Siziopikou, K.P.; Naidoo, T.; Rane, S.; Salgado, R.; Pinder, S.E.; et al. Computational pathology in cancer diagnosis, prognosis, and prediction—Present day and prospects. J. Pathol. 2023, 260, 551–563. [Google Scholar] [CrossRef]
- Kapil, A.; Failmezger, H.; Spitzmüller, A.; Chan, J.; Haneder, S.; Shumilov, A.; Barkell, A.; Oguma, T.; Inaki, K.; Suto, F.; et al. Computational pathology–based HER2 quantification to identify novel biomarkers in gastric cancer (GC). J. Clin. Oncol. 2023, 41 (Suppl. S4), 449. [Google Scholar] [CrossRef]
- Pinto, D.G.; Bychkov, A.; Tsuyama, N.; Fukuoka, J.; Eloy, C. Exploring the adoption of digital pathology in clinical settings—Insights from a cross-continent study. medRxiv 2023, preprint. [Google Scholar]
- Hanna, M.G.; Reuter, V.E.; Samboy, J.; England, C.; Corsale, L.; Fine, S.W.; Agaram, N.P.; Stamelos, E.; Yagi, Y.; Hameed, M.; et al. Implementation of Digital Pathology Offers Clinical and Operational Increase in Efficiency and Cost Savings. Arch. Pathol. Lab. Med. 2019, 143, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- CAP. Digital Pathology Codes. 2024. Available online: https://www.cap.org/advocacy/payments-for-pathology-services/digital-pathology-codes (accessed on 10 March 2025).
- CMS. Clinical Laboratory Fee Schedule. 2024. Available online: https://www.cms.gov/medicare/payment/fee-schedules/clinical-laboratory-fee-schedule-clfs (accessed on 10 March 2025).
- Browning, L.; Colling, R.; Rittscher, J.; Winter, L.; McEntyre, N.; Verrill, C. Implementation of digital pathology into diagnostic practice: Perceptions and opinions of histopathology trainees and implications for training. J. Clin. Pathol. 2020, 73, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Chlipala, E.; Elin, J.; Eichhorn, E.; Huisman, A.; Krishnamurti, M.; Sabata, B. Archival and Retrieval in Digital Pathology Systems; Digital Pathology Association: Madison, WI, USA, 2011; pp. 1–10. [Google Scholar]
- Guo, H.; Birsa, J.; Farahani, N.; Hartman, D.J.; Piccoli, A.; O’Leary, M.; McHugh, J.; Nyman, M.; Stratman, C.; Kvarnstrom, V. Digital pathology and anatomic pathology laboratory information system integration to support digital pathology sign-out. J. Pathol. Inform. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, C.; Cancian, P.; Savevski, V.; Kotha, S.R.R.; Fraggetta, F.; Graziano, P.; Di Tommaso, L. Artificial Intelligence & Tissue Biomarkers: Advantages, Risks and Perspectives for Pathology. Cells 2021, 10, 787. [Google Scholar] [CrossRef]
- Hanna, M.G.; Ardon, O.; Reuter, V.E.; Sirintrapun, S.J.; England, C.; Klimstra, D.S.; Hameed, M.R. Integrating digital pathology into clinical practice. Mod. Pathol. 2022, 35, 152–164. [Google Scholar] [CrossRef]
- Digital Pathology Market Size, Share, Trends and Forecast by Product, Type, Delivery Model, End-User, Application, and Region, 2025–2033. IMARC Group. Report ID: SR112025A1836. 2024, pp. 1–10. Available online: https://www.imarcgroup.com/digital-pathology-market (accessed on 10 March 2025).
- Ho, J.; Ahlers, S.M.; Stratman, C.; Aridor, O.; Pantanowitz, L.; Fine, J.L.; Kuzmishin, J.A.; Montalto, M.C.; Parwani, A.V. Can digital pathology result in cost savings? A financial projection for digital pathology implementation at a large integrated health care organization. J. Pathol. Inform. 2014, 5, 33. [Google Scholar] [CrossRef]
- Williams, B.J.; Bottoms, D.; Treanor, D. Future-proofing pathology: The case for clinical adoption of digital pathology. J. Clin. Pathol. 2017, 70, 1010–1018. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Sinard, J.H.; Henricks, W.H.; Fatheree, L.A.; Carter, A.B.; Contis, L.; Beckwith, B.A.; Evans, A.J.; Lal, A.; Parwani, A.V.; et al. Validating whole slide imaging for diagnostic purposes in pathology: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch. Pathol. Lab. Med. 2013, 137, 1710–1722. [Google Scholar] [CrossRef]
- Bui, M.M.; Riben, M.W.; Allison, K.H.; Chlipala, E.; Colasacco, C.; Kahn, A.G.; Lacchetti, C.; Madabhushi, A.; Pantanowitz, L.; Salama, M.E.; et al. Quantitative Image Analysis of Human Epidermal Growth Factor Receptor 2 Immunohistochemistry for Breast Cancer: Guideline from the College of American Pathologists. Arch. Pathol. Lab. Med. 2019, 143, 1180–1195. [Google Scholar] [CrossRef]
- Hanna, M.G.; Olson, N.H.; Zarella, M.; Dash, R.C.; Herrmann, M.D.; Furtado, L.V.; Stram, M.N.; Raciti, P.M.; Hassell, L.; Mayset, A.; et al. Recommendations for Performance Evaluation of Machine Learning in Pathology: A Concept Paper from the College of American Pathologists. Arch. Pathol. Lab. Med. 2024, 148, e335–e361. [Google Scholar] [CrossRef]
- John, A.; Yang, B.; Shah, R. Clinical Impact of Adherence to NCCN Guidelines for Biomarker Testing and First-Line Treatment in Advanced Non-Small Cell Lung Cancer (aNSCLC) Using Real-World Electronic Health Record Data. Adv. Ther. 2021, 38, 1552–1566. [Google Scholar] [CrossRef] [PubMed]
- Lujan, G.; Quigley, J.C.; Hartman, D.; Parwani, A.; Roehmholdt, B.; Van Meter, B.; Ardon, O.; Hanna, M.G.; Kelly, D.; Sowards, C.; et al. Dissecting the Business Case for Adoption and Implementation of Digital Pathology: A White Paper from the Digital Pathology Association. J. Pathol. Inform. 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Fraggetta, F.; L’Imperio, V.; Ameisen, D.; Carvalho, R.; Leh, S.; Kiehl, T.-R.; Serbanescu, M.; Racoceanu, D.; Della Mea, V.; Polonia, A. Best Practice Recommendations for the Implementation of a Digital Pathology Workflow in the Anatomic Pathology Laboratory by the European Society of Digital and Integrative Pathology (ESDIP). Diagnostics 2021, 11, 2167. [Google Scholar] [CrossRef]
- Thusini, S.; Milenova, M.; Nahabedian, N.; Grey, B.; Soukup, T.; Chua, K.-C.; Henderson, C. The development of the concept of return-on-investment from large-scale quality improvement programmes in healthcare: An integrative systematic literature review. BMC Health Serv. Res. 2022, 22, 1492. [Google Scholar]
- Ardon, O.; Asa, S.; Lloyd, M.; Lujan, G.; Parwani, A.; Santa-Rosario, J.; Van Meter, B.; Samboy, J.; Pirain, D.; Blakely, S.; et al. Understanding the financial aspects of digital pathology: A dynamic customizable return on investment calculator for informed decision-making. J. Pathol. Inform. 2024, 15, 100376. [Google Scholar] [CrossRef]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Sharma, A.; Carter, A.B.; Kurc, T.; Sussman, A.; Saltz, J. Twenty Years of Digital Pathology: An Overview of the Road Travelled, What is on the Horizon, and the Emergence of Vendor-Neutral Archives. J. Pathol. Inform. 2018, 9, 40. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Hanna, M.; Pantanowitz, J.; Lennerz, J.; Henricks, W.H.; Shen, P.; Quinn, B.; Bennet, S.; Rashidi, H.H. Regulatory Aspects of Artificial Intelligence and Machine Learning. Mod. Pathol. 2024, 37, 100609. [Google Scholar] [CrossRef]


| Survey Respondent Demographics | ||
|---|---|---|
| Pathologists and Lab Directors (n = 63) | ||
| Respondents | ||
| Title/Role | Laboratory director | 67% |
| Laboratory manager/supervisor | 3% | |
| Staff pathologist | 30% | |
| Geographic Region | West | 33% |
| Midwest | 16% | |
| South | 27% | |
| Northeast | 24% | |
| Lab Setting | Independent reference lab | 6% |
| Academic hospital | 37% | |
| Community hospital | 46% | |
| Academic-affiliated community hospital | 11% | |
| Current Use of Digital Pathology (DP) | Non-user but familiar with DP | 14% |
| User of DP for educational purposes only | 17% | |
| User of DP for primary diagnosis only | 11% | |
| User of DP for multiple purposes | 57% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessen, J.L.; Alexander, M.; Foroughi, O.; Brathwaite, R.; Baser, E.; Lee, L.C.; Perez, O.; Gustavsen, G. Perspectives on Reducing Barriers to the Adoption of Digital and Computational Pathology Technology by Clinical Labs. Diagnostics 2025, 15, 794. https://doi.org/10.3390/diagnostics15070794
Bessen JL, Alexander M, Foroughi O, Brathwaite R, Baser E, Lee LC, Perez O, Gustavsen G. Perspectives on Reducing Barriers to the Adoption of Digital and Computational Pathology Technology by Clinical Labs. Diagnostics. 2025; 15(7):794. https://doi.org/10.3390/diagnostics15070794
Chicago/Turabian StyleBessen, Jeffrey L., Melissa Alexander, Olivia Foroughi, Roderick Brathwaite, Emre Baser, Liam C. Lee, Omar Perez, and Gary Gustavsen. 2025. "Perspectives on Reducing Barriers to the Adoption of Digital and Computational Pathology Technology by Clinical Labs" Diagnostics 15, no. 7: 794. https://doi.org/10.3390/diagnostics15070794
APA StyleBessen, J. L., Alexander, M., Foroughi, O., Brathwaite, R., Baser, E., Lee, L. C., Perez, O., & Gustavsen, G. (2025). Perspectives on Reducing Barriers to the Adoption of Digital and Computational Pathology Technology by Clinical Labs. Diagnostics, 15(7), 794. https://doi.org/10.3390/diagnostics15070794






